Abstract
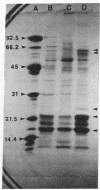
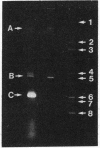
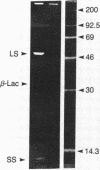
Anacystis nidulans 6301 has been transformed in the light to ampicillin resistance with the plasmid pBR322. Permeaplasts prepared by 2-hr treatment of cells with lysozyme and EDTA are transformed with a 50-fold higher efficiency than that observed for cells. beta-Lactamase is present in A. nidulans transformed either with pBR322 or the plasmid pCH1 as evidenced by hydrolysis of the beta-lactam ring of Nitrocefin in extracts of transformants. beta-Lactamase also can be immunoprecipitated from extracts of [35S]methionine-labeled pBR322 transformants and coprecipitates with ribulose-bisphosphate carboxylase. Expression of the carboxylase is apparently amplified in pBR322 transformants as is that for several soluble proteins in pCH1 transformants. Chromosomal DNA per cell is increased about 6-fold after transformation of A. nidulans 6301 with either pBR322 or pCH1. A 4.3-kilobase-pair plasmid can be isolated from pBR322 transformants in addition to the endogenous plasmids pUH24 and pUH25.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chauvat F., Astier C., Vedel F., Joset-Espardellier F. Transformation in the cyanobacterium Synechococcus R2: improvement of efficiency; role of the pUH24 plasmid. Mol Gen Genet. 1983;191(1):39–45. doi: 10.1007/BF00330887. [DOI] [PubMed] [Google Scholar]
- Daniell H., Krishnan M., Ranganathan M., Gnanam A. Radioisotopic evidence for the polypeptides associated with photosystem II. Biochem Biophys Res Commun. 1984 Dec 28;125(3):988–995. doi: 10.1016/0006-291x(84)91381-0. [DOI] [PubMed] [Google Scholar]
- Gendel S., Straus N., Pulleyblank D., Williams J. Shuttle cloning vectors for the cyanobacterium Anacystis nidulans. J Bacteriol. 1983 Oct;156(1):148–154. doi: 10.1128/jb.156.1.148-154.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Sherman L. A. A hybrid plasmid is a stable cloning vector for the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1983 Sep;155(3):966–972. doi: 10.1128/jb.155.3.966-972.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolowsky K. S., Williams J. G., Szalay A. A. Length of foreign DNA in chimeric plasmids determines the efficiency of its integration into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1984 Mar;27(3):289–299. doi: 10.1016/0378-1119(84)90073-8. [DOI] [PubMed] [Google Scholar]
- Kuhlemeier C. J., Borrias W. E., van den Hondel C. A., van Arkel G. A. Vectors for cloning in cyanobacteria: construction and characterization of two recombinant plasmids capable of transformation of Escherichia coli K12 and Anacystis nidulans R2. Mol Gen Genet. 1981;184(2):249–254. doi: 10.1007/BF00272912. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lopez P., Espinosa M., Stassi D. L., Lacks S. A. Facilitation of plasmid transfer in Streptococcus pneumoniae by chromosomal homology. J Bacteriol. 1982 May;150(2):692–701. doi: 10.1128/jb.150.2.692-701.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurquin P. F., Kleinhofs A. Effects of chloramphenicol on plant cells: potential as a selectable marker for transformation studies. Biochem Biophys Res Commun. 1982 Jul 16;107(1):286–293. doi: 10.1016/0006-291x(82)91702-8. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., van de Putte P. Construction of a hybrid plasmid capable of replication in the bacterium Escherichia coli and the cyanobacterium Anacystis nidulans. J Bacteriol. 1982 Apr;150(1):410–413. doi: 10.1128/jb.150.1.410-413.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Tomioka N., Yamada C., Sugiura M. Cloning and characterization of a plasmid DNA from anacystis nidulans 6301. Gene. 1982 Sep;19(2):221–224. doi: 10.1016/0378-1119(82)90009-9. [DOI] [PubMed] [Google Scholar]
- Simon R. D. Survey of extrachromosomal DNA found in the filamentous cyanobacteria. J Bacteriol. 1978 Oct;136(1):414–418. doi: 10.1128/jb.136.1.414-418.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassi D. L., Lopez P., Espinosa M., Lacks S. A. Cloning of chromosomal genes in Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7028–7032. doi: 10.1073/pnas.78.11.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadanier J., Hallas R., Holms J., Freiberg L. A., Bacino D. 3-amino-3-demethoxyfortimicin A and the C-2 epimeric-2-amino-3-O-demethyl-2-deoxyfortimicins A. J Antibiot (Tokyo) 1983 Mar;36(3):267–275. doi: 10.7164/antibiotics.36.267. [DOI] [PubMed] [Google Scholar]
- Tandeau de Marsac N., Borrias W. E., Kuhlemeier C. J., Castets A. M., van Arkel G. A., van den Hondel C. A. A new approach for molecular cloning in cyanobacteria: cloning of an Anacystis nidulans met gene using a Tn901-induced mutant. Gene. 1982 Nov;20(1):111–119. doi: 10.1016/0378-1119(82)90092-0. [DOI] [PubMed] [Google Scholar]
- Vasseghi H., Claverys J. P. Amplification of a chimeric plasmid carrying an erythromycin-resistance determinant introduced into the genome of Streptococcus pneumoniae. Gene. 1983 Mar;21(3):285–292. doi: 10.1016/0378-1119(83)90012-4. [DOI] [PubMed] [Google Scholar]
- Ward B., Myers J. Photosynthetic properties of permaplasts of anacystis. Plant Physiol. 1972 Nov;50(5):547–550. doi: 10.1104/pp.50.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Szalay A. A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1983 Sep;24(1):37–51. doi: 10.1016/0378-1119(83)90129-4. [DOI] [PubMed] [Google Scholar]
- Wilson C. R., Morgan A. E. Chromosomal-DNA amplification in Bacillus subtilis. J Bacteriol. 1985 Aug;163(2):445–453. doi: 10.1128/jb.163.2.445-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. Gene amplification in Bacillus subtilis. J Gen Microbiol. 1984 Jul;130(7):1613–1621. doi: 10.1099/00221287-130-7-1613. [DOI] [PubMed] [Google Scholar]
- van den Hondel C. A., Keegstra W., Borrias W. E., van Arkel G. A. Homology of plasmids in strains of unicellular Cyanobacteria. Plasmid. 1979 Jul;2(3):323–333. doi: 10.1016/0147-619x(79)90016-7. [DOI] [PubMed] [Google Scholar]
- van den Hondel C. A., Verbeek S., van der Ende A., Weisbeek P. J., Borrias W. E., van Arkel G. A. Introduction of transposon Tn901 into a plasmid of Anacystis nidulans: preparation for cloning in cyanobacteria. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1570–1574. doi: 10.1073/pnas.77.3.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]