Abstract
Elevated catecholamine levels are a well-recognized cause of various types of cardiomyopathy. Causes of catecholamine elevation include tumors, toxins, drugs, emotional stress, and sepsis. Milnacipran is a dual and equipotent inhibitor of norepinephrine and serotonin uptake. It is frequently prescribed as therapy for fibromyalgia, and the drug has a good safety profile. Herein, we report the case of a 42-year-old woman with undefined connective-tissue disease and fibromyalgia who developed a severe and reversible cardiomyopathy while taking recommended doses of milnacipran. The cardiomyopathy was associated with a hyperadrenergic state manifested by tachycardia, hypertension, and elevated plasma catecholamine levels. The discontinuation of milnacipran and the initiation of anti-failure therapy resulted in complete resolution of the cardiomyopathy in 6 months. To our knowledge, this is the first report of milnacipran as a possible cause of catecholamine-induced cardiomyopathy.
Key words: Antidepressive agents, second-generation/administration & dosage/adverse effects/therapeutic use; cardiomyopathies/diagnosis/epidemiology/etiology/therapy; catecholamines/adverse effects; fibromyalgia/drug therapy; hypertension/chemically induced; magnetic resonance angiography; milnacipran; myocardium/pathology; serotonin uptake inhibitors/adverse effects/therapeutic use; takotsubo cardiomyopathy/chemically induced; treatment outcome; ventricular dysfunction/chemically induced
Excessive levels of circulating catecholamines secondary to tumors, emotional stress, sepsis, toxins, or drugs are a well-recognized cause of global or focal (takotsubo) cardiomyopathy.1 Milnacipran, a serotonin and norepinephrine uptake inhibitor (SNRI), was recently approved as therapy for the common, chronic, unexplained-pain syndrome called fibromyalgia.2,3 In contrast with the 2 other SNRIs (venlafaxine and duloxetine), which have a higher affinity for serotonin than for norepinephrine receptors, milnacipran has a balanced ratio of potency in the inhibition of norepinephrine and serotonin uptake.2 In large clinical trials, the drug has had a good cardiovascular safety profile, causing only minor effects on heart rate and systolic blood pressure in a small percentage of patients.3,4 However, rare instances of sustained tachycardia and hypertension have been described.5,6 Herein, we report the case of a patient with undefined connective-tissue disease (CTD) who developed a severe and reversible cardiomyopathy in the presence of tachycardia and hypertension induced by milnacipran.
Case Report
In July 2007, a 42-year-old woman visited a rheumatologist in Belgium. She had widespread arthralgia without synovitis. Serology results were positive for rheumatoid factor, antinuclear antibody (titer of 1:1,000), anti-extractable nuclear antigen, and anti-ribonucleic protein. Results were negative for anti-double-stranded DNA, lupus anticoagulant, anti-Sm, anti-Jo-1, c-neutrophil cytoplasmic antibodies, anticyclic citrullinated protein antibodies, and anti-hepatitis B and C. Results for thyroid function, acute-phase proteins, and bone marrow aspiration were normal; however, an abnormal monoclonal gammopathy was detected. The patient reported no photosensitivity. She had mild symptoms of Sjögren syndrome and mild Raynaud phenomenon with normal capillaroscopic patterns. She had no history of alopecia or oral aphthae.
In March 2008, the patient was referred to a rheumatologist in the United States because of arthralgia that continued despite therapy with hydroxychloroquine (200 mg twice daily for 4 wk). Examination again revealed no synovitis or rash. Upon testing, anti-ribonucleic protein was strongly positive, and anti-Sm was now positive. Tests revealed negative results for anti-SSA, anti-SSB, and anti-double-stranded DNA antibodies; normal levels of acute-phase proteins and complement; and slight leucopenia (white cell count, 3,900/mm3). The diagnosis was mild undifferentiated CTD without internal-organ involvement, and the hydroxychloroquine therapy was continued. Upon follow-up examination in July 2008, the patient had widespread pain with tenderness and fatigue, and superimposed, unrelated fibromyalgia was strongly suspected. Testing to detect abnormal levels of muscle enzymes yielded negative results, and the patient had no proximal muscle weakness. Because of her continued widespread pain, she was started on a low dose of prednisone (5 mg/d), which improved her symptoms.
The patient returned to the United States for follow-up in June 2009. She reported photosensitivity, a sense of generalized fluid retention from the corticosteroids, and diffuse pain. Again, no synovitis was detected. She was slowly weaned from the steroids, and trial therapy with milnacipran was titrated over a few weeks to the recommended dose of 50 mg twice daily, with continuation of hydroxychloroquine. The patient's symptoms improved during the next 6 months.
In December 2009, outside the U.S., she sought emergency treatment for atypical chest pain and occipital headache. Although her cardiac enzyme levels were normal, an abnormal electrocardiogram (ECG) prompted an immediate referral to a cardiologist for detailed evaluation. In contrast with the multiple recordings of normal heart rate and blood pressure during the prior 18 months, examination now revealed sinus tachycardia (heart rate, 120 beats/min) and hypertension (blood pressure, 160/100 mmHg). Her jugular venous pressure was not elevated, and there was no evidence of peripheral edema. Cardiac auscultation revealed a prominent summation gallop and a short systolic ejection murmur, and an ECG showed sinus tachycardia, right-axis deviation, prominent voltage, and T-wave inversion in the lateral leads. A 2-dimensional echocardiogram showed normal left ventricular (LV) size and wall thickness, severe global hypokinesis, an LV ejection fraction (LVEF) of 0.30, evidence of grade 3 diastolic dysfunction, and mild mitral regurgitation with a structurally normal mitral valve. No pericardial effusion was evident.
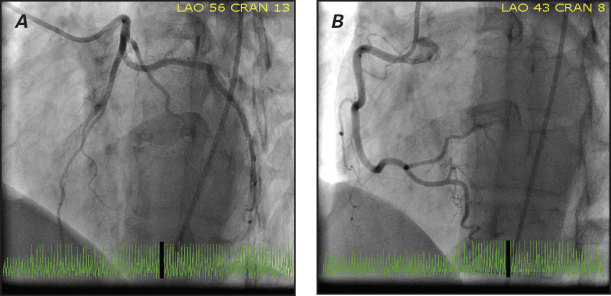
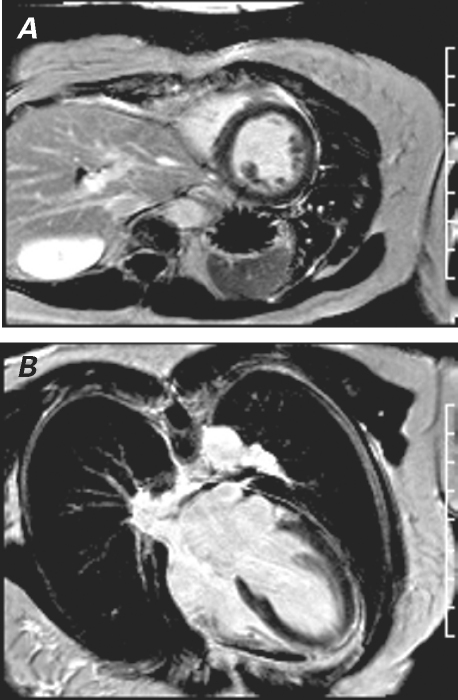
The patient was started on 2.5 mg of enalapril twice daily and 3.125 mg of carvedilol twice daily. She returned to the United States 10 days later for further evaluation. Examination revealed persistent hypertension (blood pressure, 140/95 mmHg), sinus tachycardia (heart rate, 110 beats/min), and a loud summation gallop. Extensive laboratory testing, including thyroid function tests and iron studies, yielded no unusual results. Blood samples obtained 2 weeks after this latest presentation revealed an elevated total catecholamine level of 978 pg/mL (normal, ≤504 pg/mL), an elevated norepinephrine level of 916 pg/mL (normal, ≤420 pg/mL), and an epinephrine level of 62 pg/mL (normal, ≤84 pg/mL). The patient subsequently underwent left-sided heart catheterization, which revealed angiographically normal coronary arteries (Fig. 1). A ventriculogram showed global hypokinesis with a calculated LVEF of 0.35. Detailed-protocol cardiovascular magnetic resonance (CMR) was performed, including trueFISP cine, T1- and T2-weighted black blood imaging, T2* imaging for hemochromatosis, and first-pass perfusion imaging with weighted enhancement (early and late). Findings included generalized LV enlargement with an end-diastolic volume of 135 mL and an end-systolic volume of 101 mL; severe global LV systolic dysfunction with a calculated LVEF of 0.26; minuscule amounts of pericardial fluid; no hemochromatosis or amyloidosis; and no evidence of edema, inflammation (on early gadolinium enhancement), necrosis, or fibrosis (Fig. 2).
Fig. 1 Coronary angiograms (left anterior oblique view) show normal A) left and B) right coronary arteries.
Fig. 2 Cardiovascular magnetic resonance after gadolinium inversion recovery. Late-enhancement-sequence images in A) short-axis and B) 4-chamber views show that the myocardium is normal, with no evidence of irreversible myocyte injury.
Given the absence of inflammatory tissue and infiltrative disorder, the presumptive diagnosis was catecholamine-induced cardiomyopathy secondary to milnacipran use. The patient was weaned from milnacipran over 2 weeks, with titration of an angiotensin-converting enzyme (ACE) inhibitor and an α/β-blocker to 10 mg of enalapril twice daily and 25 mg of carvedilol twice daily. This change in therapy was associated with normalization of the patient's blood pressure and heart rate. An echocardiogram 3 months after presentation showed improved LV systolic function, with the LVEF estimated at 0.45. Six months after presentation, CMR revealed an LVEF of 0.56 with an end-diastolic volume of 80 mL and an end-systolic volume of 40 mL. An echocardiogram confirmed normal systolic function with trivial mitral regurgitation, and an ECG showed resolution of the T-wave inversion. Because the patient's pain had returned after the discontinuation of milnacipran, pregabalin was added to her hydroxychloroquine therapy, and this led to modest improvement in the pain symptoms. Her serologic results remained stable, without evidence of internal-organ involvement, 4 years after initial presentation. After her LV systolic function returned to normal, she was weaned from the ACE inhibitor and α/β-blocker during the next few months. Her heart rate, blood pressure, and cardiac function remained normal 20 months after the development of the cardiomyopathy.
Discussion
Serotonin norepinephrine reuptake inhibitors are commonly prescribed as therapy for depression and for fibromyalgia.3,4 The 3 SNRIs approved in the United States are venlafaxine, duloxetine, and milnacipran. Although venlafaxine and duloxetine have a 30- and 10-fold selectivity, respectively, for serotonin, milnacipran is nonselective in blocking the uptakes of norepinephrine and serotonin.2
Patients generally tolerate SNRIs well,2–4 and milnacipran has an excellent cardiovascular safety profile, with little effect on electrophysiologic values.7,8 Clinical investigators have documented very modest increases in heart rate (3–5 beats/min) and systolic pressure (1–3 mmHg) in study subjects who took 100 to 200 mg of oral milnacipran daily; however, in rare instances, oral milnacipran has caused significant and sustained hypertension and tachycardia.3,4 Intravenous milnacipran has significantly increased heart rate (by approximately 19% in the first 50 min) and systolic blood pressure (by approximately 21% in the first 10 min).8 One patient with manic-depressive psychosis who took 100 mg/d of oral milnacipran developed a hypertensive response (blood pressure, 160/100 mmHg)5; another patient who took 150 mg/d of milnacipran developed severe hypertension, but his blood pressure fell to acceptable levels when the dose was reduced to 100 mg/d.6 The proposed mechanism of hypertension from the taking of SNRIs is increased vascular resistance, mediated by increased noradrenergic neurotransmission secondary to greater availability of norepinephrine at the postjunctional receptor.5
It is likely that our patient developed a severe, nondilated, global cardiomyopathy because of a milnacipran-induced hyperadrenergic state: milnacipran was associated with her sinus tachycardia, hypertension, and elevated plasma levels of norepinephrine. Moreover, the discontinuation of milnacipran and the institution of therapy with an ACE inhibitor and an α/β-adrenoceptor blocker resulted in normal cardiac function in 6 months and resolution of the sinus tachycardia and hypertension. At 20 months' follow-up, the patient remained normotensive without taking ACE inhibitors or α/β-blockers.
Our conclusion that a hyperadrenergic state caused our patient's cardiomyopathy conforms with the fact that elevated catecholamine levels induce cardiac dysfunction.1,9,10 In this regard, studies have shown that excess catecholamines induce numerous abnormal patterns. These include dilated global cardiomyopathy, hypertrophic cardiomyopathy, and regional wall-motion abnormalities that typically manifest themselves as apical ballooning (takotsubo cardiomyopathy).1 A recent case of takotsubo cardiomyopathy was attributed to an overdose of the SNRI venlafaxine,11 and another was associated with appropriate doses of duloxetine.12 The mechanisms of catecholamine-induced cardiomyopathy are multifactorial and not well understood.
It is conceivable, although unlikely, that myocarditis unrelated to milnacipran caused our patient's cardiomyopathy. Potential causes include viral infections, CTD, and hydroxychloroquine. Histologic evidence of myocardial involvement in CTD varies according to the type of disease; for example, the prevalence is as high as 40% in systemic lupus erythematosus.13 However, in CTD, fulminant myocarditis that results in cardiac failure is extremely rare and is usually associated with pericarditis.13–15 The findings in our patient met the clinical and serologic standards for undefined CTD, which invariably has a good prognosis and rarely progresses to a defined CTD with major organ involvement.16,17 At 4 years after diagnosis, she had not developed new symptoms, and the serologic markers showed no change that would support a diagnosis of progressive CTD (such as scleroderma or systemic lupus erythematosus) as a cause of the cardiomyopathy. The CMR findings excluded myocarditis or an infiltrative disorder. Of note, CMR is useful in detecting viral and autoimmune myocarditis, because both processes produce a marked inflammatory response that results in capillary leakage, edema, and necrosis/fibrosis. When tissue-inflammation markers (the Lake Louise consensus criteria) for CMR are used, myocarditis can be ruled out with a diagnostic accuracy of 78%.18 Because CMR showed no inflammatory response in our patient and her ventricular function became normal without immunosuppressive therapy, a viral- or immune-mediated cause was very unlikely. Rarely, hydroxychloroquine produces a cardiomyopathy characterized by ventricular hypertrophy, atrial enlargement, and pathognomonic findings on electron microscopy.19,20 However, echocardiograms in our patient revealed no features to support this diagnosis, and her cardiac function was restored with continuing hydroxychloroquine therapy.
This case shows that patients who are taking SNRIs —including milnacipran—should be carefully monitored for the appearance of a severe hyperadrenergic state manifested by sinus tachycardia and hypertension. This state may result in a catecholamine-induced cardiomyopathy that is reversible upon the discontinuation of the SNRI and the initiation of appropriate anti-failure therapy. To our knowledge, this is the first documentation of milnacipran as a possible cause of catecholamine-induced cardiomyopathy.
Footnotes
Address for reprints: Mervyn B. Forman, MD, 960 Johnson Ferry Rd., Suite 530, Atlanta, GA 30342
E-mail: formanm@bellsouth.net
References
- 1.Kassim TA, Clarke DD, Mai VQ, Clyde PW, Shakir KMM. Catecholamine-induced cardiomyopathy. Endocr Pract 2008; 14(9):1137–49. [DOI] [PubMed]
- 2.Montgomery SA. Tolerability of serotonin norepinephrine reuptake inhibitor antidepressants. CNS Spectr 2008;13(7 Suppl 11):27–33. [DOI] [PubMed]
- 3.Mease PJ, Clauw DJ, Gendreau RM, Rao SG, Kranzler J, Chen W, Palmer RH. The efficacy and safety of milnacipran for treatment of fibromyalgia. A randomized, double-blind, placebo-controlled trial [published erratum appears in J Rheumatol 2009;36(3):661]. J Rheumatol 2009;36(2):398–409. [DOI] [PubMed]
- 4.Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial [published errata appear in Clin Ther 2009;31(2):446 and Clin Ther 2009;31(7):1617]. Clin Ther 2008;30(11):1988–2004. [DOI] [PubMed]
- 5.de Toledo Ferraz Alves TC, Guerra de Andrade A. Hypertension induced by regular doses of milnacipran: a case report. Pharmacopsychiatry 2007;40(1):41–2. [DOI] [PubMed]
- 6.Yoshida K, Higuchi H, Takahashi H, Shimizu T. Elevation of blood pressure induced by high-dose milnacipran. Hum Psychopharmacol 2002;17(8):431. [DOI] [PubMed]
- 7.Periclou A, Palmer RH, Zheng H, Lindamood C 3rd. Effects of milnacipran on cardiac repolarization in healthy participants. J Clin Pharmacol 2010;50(4):422–33. [DOI] [PubMed]
- 8.Caron J, Libersa C, Hazard JR, Lacroix D, Facq E, Guedon-Moreau L, et al. Acute electrophysiological effects of intravenous milnacipran, a new antidepressant agent. Eur Neuropsychopharmacol 1993;3(4):493–500. [DOI] [PubMed]
- 9.Sutherland JA, Al Chekakie MO, Moran JF. Catecholamine-induced cardiomyopathy rapidly reversed with beta-blocker therapy. Congest Heart Fail 2009;15(4):193–5. [DOI] [PubMed]
- 10.Budhwani N, Bonaparte KL, Cuyjet AB, Saric M. Severe reversible left ventricular systolic and diastolic dysfunction due to accidental iatrogenic epinephrine overdose. Rev Cardiovasc Med 2004;5(2):130–3. [PubMed]
- 11.Christoph M, Ebner B, Stolte D, Ibrahim K, Kolschmann S, Strasser RH, Schon S. Broken heart syndrome: Tako Tsubo cardiomyopathy associated with an overdose of the serotonin-norepinephrine reuptake inhibitor venlafaxine. Eur Neuropsychopharmacol 2010;20(8):594–7. [DOI] [PubMed]
- 12.Selke KJ, Dhar G, Cohn JM. Takotsubo cardiomyopathy associated with titration of duloxetine. Tex Heart Inst J 2011;38 (5):573–6. [PMC free article] [PubMed]
- 13.Doherty NE, Siegel RJ. Cardiovascular manifestations of systemic lupus erythematosus. Am Heart J 1985;110(6):1257–65. [DOI] [PubMed]
- 14.Doria A, Iaccarino L, Sarzi-Puttini P, Atzeni F, Turriel M, Petri M. Cardiac involvement in systemic lupus erythematosus. Lupus 2005;14(9):683–6. [DOI] [PubMed]
- 15.Lundberg IE. Cardiac involvement in autoimmune myositis and mixed connective tissue disease. Lupus 2005;14(9):708–12. [DOI] [PubMed]
- 16.Mosca M, Tani C, Bombardieri S. Undifferentiated connective tissue diseases (UCTD): a new frontier for rheumatology. Best Pract Res Clin Rheumatol 2007;21(6):1011–23. [DOI] [PubMed]
- 17.Vaz CC, Couto M, Medeiros D, Miranda L, Costa J, Nero P, et al. Undifferentiated connective tissue disease: a seven-center cross-sectional study of 184 patients. Clin Rheumatol 2009;28(8):915–21. [DOI] [PubMed]
- 18.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 2009;53(17):1475–87. [DOI] [PMC free article] [PubMed]
- 19.Ratliff NB, Estes ML, Myles JL, Shirey EK, McMahon JT. Diagnosis of chloroquine cardiomyopathy by endomyocardial biopsy. N Engl J Med 1987;316(4):191–3. [DOI] [PubMed]
- 20.Costedoat-Chalumeau N, Hulot JS, Amoura Z, Delcourt A, Maisonobe T, Dorent R, et al. Cardiomyopathy related to antimalarial therapy with illustrative case report. Cardiology 2007;107(2):73–80. [DOI] [PubMed]