Abstract
Cardiac neural crest (CNC) plays a requisite role during cardiovascular development and defects in the formation of CNC-derived structures underlie several common forms of human congenital birth defects. Migration of the CNC cells to their destinations as well as expansion and maintenance of these cells are important for the normal development of the cardiac outflow tract and aortic arch arteries; however, molecular mechanisms regulating these processes are not well-understood. Fibronectin (FN) protein is present along neural crest migration paths and neural crest cells migrate when plated on FN in vitro; therefore, we tested the role of FN during the development of the CNC in vivo. Our analysis of the fate of the neural crest shows that CNC cells reach their destinations in the branchial arches and the cardiac outflow tract in the absence of FN or its cellular receptor integrin α5β1. However, we found that FN and integrin α5 modulate CNC proliferation and survival, and are required for the presence of normal numbers of CNC cells at their destinations.
Keywords: cardiac neural crest, fibronectin, integrin alpha5, proliferation, survival
1. Introduction
Neural crest cells (NCCs) are a population of multipotent progenitor cells that originate in the dorsal neural tube during embryogenesis (Crane and Trainor, 2006). In the dorsal neural tube, NCC progenitors undergo the epithelial-to-mesenchymal transition (EMT), detach from the neural tube and migrate to their diverse destinations. Depending on the axial position, NCCs give rise to a wide array of cell types in the embryo, including neurons, glia, vascular smooth muscle cells (VSMCs), cranial bones and cartilage (Le Douarin, 1982).
In chick, a subpopulation of the cranial NC originating from the region of the dorsal neural tube between the mid-otic placode and the posterior boundary of the third somite is termed the cardiac neural crest (CNC). CNC gives rise to VSMCs around aortic arch arteries and the distal portions of aorta and pulmonary artery (Hutson and Kirby, 2007). The importance of the CNC in cardiovascular development was first recognized in seminal studies performed in chick, showing that the ablation of a portion of the dorsal neural tube containing CNC progenitors resulted in aberrant septation and positioning of the cardiac outflow tract, absence of CNC-derived VSMCs and defective remodeling of pharyngeal arch arteries into their canonical mature asymmetric arrangement. These defects resemble some of the most common and severe forms of human congenital cardiovascular disorders, including those found in patients with DiGeorge syndrome (Hutson and Kirby, 2007). Similar to the chick, mouse NC progenitors that give rise to the CNC originate between the pro-rhombomere c and the fourth somite (Chan et al., 2004).
In vitro experiments performed with NCCs isolated from different axial levels of the neural tube showed that depending on their site of origin, NCCs exhibit different migratory responses to extracellular matrix (ECM). These experiments also showed that components of the ECM and levels and repertoire of integrins expressed by NCCs modulate NCC migratory behavior (Delannet et al., 1994; Strachan and Condic, 2003; Testaz et al., 1999; Xu et al., 2006). While there are examples indicating that some ECM components (e.g. laminin alpha 5 and fibulin-1) modulate NC migratory paths in vivo (Coles et al., 2006; Cooley et al., 2008), the functions of many other components of the ECM during the development of the NC are not completely understood.
Fibronectin (FN) is an essential component of the ECM present along the paths of NCC transit (Duband and Thiery, 1982; George et al., 1997; Mayer et al., 1981; Rovasio et al., 1983). The appreciation that both the FN gene and the neural crest are unique to vertebrates led to the hypothesis that FN could have evolved to play an important role during the development of the neural crest and its derived lineages (Hynes and Zhao, 2000; Whittaker et al., 2006). While experiments in vitro indicate that FN serves as a permissive substratum for NCC migration (Rovasio et al., 1983) the function of FN during NC development in vivo is not known.
In order to determine the role of FN in the development of the neural crest, we performed lineage-tracing experiments to follow the fate of CNC precursors in embryos that lack FN or its cellular receptor, integrin α5. While we observed extensive migration of cranial NCCs, including CNCCs in the absence of FN or integrin α5, our experiments also indicate that there is a significant deficiency in the number of CNCCs in the mutant embryos. Our experiments show that this deficiency is not due to defective formation of the CNC or defective exit of CNC precursors from the neural tube, but rather to the depletion of the CNC progenitor pool within the neural tube and decreased proliferation and survival of CNCCs. Our studies are the first to demonstrate the requisite role of FN and integrin α5 during the ontogeny of the CNC.
2. Results
2.1. Fibronectin synthesis is upregulated in CNC progenitors at the time corresponding with their expansion and the onset of migration
Prior studies in chick and frog showed that NCC progenitors within the dorsal neural tube as well as NCCs exiting the neural tube are surrounded by FN protein (Alfandari et al., 2003; Duband and Thiery, 1982; Le Douarin, 1982; Mayer et al., 1981). However, the cellular source(s) of FN during NC ontogeny are not known. Therefore, we used in situ hybridization to determine the spatio-temporal localization of mouse FN mRNA and immunofluorescence (IF) to examine FN protein expression at different embryonic stages corresponding with induction, expansion and migration of CNCCs.
We first assayed the presence of FN mRNA and protein in embryos with five somites, a time-point when the first known markers (e.g. Pax3) are already expressed by the CNC progenitors in the dorsal neural folds (Goulding et al., 1991). At this stage, we did not detect FN mRNA within the neural folds in wild-type embryos (n=6 embryos), while FN was abundantly transcribed in other embryonic locations such as the foregut endoderm, splanchnic mesoderm and endocardium (Fig. 1A, B). Since FN is a secreted protein, we used immunofluorescence (IF) microscopy to assay its spatio-temporal distribution. Concordant with the FN mRNA expression data, we did not detect FN protein in the dorsal neural tube in embryos with 5 somites, even though other embryonic locations were abundant with FN protein (Fig. 1C). Taken together, these experiments show that FN mRNA and protein are undetectable in the dorsal neural tube prior to CNC formation.
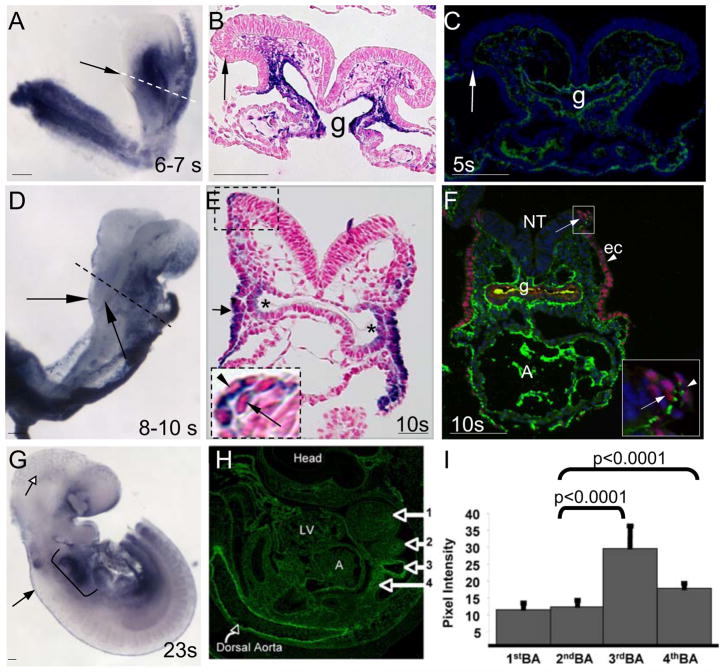
Figure 1. Dynamic expression of fibronectin mRNA during CNC development.
A, B. In situ hybridization shows that FN1 mRNA is not detectable within the dorsal neural folds in embryos with 6 to 7 somites (black arrow), while it is highly expressed in the region dorsal to the heart. Dotted line in A indicates an approximate plane of section shown in B. C. Similarly, FN protein is not detectable at the dorsal neural tube in embryos with 5 somites (arrow), while it is abundant in mesenchyme, around the gut (g) and endocardium. D. FN1 mRNA is expressed at the edges of the dorsal neural tube in 8–10 somite embryos (black arrows). Dotted line is an approximate plane of section shown in E. E. FN mRNA is expressed by the dorsal neural ectoderm (long arrow in the inset) and the surface non-neural ectoderm (arrowhead in the inset). * marks FN mRNA in pharyngeal pocket endoderm and short arrow points at pharyngeal ectoderm. F. Transverse section through a region containing CNCCs in a 10 somite wild-type embryo. FN protein (green) is present between TFAP2α+ neural (arrow, inset) and non-neural, surface (arrowhead, inset) ectoderm. G. FN1 mRNA expression is maintained at E9.5 in the dorsal neural tube (black arrow) and within the region of branchial arches 3, 4, and 6 (bracket). FN1 mRNA is also expressed by microvascular endothelial cells in the head (open arrow). H. Sagittal section through E9.5 embryo showing expression of FN protein (green). I. FN protein is enriched in branchial arches 3 and 4, compared with arches 1 and 2 (marked by arrows in H). LV – left ventricle, A – atrium. Scale bars are 100 μm.
By the 10th somite stage, CNCCs have begun exiting from the dorsal neural tube (Chan et al., 2004; Serbedzija et al., 1992; Stottmann et al., 2004). Examination of FN synthesis in 10–11 somite embryos showed that FN mRNA is up-regulated in the top few cell layers of the dorsal neural tube as well as in the cells of apposing surface ectoderm (Figure 1D, E). Concomitantly, FN protein is localized between the surface ectoderm and the neural epithelium containing NC progenitors (Fig. 1F). This localization pattern of FN protein is similar with that observed during NC development in chick and frog (Alfandari et al., 2003; Mayer et al., 1981). FN protein is also localized within the embryonic mesenchyme where it could interact with migrating CNCCs (Supplementary Fig. S1) (Duband and Thiery, 1982; Mayer et al., 1981; Peters and Hynes, 1996).
FN synthesis by NC progenitors is maintained in embryos at E9.5 (23 somites) (Fig. 1G, black arrow) and NCCs exiting the neural tube also synthesize FN (Supplementary Fig. S2A). FN synthesis is downregulated within the arch mesenchyme while within the cardiac outflow tract, mesenchymal cells (presumptive CNCCs) and endocardial cells express FN mRNA (Sup. Fig. S2B, B′). Taken together, our studies indicate that FN synthesis is induced in CNC precursors within the dorsal neural tube at the time corresponding with their expansion and exit (Conway et al., 2000; Serbedzija et al., 1992) and suggest a potential role for FN during these processes.
In mice, CNCCs destined for the cardiac outflow tract, migrate mainly through the fourth pharyngeal arch (Chan et al., 2004) and CNCCs remaining within the third, fourth and sixth pharyngeal arches give rise to VSMCs of aortic arch arteries (Jiang et al., 2000). In embryos with 6–23 somites, FN mRNA is highly enriched in the pharyngeal pocket endoderm and pharyngeal ectoderm, the areas of future pharyngeal arches 3, 4 and 6 (Figs. 1E, G and Supplementary Fig. S2B). In order to determine whether enrichment of FN mRNA within this region corresponds with enrichment in FN protein, we performed IF microscopy and quantified fluorescent pixel intensity within the pharyngeal arches of E9.5 embryos (Fig. 1H). This analysis showed that similar to FN mRNA, FN protein is enriched in the mesenchyme of pharyngeal arches 3 and 4 compared with arches 1 and 2 (Fig. 1H, I). Taken together our studies show that FN protein is expressed by and is localized to tissues important for the development of the CNC.
2.2. CNCCs arrive at their destinations in the branchial arches and the cardiac outflow tract in the absence of FN
To study the function of FN in CNC development we performed lineage-tracing experiments to determine the fate of the CNC in the absence of FN. For these experiments, we utilized Wnt1-Cre transgenic and R26R reporter strains of mice to mark the NC (Jiang et al., 2000; Soriano, 1999). Analysis of β-gal staining of E9.5 FN-null; Wnt1-Cre/+; R26R/+ embryos showed that the overall distribution of cranial NCCs (which include the CNC) was not severely perturbed by the absence of FN (Fig. 2A, B). However, we observed an interruption in the NC distribution at the site of putative trigeminal ganglia (Fig. 2B, asterisk) in each of the FN-null; Wnt1-Cre/+;R26R/+ embryos (n=4) compared with controls. This could be due to the absence or truncation of the hindbrain in FN-null mutants (data not shown) or, alternatively, this interruption may indicate that FN modulates migration of a subset of cranial NCCs. Notably, by E9.5, NCCs in FN-null mutants filled pharyngeal arches to their furthest and ventral-most extent (Fig. 2A′, B′) and entered into the cardiac outflow tract (Fig. 2A″, B″). Since CNCCs begin entering the cardiac outflow tract at about E9.5 in wild-type embryos (Jiang et al., 2000; Waldo et al., 1999), our observations indicate that CNCCs in FN-null mutants have reached their distal-most destinations within the pharyngeal arches and the heart at the expected embryonic stages of development. Taken together, these fate-mapping studies show that NCCs migrate into the pharyngeal arches and the cardiac outflow tract in the absence of FN.
Figure 2. Distribution of NCCs in control, FN and integrin α5-null embryos.
A, B. Lineage tracing of the NC in control and FN-null embryos at E9.5. Wnt1-Cre transgenic (A, B), Pax3Cre/+ knock-in (C, D) and R26R reporter strains were used to detect the NCCs and their descendants. Brackets mark pharyngeal regions containing CNCCs; asterisks in A and D mark interruption in NCC distribution at sites of putative trigeminal ganglia in the mutants. Dotted lines mark approximate planes of section shown in A″–D″. Asterisk in D″ marks a blood vessel contiguous with the common cardinal vein in serial sections caudal to the one shown. A–D. Distributions of NCCs in the head and branchial arches are similar between control and mutant embryos. A′–D′. Magnified views of branchial arches show that NCCs fill the arches to their ventral-most extent. Arrows point to the ventral pharyngeal ectoderm, the furthest border of the arches and show that NCCs have reached this border in controls and mutants. A″–D″. CNCCs entered the cardiac outflow tract in control and mutant embryos (arrows). Scale bars are 100 μm.
2.3. Absence of fibronectin leads to depletion of the CNC
Sparse β-gal staining in whole FN-null; Wnt1-Cre/+; R26R/+ embryos compared with controls, suggested that there were fewer NCCs in these mutants (Fig. 2A, B). To test the idea that FN was required for the presence of normal numbers of NCCs, we focused on the CNC population of the cranial NC and quantified the numbers of CNCCs per section in serial transverse sections in the pharyngeal region located between the otic pit and the atria. Analysis of control and FN-null littermates indicated that the average number of CNCCs per section was three to six-fold lower in FN-null embryos than in controls (Fig. 3A).
Figure 3. FN and integrin α5 are required for the presence of normal numbers of CNCCs in branchial arches at E9.5.
A. Quantification of CNCCs located outside of the neural tube in 2 control (C1 and C2, closed bars) and 3 FN-null (N1–N3, open bars) embryos. Serial sections caudal to the otic vesicle were analyzed. C1 and C2: three sections for each embryo through 30 – 50 μm of tissue were counted; N1: nine step sections, 5 μm in thickness, through 90 μm of tissue were analyzed; N2: six sections through 60 μm of tissue were analyzed; N3: seven sections through 70 μm of tissue were analyzed. B. Quantification of TFAP2α+ cells in embryonic mesenchyme at each branchial arch level in control (closed bars) and FN-null (open bars) embryos at E9.5. Total of 17 sections from FN-null (spanning about 200 μm of tissue and 13 sections from control embryo (covering approximately 150 μm of tissue) were analyzed. p< 0.001 for all null vs control comparisons. BA – branchial arch. Number of cells were counted in each section and averaged among sections. C, D. Quantification of CNCCs outside the neural tube in Pax3Cre/+ (C) and Wnt1-Cre (D) labeled control and integrin α5-null embryos. Serial sections caudal to the otic vesicle were analyzed. C. Pax3Cre/+ fate mapping. Average number of CNCCs per section is plotted. C1: 9 step sections and C2: 5 step sections were analyzed; N1: 31 serial sections spanning about 300 μm of tissue were analyzed, N2: 13 step sections, N3: 14 step sections were analyzed. D. Wnt1-Cre fate mapping. Average number of CNCCs per section is plotted. C1: 10 sections and N1: 9 sections were examined.
We confirmed this decrease with an independent method by detecting NCCs utilizing antibodies recognizing the transcription factor AP2α (TFAP2α). At E8.5, TFAP2α is expressed by NCC progenitors in the dorsal neural tube and by NCCs. At E9.5, TFAP2α is expressed by NCCs and some NC derivatives at E9.5 (Conway et al., 2000). In order to quantify the number of NCCs outside the neural tube in E9.5 FN-null and control embryos, we counted the number of TFAP2α-positive mesenchymal cells per section excluding the cells of the surface ectoderm. Using this method, we also observed approximately a three-fold decrease in the number of NCCs at each branchial arch level (Fig. 3B). Taken together, these studies indicate that FN is required for the presence of normal numbers of CNCCs.
2.4. Fibronectin is not required for specification of the NC and pharyngeal lineages
The presence of LacZ-positive cells in FN-null embryos following lineage tracing analyses indicated that FN is not required to induce the NC lineage. This conclusion is also supported by the observation that transcription factors Foxd3 and Pax3, which are the earliest genes known to be expressed by the NC progenitors as well as Cx43 transcripts are expressed in the dorsal neural tube of FN-null embryos (Fig. 4).
Figure 4. Expression of neural crest markers at E8.5 does not depend on FN.

In situ hybridization detection of Pax3, Foxd3 and Cx43 transcripts. Arrows point at the neural tubes. FN-null and control embryos were photographed at the same magnification. Scale bars are 100 μm.
It has been established that the pharyngeal endoderm, mesoderm and ectoderm play essential roles during the development of CNC-derived lineages (Calmont et al., 2009; Goddeeris et al., 2007; High et al., 2009; Macatee et al., 2003; Park et al., 2006; Park et al., 2008; Zhang et al., 2005). Therefore, in this study, we addressed tissue-specific expression of genes normally present within lineages of the pharynx. Our IF studies demonstrated that similar to controls, pharyngeal endoderm and mesoderm express the transcription factor Isl1, and pharyngeal ectoderm expresses both Isl1 and TFAP2α (Figs. 6, 7 and Fig. S3). Taken together, these studies and work prior to ours (George et al., 1997; George et al., 1993; Georges-Labouesse et al., 1996) indicate that decreased abundance of CNCCs in FN-null embryos is not due to misspecification of pharyngeal, NC or other embryonic lineages.
Figure 6. Defective survival of the cranial NC in the absence of FN at E9.5.
Transverse sections through control (A) and FN-null (B) embryos are shown. NC and surface ectoderm (arrows in B) are detected using anti-TFAP2α antibody (red). Apoptosis was detected using anti-cleaved caspase 3 antibody. Note extensive apoptosis in the neural tube (marked by the white dotted line) and in the NC in FN-null embryo (B) while other tissues are relatively apoptosis-free. Arrowheads point at double-positive cells. C. Quantification of NC apoptosis in control and FN-null embryos. D. Quantification of survival of pharyngeal Isl1+ cells (mesoderm) in control and FN-null embryos shows that absence of FN does not significantly affect survival of pharyngeal arch mesoderm, even though extensive death is observed within the NCCs.
Figure 7. Proliferation and survival of the CNC is regulated by FN and integrin α5 at E8.5.
A, B. Whole mount staining of E8.75 using anti-cleaved caspase 3 antibody shows minimal overall apoptosis in FN-null embryos (B) compared with control embryos (A). Arrows point at apoptotic cells (dark brown spots). Older FN-null embryos show enrichment in apoptosis (reactivity with anti-cleaved caspase 3 antibody) in the neural tube segment located dorsal to the heart (C, D). E–L. Staining of transverse sections using the antibody to TFAP2α (red) to mark the NC and surface ectoderm (ec) in control, FN-null and integrin α5-null embryos. Apoptosis was detected using anti-cleaved caspase 3 antibody (green in E, I, G and K); Mitosis was detected using anti-pHH3 antibody (green in F, F′, F″, J, J′, H and L). Boxes in F and J are expanded in F′, F″, and J′, respectively. Neural and non-neural ectoderm are marked by dotted lines in F″, H, I, and L. Arrows point to examples of double-positive cells. Boxes in I and K show apoptotic CNC progenitors and CNCCs, respectively. Boxes in F′, F″ and H show mitotic CNC progenitors. Panel in J′ shows mitotic CNCCs. ec-surface ectoderm, g-gut, NT-neural tube, OFT-outflow tract. Scale bars are 100 μm.
2.5. Exit of CNC progenitors from the neural tube does not depend on FN
The decrease in the number of CNCCs observed at E9.5 could be due to defective emigration of CNC progenitors from the neural tube earlier in embryonic development. If this were the case, we would have observed accumulation of CNCCs in the dorsal neural tube of FN-null embryos compared with controls. In mice, emigration of CNCCs from the neural tube is already evident by the 10th somite stage, corresponding with approximately E8.5 of development (Chan et al., 2004; Serbedzija et al., 1992). At this stage, we identified FN-null embryos that were morphogenetically similar to controls (Fig. 5A, B). FN-null embryos lack somites (George et al., 1993; Georges-Labouesse et al., 1996) and therefore, we used an alternative approach (Downs and Davies, 1993) and staged the null and control embryos by their overall morphology. In order to determine whether the absence of FN resulted in accumulation of CNC progenitors within the neural tube during early stages of CNC development and before morphogenetic abnormalities in FN-nulls became grossly evident, we quantified the number of TFAP2α+ cells per section within the dorsal neural tube in FN-null embryos that looked similar to controls containing 10–11 somites. Our quantifications showed that the average number of CNC progenitors was 20% lower in FN-null embryos compared with controls. We think that this decrease is due to the already ongoing depletion of the CNC progenitor pool because of defective proliferation and survival of these cells in FN-null mutants (see sections 2.6 and 2.7 below). Nonetheless, these quantifications demonstrate the absence of accumulation of CNC progenitors within the dorsal neural tube in FN-null embryos.
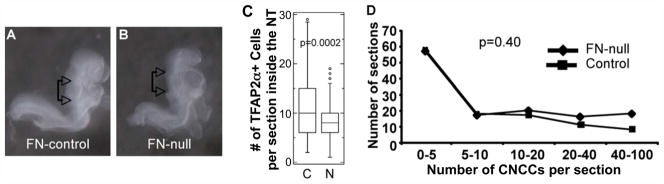
Figure 5. FN is not essential for the emigration of CNC progenitors from the neural tube.
A–D. Quantification of CNC progenitors in neural tubes of control and FN-null embryos. A, B. FN-null (n=6) and control 10–11 somite embryos (n=6) similar in size and shape were analyzed in this experiment. C. 129 transverse sections from 6 control and 129 transverse sections from 6 null embryos isolated at E8.5 were examined. The mean number of CNC progenitors per section was 8.4 +/− 4 cells (median=8) in FN-nulls (1021 TFAP2α+ cells were analyzed). The mean number of CNC progenitors per section in controls was 10.8 +/− 6 cells per section (median=10), (1373 TFAP2α+ cells were analyzed), p<0.0002, (Student’s t test). D. Control and FN-null embryos contain comparable distributions of CNCCs outside the neural tube at E8.5. Chi-square test.
To complement the above analysis, we quantified distribution of CNCCs in the mesenchyme of FN-null and control embryos analyzed above. If there were defective exit of CNC progenitors from the neural tube, we would expect altered distribution of CNCCs outside of the neural tube. The onset of NC exit from the neural tube proceeds in rostral to caudal order, thus rostral sections from an embryo are expected to contain more NCCs within their mesenchyme compared with caudal sections. We counted the number of CNCCs per section in each of the six control and six FN-null embryos isolated at E8.5. The results were binned according to the number of observed CNCCs per section and plotted against the y-axis designating the number of sections contained within each bin. The resultant distributions of CNCCs were similar between FN-null and control embryos (Fig. 5D). Taken together, these complementary analyses indicate that FN is not required for the exit of CNC progenitors from the neural tube and suggest that the process of epithelial-to-mesenchymal transition does not depend on FN.
2.6. Fibronectin is essential for the survival of CNC progenitors
Cell adhesion to FN is required for growth factor-mediated cell survival of normal non-transformed cells in vitro (Matter and Ruoslahti, 2001; Zhang et al., 1995). Our initial experiments demonstrated extensive cells death primarily in the neural tube and the NC of FN-null embryos at E9.5 (Figs. 6A–C). Since apoptosis within the Isl1+ mesodermal cells in the pharyngeal arches did not yield statistically significant differences between the null and control embryos (Figs. 6D), our data suggest a selective role for FN in the survival of cells of the neural tube and the NC at E9.5.
Since we observed enrichment in NC apoptosis at E9.5, we reasoned that the deficiency in the CNC in FN-null embryos at this stage could be a result of decreased survival of CNC progenitors earlier in embryonic development, e.g. at E8.5. To test this hypothesis, we isolated FN-null embryos at E8.5 that were similar in size and shape to controls having 10 -11 somites. CNC progenitors reside between the otic pit and the fourth somite in mice (Chan et al., 2004), and therefore, we obtained serial sections through this region of control embryos and through a comparable region of the nulls. Sections were then co-stained to detect both the NC and apoptotic cells (Fig. 7). FN-null mutants contained four-fold more apoptotic CNC progenitors than controls within the dorsal neural tube, p<0.001 (Fig. 8A). We also detected a ten-fold increase in the apoptosis of TFAP2α+ cells outside the neural tube, p<0.0001 (Fig. 8A). Concordant with the above findings, detection of apoptosis using the TUNEL assay indicated increased apoptosis in CNC progenitors and CNCCs in E8.5 FN-null embryos relative to controls (data not shown).
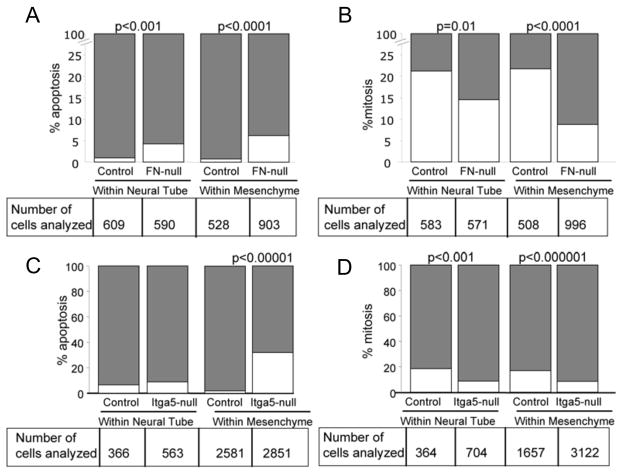
Figure 8. Quantitative analysis of CNC apoptosis and proliferation in FN-null and integrin α5-null embryos at E8.5.
A, B. Decreased survival and proliferation of CNC progenitors and CNCCs in the absence of FN. A. Survival. White bars represent the percent of cleaved caspase 3-positive CNC progenitors or CNCCs and grey bars represent the percent of cleaved caspase 3-negative TFAP2α+ cells. B. Proliferation. White bars represent the percent of pHH3+ TFAP2α+ cells within the dorsal neural tube or mesenchyme and grey bars represent the percent of pHH3- TFAP2α+ cells. Statistics were performed using Z-test for two proportions, chi-square test or Fisher exact test, with similar results. C, D. Survival and proliferation of the CNC in integrin a5-null mutants and controls. The numbers of analyzed TFAP2α+ CNCCs are listed below each graph. Quantification of survival and proliferation are described in the Methods section and were similar to the analysis performed on FN-null and control embryos. Larger number of CNCCs in the mesenchyme of control and integrin α5-null embryos is due to the more advanced developmental stage of these embryos compared with FN-null and control set of embryos.
At E8.5 we observed the presence of some apoptotic cells in the pharyngeal endoderm and ectoderm in addition to the CNC of FN-null embryos. However, whole mount immunohistochemistry performed on embryos using the antibody to cleaved caspase 3 and examination of serial sections stained with this antibody indicated very low overall levels of apoptosis in the mutants and controls (Fig. 7A–D, E, I). These experiments attest to the relative health of the analyzed FN-null mutants. Taken together, our studies indicate that FN is required for the survival of CNC progenitors and their descendants.
2.7. Fibronectin is essential for proliferation of CNC progenitors
Cell binding to FN is required for growth factor-mediated proliferation of normal untransformed cells in vitro. (Assoian and Schwartz, 2001; Roovers et al., 1999; Schwartz and Assoian, 2001). In order to determine whether FN is required for CNC proliferation in vivo, we labeled NCCs with the antibody against TFAP2α and assayed cell proliferation by using the antibody to phosphohistone H3 in FN-null embryos isolated at E8.5 and in controls containing 10 to 11 somites. We found that at this early stage of CNC ontogeny, FN-null embryos contained fewer mitotic CNC progenitors and CNCCs (Fig. 7F, F′, F″, J, J′ and Fig. 8B). Our quantitative studies indicate that FN plays a requisite role in maintenance and expansion of the CNC both within and outside of the dorsal neural tube.
2.8. CNCCs migrate to branchial arches and the cardiac outflow tract in the absence of integrin α5
Integrins comprise a major group of cell surface receptors mediating cellular migration, survival and proliferation upon binding to FN. A number of integrin heterodimers (αvβ3, αvβ1, α8β1, α5β1, α4β1) bind FN in vitro (Humphries et al., 2006; Saoncella et al., 1999). Individual deletion of integrin α4, αv, or α8 permits embryonic development past mid-gestation and/or until birth, while the deletion of integrin α5 leads to early embryonic lethality with similar (albeit a slightly milder) embryonic lethal phenotype as FN-null embryos (Bader et al., 1998; Muller et al., 1997; Yang et al., 1999). Since the phenotypes of integrin α5-null and FN-null embryos are similar and since integrin α5 directly binds FN in vitro and activates intercellular signaling pathways modulating cell migration, survival and proliferation, integrin α5 is thought to function as a major FN receptor during early embryogenesis (Yang et al., 1999).
To uncover the potential downstream players mediating the function of FN during CNC development, we asked whether integrin α5 was similarly required for the formation of quantity of the CNC. To answer this question, we performed lineage-tracing experiments utilizing either Wnt1-Cre or Pax3Cre/+ strains to mark the NC in integrin α5-null mutants. Our experiments indicated that extensive migration of NCCs took place in the absence of integrin α5 (Fig. 2C, D, C′, D′), including migration of CNCCs into the cardiac outflow tract (Fig. 2C″, D″). We also detected CNCCs within the cardiac outflow tracts of integrin α5-null embryos at E9.5 using the antibody to TFAP2α (Fig. 7K). This experiment serves as an independent confirmation that CNCCs successfully reach and enter the cardiac outflow tract in integrin α5-null embryos and indicate that NCCs are capable of long-range migration in the absence of integrin α5.
Whole mount staining of embryos in which the NC was marked with the use of Pax3Cre/+ knock-in or Wnt1-Cre transgenic alleles suggested that similar to FN-null embryos, integrin α5-nulls contained fewer CNCCs in branchial arches (Fig. 2C′, D′ and data not shown). Quantitative analysis of sections indicated that this was indeed the case (Fig. 3C, D). Taken together, our experiments indicate that while both FN and integrin α5 are dispensable for the gross migration of the CNCCs to their target destinations, they are required for the presence of normal numbers of CNCCs.
2.9. Integrin α5 is required for survival and proliferation of the CNC
Prior studies indicated that integrin α5 is required for the survival of cranial NCCs within the second branchial arch (Goh et al., 1997). Therefore, we tested whether integrin α5 was also required for the survival of the CNC, a different population of the cranial NC. We observed statistically significant increase in apoptosis of the CNC outside the neural tube in integrin α5-null embryos (Figs. 7G, K and 8C). However, we did not find an appreciable decrease in survival of CNC progenitors within the neural tube of integrin α5-null embryos. Possibly, other FN-binding integrins in the dorsal neural tube mediated survival of CNC progenitors in the absence of integrin α5. Consistent with our results, isolated integrin α5-null trunk NCCs can survive in vitro during the initial four days of culture and express additional integrins (Haack and Hynes, 2001). Quantification of proliferation in CNC progenitors within the dorsal neural tube and their descendants within the embryonic mesenchyme, demonstrated a significant decrease in the proportion of mitotic CNC progenitors and CNCCs in integrin α5-null embryos (Figs. 7H, L and 8D). Taken together, these data indicate that similar to FN, integrin α5 is required for proliferation of CNC progenitors and for the proliferation and survival of the CNC.
Prior studies extensively characterized gene expression in integrin α5-null embryos and determined that the spatio-temporal gene expression patterns as well as patterning of the dorsal neural tube caudal to the otic pit were similar between integrin α5-null and control embryos (Goh et al., 1997). Moreover, these studies showed that absence of integrin α5 does not lead to significant differences in proliferation and survival of cells in mesodermal tissues (Goh et al., 1997). Taken together, prior work and our studies indicate that deficiency in CNCC numbers, proliferation and survial is not due to mispartening of embryonic tissues and the neural tube in integrin α5-null embryos, and that integrin α5 plays a selective role in proliferation and survival of the NC.
3. Discussion
The importance of FN in the development of the NC was hypothesized based on observations that FN protein is present along the neural crest transit routes in vivo, on studies implicating FN in NC migration in vitro, and on evolutionary studies implicating FN in the development of vertebrate-specific features such as the neural crest. Our work is the first to specifically examine the function of FN during neural crest development in vivo. Using a genetic fate mapping approach, we found that CNCCs reached the branchial arches and invaded the cardiac outflow tract in the absence of FN, suggesting that FN is not required for the migration of the CNC. Instead, we observed a decrease in the number of CNCCs at their destinations and found that proliferation and survival of CNC progenitors and their descendants were compromised in the absence of FN. These data indicate that in vivo, FN is an important regulator of CNC abundance and suggest that it regulates this process by modulating proliferation and survival of the CNC.
In order to understand how FN functions during CNC development, we examined the role of integrin α5 (a major transducer of FN signals) in CNC migration, proliferation and survival. Our fate mapping analysis showed that similar to FN, integrin α5 is not required for the CNC to reach and fill branchial arches and to invade the cardiac outflow tract. However, integrin α5 is important for regulating CNC numbers and facilitates proliferation and survival of the CNC, suggesting that α5β1 transduces FN proliferation and survival signals into the CNC during embryonic development.
In agreement with our findings, earlier studies showed that mouse integrin α5-null NCCs migrated along ventral paths when injected into the dorsal neural tube of a chick host embryo (Haack and Hynes, 2001). Thus two independent assays, fate mapping (this study) and heterologous transplantation (Haack and Hynes, 2001), indicate that integrin α5 is dispensable for the gross migration of the NC.
Concordant with our studies, earlier studies also indicated compromised survival of a subset of cranial NCCs in integrin α5-null embryos while proliferation and survival of mesodermal tissues were not significantly defective in these mutants, indicating a specific role for integrin α5 during the development of the NC (Goh et al., 1997). Finally, in accord with our conclusion that integrin α5 regulates CNC proliferation in vivo, analysis performed in vitro indicated that trunk NCCs isolated from integrin α5-null embryos failed to proliferate even in the presence of transforming oncogenes, SV40 large and small T antigens (Haack and Hynes, 2001). Taken together these experiments demonstrate an essential role for FN and integrin α5 in maintaining CNC cell number by modulating CNC proliferation and survival.
Extracellular environment is thought to play a major role in NC ontogeny (Duband and Thiery, 1987; Hynes, 1990; Le Douarin, 1982). In order to gain an insight into cellular and molecular roles of FN during NC development, we performed in situ hybridization and IF studies to determine which cell types synthesize FN and to determine the localization of FN protein during NC development. These experiments led to a novel observation that FN mRNA is not produced uniformly by all cells and that FN protein is enriched near the sites of its synthesis in the developing embryo. Analysis of the published literature also indicates that other components of the ECM are not uniformly distributed throughout the embryo. For example, laminin α5 and fibulin-1 transcripts are enriched in distinct embryonic loci; and a number of ECM proteins implicated in NC development are also highly localized within distinct embryonic regions (Coles et al., 2006; Cooley et al., 2008; Duband and Thiery, 1987). These studies indicate that NCCs encounter both different types and different concentrations of ECM proteins during their long-range transit and imply that these differences may be important during the development of the NC and its derivatives. Indeed, absence of laminin α5 chain leads to the widening of NC streams (Coles et al., 2006). Similarly, the absence of fibulin-1 leads to widening and fusion of some NC streams and gives rise to severe defects in the positioning of the cardiac outflow vessels, patterning of aortic arch arteries and NC survival (Cooley et al., 2008). Our studies demonstrated that FN mRNA and protein are upregulated in the dorsal neural tube at the time of expansion and exit of CNC progenitors. FN is also upregulated within the branchial arch regions into which and through which CNCCs migrate. We hypothesize that FN expressed by CNC progenitors and by the tissues comprising branchial arches 3 and 4 play specific roles during CNC development. Future studies using conditional mutagenesis will provide important insights into cell-autonomous and non-cell autonomous functions of FN during the development of the NC.
4. Experimental Procedures
4.1. Mouse strains
All experiments involving vertebrate animals were performed according to the NIH guidelines and were approved by the institutional animal care and use committee. Animals heterozygous for the null alleles of fibronectin (FN+/−) or integrin α5 (Itga5+/) on C57BL6/J genetic background were obtained from Dr. Richard Hynes (MIT) (George et al., 1993; Yang et al., 1993). Wnt1-Cre transgenic strain, Pax3Cre/+ knock-in strain and R26R reporter mice were obtained from the Jackson labs (Engleka et al., 2005; Jiang et al., 2000; Lang et al., 2005; Soriano, 1999).
4.2. Lineage tracing analysis
FN+/−;R26R/R26R females were mated with FN+/−; Wnt1-Cre males and embryos were dissected at E9.5. Similarly, Itga5+/−; R26R/R26R females were mated with either Itga5+/−; Wnt1-Cre or Itga5+/−; Pax3Cre/+ males to obtain embryos at E9.5. Embryos were dissected in ice-cold phosphate buffered saline (PBS) and stained using bluo-gal as the substrate (#B2904, Sigma-Aldrich) and a standard protocol to detect β-gal enzymatic activity. Staining reactions were allowed to proceed for two days at 37°C. Embryos were then photographed using Zeiss Stemi 2000-C stereomicroscope and DFC420 digital camera (Leica). Embryos were dehydrated, embedded into paraffin and serially sectioned in transverse orientation. Serial sections (10–15 microns apart) between the otic pit and the atria were used to quantify the numbers of CNCCs per section. Alternatively, FN-null and control embryos were collected from matings between FN+/− animals and serial sections (10–15 microns apart) were stained using mouse monoclonal antibody recognizing TFAP2α clone 3B5, generated by Trevor Williams and obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA).
4.3. In situ hybridization
In situ hybridizations were performed according to a standard protocol (Henrique et al., 1995), obtained from the Dr. Janet Rossant’s lab web page, http://www.sickkids.ca/research/rossant/protocols/Conlon/WM2_Henrique.pdf. Plasmids encoding Foxd3 and Pax3 probes were obtained from Drs. Patricia Labosky (Vanderbilt University) and Andrew McMahon (Harvard University), respectively. Plasmid encoding Cx43 probe was generated using primers: 5′-gaattcgtcgacGAGGTGCCCAGACATGGGTG and 5- gatatgcggccgcGACACCACCAGCATGAAGATG. These primers were used to amplify 662 bp of the 2nd exon by PCR utilizing mouse genomic DNA. The resulting fragment was digested with NotI and SalI and subcloned into pBluescript. For anti-sense probe preparation, the plasmid was cut with SalI and transcribed using T7 polymerase (Promega) and DIG RNA labeling mix (Roche).
4.4. Detection of fibronectin and integrin α5 proteins
All stainings were performed on tissues fixed in 4% PBS-buffered paraformaldehyde and embedded into paraffin. FN protein was detected using rabbit polyclonal antibody 297.1 (a gift from Dr. Richard Hynes) and Alexa Fluor 488 secondary anti-rabbit IgG (Invitrogen). Fluorescence intensity was quantified using the rectangle tool in ImageJ, according to the software specifications. Double detection of FN and TFAP2α were performed using paraffin sections, antigen retrieval in 10 mM Citrate buffer, anti-FN antibody obtained from Millipore (cat # AB2033, at 1:200 dilution) and the anti-TFAP2α antibody at the dilution of 1:50. Use of the latter FN antibody on sections from FN-null embryos gave minimal background staining (Supplemental Fig. 1B). Additional control stainings were performed using normal IgG from naïve rabbits.
4.5. Immunofluorescent analysis of proliferation and apoptosis
FN-null and control embryos were isolated from FN+/− matings at E8.5. FN-null embryos that were similar in size and shape to control embryos having 10–11 somites were chosen for our analyses. Embryos were fixed in 4% paraformaldehyde overnight at 4 °C and stored at 4 °C in 70% ethanol until use. Embryos were dehydrated, embedded into paraffin and serially sectioned in transverse orientation. Serial sections between the otic vesicle and atria (thirty-fourty 5-μm thick sections 5–10 micron apart, per embryo) were analyzed using immunofluorescence microscopy following antigen retrieval in 0.01 M NaCitrate, pH 6.0. NCCs were detected using mouse monoclonal anti-TFAP2α antibody (1:50). Rabbit polyclonal antibodies recognizing cleaved caspase 3 (#9661) or anti-phosphohistone H3 antibody (#9701) were purchased from Cell Signaling Technology (Danvers, MA). Fluorescent anti-rabbit IgG conjugated to Alexa-Fluor 488 and anti-mouse IgG conjugated to AlexaFluor 555 (Invitrogen) were used as secondary antibodies. TUNEL staining was performed using In situ cell death detection kit (Roche, #11684795910). Slides were mounted using ProLong® Gold anti-fade reagent with DAPI (Invitrogen) and imaged using Zeiss Axiophot epifluorescence microscope equipped with Hamamatsu digital camera. Alternatively, slides were imaged using Zeiss LSM510 confocal microscope. Integrin α5-null and control embryos of similar size and shape were isolated at E9.0 (the number of somites in control embryos ranged from 17 to 19). These embryos were processed in the manner similar to the FN-null and control samples described above. Serial sections 10–15 microns apart were used for quantification to avoid double-counting of cells. Statistical analyses were performed using Z-test for two proportions http://dimensionresearch.com/resources/calculators/ztest.html and/or chi-square test. Data in Fig. 5C was analyzed using Student’s t test, and the box plot was generated using a web-based program (http://www.physics.csbsju.edu/stats/t-test_bulk_form.html). The box represents the interquartile range (IQR, central 50% of the data points), horizontal lines inside the boxes represent the median values, and vertical bars represent a spread of 1.5× IQR, while dots represent outliers, which were included into the calculation of significance.
Supplementary Material
Figure S1. A. Confocal IF microscopy shows co-localization of CNCCs (detected using anto-TFAP2α antibody, red nuclei) and FN (green) in the same plane. Magnified box 1 shows localization of FN (green) between non-neural surface ectoderm (arrowhead) and CNC progenitors (red, arrow). Magnified box 2 shows mesenchymal CNCCs (red, arrow) surrounded by FN (green). op-otic pit. B. Control. Staining of a section from FN-null embryos using equivalent concentration of anti-TFAP2α antibody (red), anti-FN antibody (green) and DAPI. Arrow points at the branchial arch mesenchyme. Arrowhead points to the non-neural surface ectoderm. Note that anti-FN antibody produces minimal staining in the absence of FN.
Figure S2. Expression of FN mRNA at E9.5. A. CNC progenitors and CNCCs express FN mRNA (arrows). B. FN mRNA is enriched in pharyngeal ectoderm (black arrow) and pharyngeal pocket endoderm, asterisks. Presumptive CNCCs inside the outflow tract (open arrows) also express FN mRNA, while mesenchymal cells lateral to dorsal aorta and in pharyngeal arches (magnified in panel B′) express low levels of FN mRNA. dAo – dorsal aorta. Endothelial cells in panel B are marked by the black arrowheads.
Figure S3. Pharyngeal ectoderm (ec, small arrow), endoderm (en, arrowhead) and mesoderm (notched arrowhead) are marked by the expression of Isl1 in control and FN-null embryos at E9.5. Long arrows point at the outflow tract myocardium expressing Isl1 in FN-null and controls.
Acknowledgments
We thank Dr. Richard Hynes for providing FN and integrin α5 mutant mouse strains and rabbit polyclonal antibody to FN. Drs. Patricia Labosky and Andrew McMahon for plasmids encoding Foxd3 and Pax3 probes. We are grateful to Drs. Heidi Stuhlmann and Yutaka Nibu for the use of their microscopes and imaging equipment. We thank Shivaprasad Bhuvanendran in the Bio-Imaging resource center at the Rockefeller University for help with confocal imaging, and thank Drs. Bruce Gelb, Ann Foley, Nathan Astrof, and Monn Monn Myat for critical reading of the manuscript. This work was supported in part by the American Heart Association Scientist Development grant # 0835556D to S.A. and by the start-up funds to S.A. from the Division of Cardiology, Weill Cornell Medical College.
References
- Alfandari D, Cousin H, Gaultier A, Hoffstrom BG, DeSimone DW. Integrin alpha5beta1 supports the migration of Xenopus cranial neural crest on fibronectin. Dev Biol. 2003;260:449–64. doi: 10.1016/s0012-1606(03)00277-x. [DOI] [PubMed] [Google Scholar]
- Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11:48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–19. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Calmont A, Ivins S, Van Bueren KL, Papangeli I, Kyriakopoulou V, Andrews WD, Martin JF, Moon AM, Illingworth EA, Basson MA, et al. Tbx1 controls cardiac neural crest cell migration during arch artery development by regulating Gbx2 expression in the pharyngeal ectoderm. Development. 2009;136:3173–83. doi: 10.1242/dev.028902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WY, Cheung CS, Yung KM, Copp AJ. Cardiac neural crest of the mouse embryo: axial level of origin, migratory pathway and cell autonomy of the splotch (Sp2H) mutant effect. Development. 2004;131:3367–79. doi: 10.1242/dev.01197. [DOI] [PubMed] [Google Scholar]
- Coles EG, Gammill LS, Miner JH, Bronner-Fraser M. Abnormalities in neural crest cell migration in laminin alpha5 mutant mice. Dev Biol. 2006;289:218–28. doi: 10.1016/j.ydbio.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Bundy J, Chen J, Dickman E, Rogers R, Will BM. Decreased neural crest stem cell expansion is responsible for the conotruncal heart defects within the splotch (Sp(2H))/Pax3 mouse mutant. Cardiovasc Res. 2000;47:314–28. doi: 10.1016/s0008-6363(00)00098-5. [DOI] [PubMed] [Google Scholar]
- Cooley MA, Kern CB, Fresco VM, Wessels A, Thompson RP, McQuinn TC, Twal WO, Mjaatvedt CH, Drake CJ, Argraves WS. Fibulin-1 is required for morphogenesis of neural crest-derived structures. Dev Biol. 2008;319:336–45. doi: 10.1016/j.ydbio.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JF, Trainor PA. Neural crest stem and progenitor cells. Annu Rev Cell Dev Biol. 2006;22:267–86. doi: 10.1146/annurev.cellbio.22.010305.103814. [DOI] [PubMed] [Google Scholar]
- Delannet M, Martin F, Bossy B, Cheresh DA, Reichardt LF, Duband JL. Specific roles of the alpha V beta 1, alpha V beta 3 and alpha V beta 5 integrins in avian neural crest cell adhesion and migration on vitronectin. Development. 1994;120:2687–702. doi: 10.1242/dev.120.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs KM, Davies T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development. 1993;118:1255–66. doi: 10.1242/dev.118.4.1255. [DOI] [PubMed] [Google Scholar]
- Duband JL, Thiery JP. Distribution of fibronectin in the early phase of avian cephalic neural crest cell migration. Dev Biol. 1982;93:308–23. doi: 10.1016/0012-1606(82)90120-8. [DOI] [PubMed] [Google Scholar]
- Duband JL, Thiery JP. Distribution of laminin and collagens during avian neural crest development. Development. 1987;101:461–78. doi: 10.1242/dev.101.3.461. [DOI] [PubMed] [Google Scholar]
- Engleka KA, Gitler AD, Zhang M, Zhou DD, High FA, Epstein JA. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol. 2005;280:396–406. doi: 10.1016/j.ydbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–81. [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–91. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse EN, George EL, Rayburn H, Hynes RO. Mesodermal development in mouse embryos mutant for fibronectin. Dev Dyn. 1996;207:145–56. doi: 10.1002/(SICI)1097-0177(199610)207:2<145::AID-AJA3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Goddeeris MM, Schwartz R, Klingensmith J, Meyers EN. Independent requirements for Hedgehog signaling by both the anterior heart field and neural crest cells for outflow tract development. Development. 2007;134:1593–604. doi: 10.1242/dev.02824. [DOI] [PubMed] [Google Scholar]
- Goh KL, Yang JT, Hynes RO. Mesodermal defects and cranial neural crest apoptosis in alpha5 integrin-null embryos. Development. 1997;124:4309–19. doi: 10.1242/dev.124.21.4309. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Chalepakis G, Deutsch U, Erselius JR, Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. Embo J. 1991;10:1135–47. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack H, Hynes RO. Integrin receptors are required for cell survival and proliferation during development of the peripheral glial lineage. Dev Biol. 2001;233:38–55. doi: 10.1006/dbio.2001.0213. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–90. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- High FA, Jain R, Stoller JZ, Antonucci NB, Lu MM, Loomes KM, Kaestner KH, Pear WS, Epstein JA. Murine Jagged1/Notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J Clin Invest. 2009;119:1986–96. doi: 10.1172/JCI38922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson MR, Kirby ML. Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol. 2007;18:101–10. doi: 10.1016/j.semcdb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Fibronectins. New York: Springer-Verlag; 1990. [Google Scholar]
- Hynes RO, Zhao Q. The evolution of cell adhesion. J Cell Biol. 2000;150:F89–96. doi: 10.1083/jcb.150.2.f89. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–16. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Lang D, Lu MM, Huang L, Engleka KA, Zhang M, Chu EY, Lipner S, Skoultchi A, Millar SE, Epstein JA. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433:884–7. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM. The neural crest. Cambridge: Cambridge University Press; 1982. [Google Scholar]
- Macatee TL, Hammond BP, Arenkiel BR, Francis L, Frank DU, Moon AM. Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development. 2003;130:6361–74. doi: 10.1242/dev.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter ML, Ruoslahti E. A signaling pathway from the alpha5beta1 and alpha(v)beta3 integrins that elevates bcl-2 transcription. J Biol Chem. 2001;276:27757–63. doi: 10.1074/jbc.M102014200. [DOI] [PubMed] [Google Scholar]
- Mayer BW, Jr, Hay ED, Hynes RO. Immunocytochemical localization of fibronectin in embryonic chick trunk and area vasculosa. Dev Biol. 1981;82:267–86. doi: 10.1016/0012-1606(81)90451-6. [DOI] [PubMed] [Google Scholar]
- Muller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 1997;88:603–13. doi: 10.1016/s0092-8674(00)81903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–33. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Watanabe Y, Smyth G, Miyagawa-Tomita S, Meyers E, Klingensmith J, Camenisch T, Buckingham M, Moon AM. An FGF autocrine loop initiated in second heart field mesoderm regulates morphogenesis at the arterial pole of the heart. Development. 2008;135:3599–610. doi: 10.1242/dev.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, Hynes RO. Fibronectin isoform distribution in the mouse. I. The alternatively spliced EIIIB, EIIIA, and V segments show widespread codistribution in the developing mouse embryo. Cell Adhes Commun. 1996;4:103–25. doi: 10.3109/15419069609010766. [DOI] [PubMed] [Google Scholar]
- Roovers K, Davey G, Zhu X, Bottazzi ME, Assoian RK. alpha 5beta 1 Integrin Controls Cyclin D1 Expression by Sustaining Mitogen-activated Protein Kinase Activity in Growth Factor-treated Cells. Mol Biol Cell. 1999;10:3197–3204. doi: 10.1091/mbc.10.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovasio RA, Delouvee A, Yamada KM, Timpl R, Thiery JP. Neural crest cell migration: requirements for exogenous fibronectin and high cell density. J Cell Biol. 1983;96:462–73. doi: 10.1083/jcb.96.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoncella S, Echtermeyer F, Denhez F, Nowlen JK, Mosher DF, Robinson SD, Hynes RO, Goetinck PF. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci U S A. 1999;96:2805–10. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114:2553–60. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development. 2004;131:2205–18. doi: 10.1242/dev.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan LR, Condic ML. Neural crest motility and integrin regulation are distinct in cranial and trunk populations. Dev Biol. 2003;259:288–302. doi: 10.1016/s0012-1606(03)00187-8. [DOI] [PubMed] [Google Scholar]
- Testaz S, Delannet M, Duband J. Adhesion and migration of avian neural crest cells on fibronectin require the cooperating activities of multiple integrins of the (beta)1 and (beta)3 families. J Cell Sci. 1999;112 ( Pt 24):4715–28. doi: 10.1242/jcs.112.24.4715. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Lo CW, Kirby ML. Connexin 43 expression reflects neural crest patterns during cardiovascular development. Dev Biol. 1999;208:307–23. doi: 10.1006/dbio.1999.9219. [DOI] [PubMed] [Google Scholar]
- Whittaker CA, Bergeron KF, Whittle J, Brandhorst BP, Burke RD, Hynes RO. The echinoderm adhesome. Dev Biol. 2006;300:252–66. doi: 10.1016/j.ydbio.2006.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Francis R, Wei CJ, Linask KL, Lo CW. Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development. 2006;133:3629–39. doi: 10.1242/dev.02543. [DOI] [PubMed] [Google Scholar]
- Yang JT, Bader BL, Kreidberg JA, Ullman-Cullere M, Trevithick JE, Hynes RO. Overlapping and independent functions of fibronectin receptor integrins in early mesodermal development. Dev Biol. 1999;215:264–77. doi: 10.1006/dbio.1999.9451. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cerrato F, Xu H, Vitelli F, Morishima M, Vincentz J, Furuta Y, Ma L, Martin JF, Baldini A, et al. Tbx1 expression in pharyngeal epithelia is necessary for pharyngeal arch artery development. Development. 2005;132:5307–15. doi: 10.1242/dev.02086. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci U S A. 1995;92:6161–5. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A. Confocal IF microscopy shows co-localization of CNCCs (detected using anto-TFAP2α antibody, red nuclei) and FN (green) in the same plane. Magnified box 1 shows localization of FN (green) between non-neural surface ectoderm (arrowhead) and CNC progenitors (red, arrow). Magnified box 2 shows mesenchymal CNCCs (red, arrow) surrounded by FN (green). op-otic pit. B. Control. Staining of a section from FN-null embryos using equivalent concentration of anti-TFAP2α antibody (red), anti-FN antibody (green) and DAPI. Arrow points at the branchial arch mesenchyme. Arrowhead points to the non-neural surface ectoderm. Note that anti-FN antibody produces minimal staining in the absence of FN.
Figure S2. Expression of FN mRNA at E9.5. A. CNC progenitors and CNCCs express FN mRNA (arrows). B. FN mRNA is enriched in pharyngeal ectoderm (black arrow) and pharyngeal pocket endoderm, asterisks. Presumptive CNCCs inside the outflow tract (open arrows) also express FN mRNA, while mesenchymal cells lateral to dorsal aorta and in pharyngeal arches (magnified in panel B′) express low levels of FN mRNA. dAo – dorsal aorta. Endothelial cells in panel B are marked by the black arrowheads.
Figure S3. Pharyngeal ectoderm (ec, small arrow), endoderm (en, arrowhead) and mesoderm (notched arrowhead) are marked by the expression of Isl1 in control and FN-null embryos at E9.5. Long arrows point at the outflow tract myocardium expressing Isl1 in FN-null and controls.