Abstract
West Nile virus (WNV) –induced encephalitis has been a public health concern in North America over the past decade. No therapeutics or vaccines are available for human use. Studies in animal models have provided important information for investigations of WNV pathogenesis and the host immune response in humans. This article will give an overview of the role of γδ T cells, one of the non-classical T cell subsets in the murine model of WNV encephalitis.
Keywords: West Nile virus, Encephalitis, Gamma/delta T cells
1. Introduction
West Nile virus (WNV), a plus-sense, single-stranded neurotropic flavivirus, is now the most widely distributed arbovirus in the world, occurring on all continents except Antarctica (Kramer et al., 2008). It was originally isolated in Africa and later caused epidemics with mainly febrile illness in humans in Europe, Africa, the Middle East, and parts of Asia. In North America, a more virulent WNV strain was detected in 1999 and has caused annual outbreaks of viral encephalitis (Campbell et al., 2002; Pletnev et al., 2006). WNV is maintained in an enzootic cycle that involves mosquitoes and birds, with humans and horses as incidental hosts. Human infection results primarily from mosquito bites (Campbell et al., 2002). Additionally, blood transfusion, organ transplantation, breast feeding and in utero or occupational exposure were reported to be associated with WNV transmission in humans (2002a; 2002b; Alpert et al., 2003; Charatan, 2002). WNV infection of the central nervous system (CNS, neuroinvasive disease) commonly presents as encephalitis, meningitis or acute flaccid paralysis. Those at highest risk of developing WNV-induced encephalitis are the elderly (> 70 years) and immuno-suppressed persons. At present, there is no specific therapeutic agent for treatment of the infection or an approved vaccine for its prevention.
2. Pathogenesis of WNV-induced encephalitis
Studies in animal models, including mice, hamsters and monkeys, have provided important information for investigators of WNV pathogenesis and host immune response in humans (Davis et al., 2001; Kramer and Bernard, 2001; Ratterree et al., 2004; Xiao et al., 2001). Following a brief period of viremia, WNV can gain access to the CNS, a process called neuroinvasion that may turn a mild viral infection into severe lethal encephalitis (Ben-Nathan et al., 1996; Diamond et al., 2003a; Halevy et al., 1994). In the susceptible host, WNV is neuroinvasive and neurovirulent (i.e., able to infect the CNS, replicate in some of its cells and injure them) (Ben-Nathan et al., 1996; Halevy et al., 1994). Although how WNV crosses the blood brain barrier (BBB) is not clearly understood, it has been suggested that WNV infects the CNS in part via hematogenous spread, e.g., an increased viral burden in the serum correlates with earlier viral entry into the brain (Diamond et al., 2003b). Systemic WNV replication induced-proinflammatory cytokines, including tumor necrosis factor (TNF-α) and macrophage migration inhibitory factor, could modulate the permeability of the BBB, which may further enable viral entry into the brain and induce lethal encephalitis (Arjona et al., 2007; Wang et al., 2004). Leukocyte migration across the brain endothelial layer also accelerates BBB breakdown (Dietrich, 2002). In WNV-infected mice, innate immune cells, including microglia or macrophages, NK cells, plasmacytoid DCs, and neutrophils, greatly expand as the virus invades the brain, followed by B and T cell infiltration (Brehin et al., 2008). WNV might also cross the BBB and enter the CNS by being carried by infected infiltrating T cells (Wang et al., 2008). Overall, it appears critical to control virus dissemination in the periphery at the early stages of WNV infection. Once inside the brain, WNV-induced CNS disease might be caused by neuronal degeneration, a direct result of viral infection, and/or by bystander damage from the immune response to the pathogen, including lymphocyte and macrophage/microglia responses (Sampson and Armbrustmacher, 2001; Shrestha et al., 2003; Wang et al., 2003b; Xiao et al., 2001).
3. Host Immunity to WNV infection
The murine model has been used as an effective in vivo experimental model to investigate host immunity to WNV infection in humans. Both type 1 and type 2 interferons (IFNs), including IFN-α, IFN-β, and IFN-γ, participate in the control of viral infections and provide protective immunity against lethal WNV encephalitis (Anderson and Rahal, 2002; Katze et al., 2002; Lucas et al., 2003; Samuel and Diamond, 2005; Shahar et al., 1990; Shrestha et al., 2006; Wang et al., 2003a). B cells and specific antibodies are critical in the control of disseminated WNV infection, but are not sufficient to eliminate it from the host (Diamond et al., 2003b; Roehrig et al., 2001). Macrophages, B cells and dendritic cells (DCs) are the antigen-presenting cells (APCs) involved during systemic WNV infection (Kulkarni et al., 1991). Among them, DCs represent the most important APCs exhibiting the unique capacity to initiate primary T cell responses. In particular, during cutaneous WNV infection, the bone marrow-derived epidermal DCs (Langerhans cells) are important APCs in the skin — where the pathogen is naturally deposited during mosquito transmission of the virus (Byrne et al., 2001; Johnston et al., 2000). These cells migrate from the epidermis by an IL-1β-dependent pathway and accumulate in the local draining lymph nodes, thereby playing an important role in T-cell activation and proliferation (Byrne et al., 2001). T cell receptor β (TCRβ)-deficient (TCRβ−/−) mice were recently shown (Wang et al., 2003a) to have an increased mortality to WNV infection, compared with that in control animals, which indicates that αβ ibute to host survival. CD4+ αβ T cells respond vigorously in the periphery (Kulkarni et al., 1991), and provide help for antibody responses and sustain WNV-specific CD8+ T cell responses in the CNS that enable viral clearance (Sitati and Diamond, 2006). In comparison, CD8+ αβ T cell responses have been observed in both the spleen and brain following WNV infection (Liu et al., 1989). They are critical in clearing WNV infection from tissues and preventing viral persistence. In this review, we will summarize the role of one of the non-classical T cells--namely γδ T cells, in WNV-induced encephalitis, either by directly responding or regulating other immune factors.
4. γδ T cells control WNV dissemination in the periphery
γδ T cells comprise a minority of the CD3+ T cells in lymphoid tissue and blood, but are well represented at epithelial and mucosal sites (Hayday, 2000). They display some unique features, including a lack of major histocompatibility complex (MHC) restriction and the potential capacity to respond to antigens without a requirement for conventional antigen processing, which together suggest a role in innate immunity against pathogen infection (Wang et al., 2001). γδ T cells are critical in the early control of WNV dissemination. TCRδ −/− mice, which are deficient in γδ T cells, had elevated viremia, which led to a greater dissemination of the pathogen to the CNS and, hence, to more severe encephalitis. Accordingly, they were much more susceptible to WNV infection than were the wild-type controls (Wang et al., 2003a). Although both peripheral αβ and γδ T cells quickly expanded at day 2 post- WNV infection, the latter ones more dramatically increased. IFN-γ has multiple mechanisms of viral control, including cell recruitment and activation, polarization of T cell responses, up-regulation of antigen processing and presentation, and direct antiviral action (Guidotti and Chisari, 2001). Additionally, IFN-γ was reported to limit myeloid cell infection in vitro (Shrestha et al., 2006) and shown to be one of the major cytokines produced by γδ T cells in several viral infection systems (Cai and Tucker, 2001; Ninomiya et al., 2000; Sciammas and Bluestone, 1999; Selin et al., 2001). At the early stages of WNV infection, γδ T cells are considered the major resource to produce IFN-γ, which partially contributes to their protective effect in host immunity. As a consequence, adoptive transfer of splenocytes from either wild-type or TCRβ−/− mice, which are deficient in αβ T cells, but not γδ T cells, enhance host survival from lethal WNV infection, whereas transfer of the splenocytes from TCRβ−/−IFNγ−/− mice, which have a defect in the IFN-γ-producing capacity of γδ T cells, did not affect host susceptibility (Wang et al., 2003a). In a separate study (Shrestha et al., 2006), irradiated mice reconstituted with IFN-γ-deficient γδ T cells were shown to have significantly higher levels of viral loads in blood and brains during WNV infection than mice reconstituted with IFN-γ-sufficient γδ T cells. Vα1+ cells, one of the two major subpopulations of peripheral γδ T cells, expanded significantly during WNV infection and were the major γδ subset producing IFN-γ. Mice depleted of Vγ1+ cells had enhanced viremia and higher mortality to WNV encephalitis (Welte et al., 2008). Similar to humans, aged mice were more susceptible to WNV infection than were young mice. Vγ1+ cells of aged mice had a slower and reduced response to WNV infection, when compared to that of young adult mice, which might partially contribute to their enhanced host susceptibility to the viral encephalitis. The total number of Vγ1+lFN-γ+ cells in young mice was much higher than in aged mice due to differences in Vγ1+ T cell expansion (Welte et al., 2008). The mechanisms by which IFN-γ limits neuroinvasiveness remain undefined.
Cytolytic function is another important mechanism of viral control attributed to γδ T cells (Sciammas et al., 1997; Selin et al., 2001; Tseng and Klimpel, 2002). In WNV-infected mice, cytolytic activity against infected target cells was detected as early as day 4, peaked at day 5, and declined rapidly after day 7 (Kesson et al., 1987). The expression of perforin, an intracellular protein, reflects the cytolytic activity of these cells (Chang and Braciale, 2002). TCRδ−/− mice showed reduced levels of intracellular perforin in total splenocytes at day 6 post-WNV infection, implying that they either directly or indirectly enhanced cytolytic activity (Wang et al., 2003a).
In summary, γδ T cells are involved in the control of WNV dissemination with different functional significance at different points during the course of infection.
5. Subsets of γδ T cells also play a pathogenic role during WNV infection
γδ T cells are divisible into functionally distinct subsets in human and mouse, which have direct and indirect effects on host immunity to pathogen infection (Bank et al., 1986). Vγ1+ T cells and Vγ4+ T cells are the two major subpopulations of splenic γδ T cells in mice. Evidence for the opposite roles of Vγ1+ T cells and Vγ4+ T cells in the resolution of pathogen infection has been reported in studies of Coxsackie virus (Huber et al., 2000) and in cytomegalovirus infection (Ninomiya et al., 2000). Vγ4+ T cells have been found to be involved in WNV pathogenesis, as their depletion resulted in a decreased viral load in the brain and ultimately a lower mortality to WNV-induced encephalitis. Aged mice maintain a higher content of Vγ4+ cells, which might lead to an increased susceptibility to WNV infection (Welte et al., 2008). Vγ4+ T cells of young adult mice expanded modestly immediately following WNV infection and had a higher potential for producing proinflammatory cytokines, such as TNF-α and IL-17 (Welte et al., 2008). TNF-α is known to be involved in BBB compromise and WNV entry into the brain (Wang et al., 2004). Indeed, depletion of Vγ4+ cells reduced TNF-α levels in the CNS, and this was accompanied by a decreased viral load in the brain at the peak of CNS infection and a lower mortality in cases of WNV encephalitis. Nevertheless, in vivo blocking of IL-17 signaling led to no differences in host susceptibility to WNV infection and in the viral load in blood and brain or in inflammatory cell responses between IL-17 neutralization antibody-treated mice and controls (Welte et al., 2011). Although IL-17-producing γδ T cells were reported to play a key role in the pathogenesis of several disease models (Flierl et al., 2008; Roark et al., 2007), their role in WNV encephalitis remains undefined.
Vγ4+ cells also negatively regulate host protective immunity during WNV infection. Vγ4+ cells were reported to suppress Vγ1+ T cell expansion by producing TGF-β, which has multiple effects on host immunity. First, this suppression could directly lead to higher viremia, more virus dissemination into the CNS, and induction of encephalitis (Welte et al., 2008). Second, it could indirectly promote IL-10 levels in WNV-infected mice. IL-10, which is predominantly produced by CD4+αβ+ T cells, plays a pathogenic role in host immunity to WNV infection (Bai et al., 2009; Schneider et al., 2007). Vγ1+ cells were previously reported to reduce the IL-10-producing CD4+CD25+ T cells in the lungs of ovalbumin-sensitized and challenged mice (Hahn et al., 2008). Thus, the suppressive effect of Vγ4+ cells on Vγ1+ cell expansion may indirectly contribute to higher IL-10 levels during WNV infection. Indeed, there was significant reduction in IL-10 production either by splenic T cells or in the blood of WNV-infected Vγ4+ T cell-depleted mice (Welte et al., 2011). Lastly, WNV-induced CNS disease is partially caused by bystander damage from lymphocyte and macrophage/microglia responses (Sampson and Armbrustmacher, 2001; Shrestha et al., 2003; Wang et al., 2003b; Xiao et al., 2001). γδ T cells, and in particular, Vγ1+ T cells have been associated with a role in the resolution of inflammation (Huber et al., 2000; O'Brien et al., 2000). In an experimental model of autoimmune encephalomyelitis, γδ T cells regulated inflammation in the CNS by targeting IFN-γ-producing encephalitogenic T cells (Ponomarev and Dittel, 2005; Ponomarev et al., 2004). In another study, Vγ1+ T cells were shown to be cytotoxic for Listeria-activated macrophages via a FasL-dependent mechanism (Dalton et al., 2004). Although their presence was undetectable in WNV-infected mouse brains, enhanced levels of Vγ1+ T cells were noted significantly in the brains of Vγ4+ T cell-depleted mice at the late stages of infection (Figure 1). In accordance with these results, Vγ4+ cell-depleted mice displayed a reduced inflammation in the CNS, with significantly reduced levels of macrophages/monocytes (CD11b+) and CD8+ T cells at the later stages of infection (Welte et al., 2011). Together, these findings indicate that higher levels of Vγ1+ T cells in the brain may help to reduce the inflammatory responses.
Figure 1. Levels of Vγ1 expression in brains of control and Vγ4-T cell-depleted mice following WNV infection.
PCR amplification of Vγ1 (top panel) or β-actin (bottom panel) of WNV-infected mice brains harvested at day 7 post-infection.
6. γδ T cells regulate the adaptive immune response to WNV infection by promoting DC maturation and activation
The characteristics of γδ T cells in adaptive immunity to bacterial infection have been described in both human and primate models (Eberl et al., 2005; Shen et al., 2002). The depletion of γδ T cells in WNV primarily-infected mice before development of a secondary infection does not affect host susceptibility (Wang et al., 2006). This phenomena suggests that γδ T cells may not be directly involved in memory response to WNV infection, and also do not influence the development of antibody responses during primary and secondary infections with WNV. Nevertheless, TCRδ−/− mice displayed a numeric and functional reduction in CD4+ and CD8+ memory T cell responses (Wang et al., 2006); Welte T. & Wang T. et al. unpublished results), which may be indicative of a role of these cells in regulating memory T cell development during WNV infection. DCs are one of the most important antigen-presenting cells, as they exhibit the unique capacity to initiate primary T cell responses. The crosstalk between γδ T cells and DCs are known to contribute to DC maturation (Collins et al., 2005; Ismaili et al., 2002; Leslie et al., 2002; Munz et al., 2005). During WNV infection, splenic DCs of TCRδ−/− mice displayed lower levels of CD40, CD80, CD86 and MHC class II expression and interleukin-12 (IL-12) production than those of wild-type mice. Naïve DCs co-cultured with non-infected γδ T cells have enhanced levels of co-stimulatory molecules and MHC class II expression, which may mean that interactions between γδ T cells and DC are necessary for DC maturation. γδ T cells appeared to support a low, but significant, level of WNV replication (Fang et al., 2010). WNV-infected γδ T cells produce pro-inflammatory cytokines, including IFN-γ, TNF-α and IL-6, which presumably contribute to DC maturation. Upregulation of co-stimulatory molecules and MHC class II expression was significantly higher on DCs that were co-cultured with WNV-infected γδ T cells than with non-infected γδ T cells, which further demonstrates that these secreting factors from WNV-infected γδ T cells are also important for promoting DC maturation and initiating CD4+ T cell priming (Fang et al., 2010). The mechanisms by which γδ T cells are activated during WNV infection and produced proinflammatory cytokines are not fully understood. It is suggested that WNV infection of γδ T cells could induce TLRs (Daffis et al., 2008; Town et al., 2009; Wang et al., 2004) or the non-TLR innate immune receptors, such as RIG-I and MDA5, which have been reported to be involved in WNV recognition (Fredericksen and Gale, 2006; Fredericksen et al., 2008). Alternatively, γδ T cells and WNV-permissive DCs may exert regulatory influences on each other as reported in human models. For example, induction of human γδ T cells by poly I: C, a ligand for TLR3, depends on DCs mediated by Type-1 IFNs (Kunzmann et al., 2004).
7. γδ T cells in other viral encephalitis models
Functions of γδ T cells have been studied in several other viral encephalitides models, including both DNA and RNA viruses. In the murine model of herpes simplex virus type 1 (HSV-1), a neurotropic DNA virus by footpad or ocular injection, the virus replicates at the site of infection and is transmitted to sensory ganglia, where it establishes latency, if not regulated, to the CNS where it can cause encephalitis. γδ T cells were shown to directly mediate host protection by decreasing HSV-1 replication early during infection and restricting its progression into the brain. These efforts led to a greatly reduced mortality by preventing the development of lethal viral encephalitis (Sciammas et al., 1997). Although the mechanisms by which γδ T cells protect the host against HSV-1 infection are not yet fully understood, isolation of IFN-γ– producing γδ T cells in the trigeminal ganglion of HSV-1 infected mice may be indicative of a role of these cells in mounting antiviral effector functions (Kodukula P, Sciammas, R, JA Bluestone and R Hendricks, unpublished data). In comparison to HSV-1 model, γδ T cells seem to be dispensable in control of infection by alphaviruses, the other neurotropic RNA viruses. For example, mice deficient of αβ T cells, but not γδ T cells, have a decreased mortality to infection with Sindbis virus, a neurovirulent alphavirus infection, suggesting a role of αβ T cells but not of γδ T cells in viral pathogenesis (Rowell and Griffin, 2002). Whereas, TCRδ−/− mice immunized with a chimeric alphavirus vaccine candidate were protected from lethal intranasal challenge with Venezuelan equine encephalitis virus (VEEV), another alphavirus, the virus was found to persist in the brain for up to 28 days following inoculation. Moreover, virus clearance was not affected in surviving animals lacking a functional IFN-γ receptor. These results in the VEEV model, in contrast with the findings in the WNV infection model, possibly indicate that alphavirus-mediated immunity to VEEV is partially independent of γδ T cells, as well as IFN-γ receptor signaling, during early antiviral control (Paessler et al., 2007).
8. Summary and Future Directions
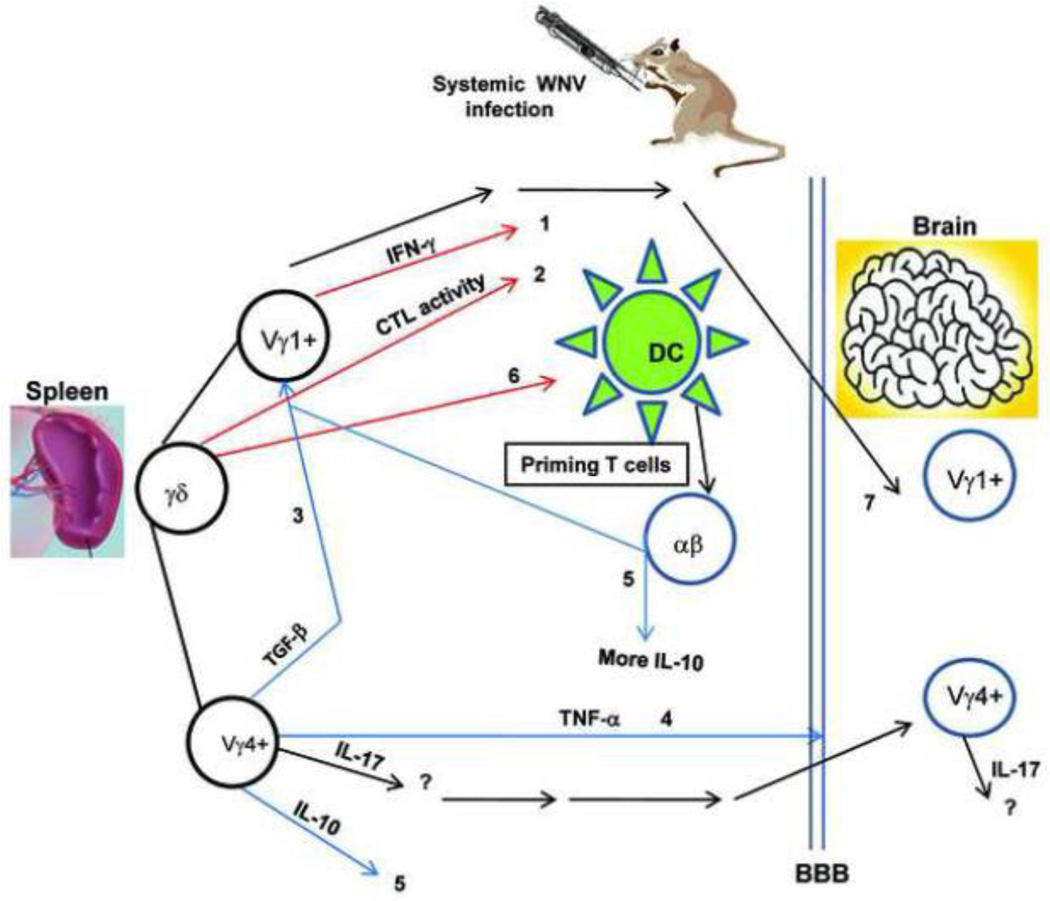
In summary, γδ T cells play a unique role in both protective immunity and viral pathogenesis during WNV infection. γδ T cells, mainly Vγ1+ cells, are involved in immediate control of WNV dissemination, partially due to their IFN-γ-producing activity. γδ T cells also contribute to protective immunity by enhancing cytolytic activity against WNV-infected target cells, while Vγ4+ cells, another subpopulation of γδ T cells, play a pathogenic role via production of both pro-inflammatory and regulatory cytokines during WNV infection, leading to a higher viremia and/or more inflammatory responses in the brain. Moreover, γδ T cells may recognize WNV and are activated to produce pro-inflammatory cytokines directly via PRRs or indirectly via other immune cells. WNV-activated γδ T cells will promote DC maturation and activation, which will ultimately prime T cell responses to WNV infection (Figure 2). Current findings imply that γδ T cells could be important in both WNV treatment and vaccine development for the potential target population. In a recent study (Wang et al., 2009), oral administration of active hexose-correlated compound (AHCC), an extract of Lentinula edodes of the Basidiomycete family of fungi rich in α-glucans, attenuated viremia and mortality following lethal WNV infection in young adult mice partially via enhancement of γδ T cell expansion. AHCC administration in the more susceptible aged mice also enhanced the protective Vγ1+ T cell response, as well as WNV-specific IgG production, which together led to attenuated viremia levels but to no differences in mortality rate (Wang et al., 2009).
Figure 2. Role of γδ T cells in West Nile virus-induced encephalitis.
1) limit WNV dissemination, 2) kill WNV-infected target cells, 3) suppress Vγ1+ T cell proliferation, 4) enhance BBB permeability, 5) increase IL-10 levels directly by production or indirectly via suppression of Vγ1+ T cell expansion, 6) promote DC maturation and 7) a potential anti-inflammatory role for infiltrating Vγ1+ T cells in the CNS. Colored arrow lines each represent a different effect of γδ T cells in WNV encephalitis (red: protective; blue: pathogenic; black: speculative or undefined).
The role of γδ T cells in human WNV infection remains undefined. Though human and mouse γδ T cells show differences in the subsets and ligand recognition, they share a substantial similarity in effector function and their protective role in pathogen infection (Girardi, 2006). The exploration of parallel activities mediated by murine γδ T cells will provide insights into immunosurveillance and immune regulation studies of WNV diseases in humans. Current understanding of the biological role of γδ T cell receptors during pathogen infection remains elusive. Future directions will also focus on an understanding of the underlying mechanism by which γδ T cells are activated during WNV infection.
Acknowledgements
I thank the members of the Wang lab for their contribution to understanding γδ T cells in West Nile virus infection. I also wish to thank Dr. Thomas Welte for allowing me to use his unpublished results. I thank Ms. Mardelle Susman for help in preparing this manuscript. This work was supported by NIH grant R01AI072060 (to T Wang).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Laboratory-acquired West Nile virus infections--United States, 2002. MMWR Morb Mortal Wkly Rep. 2002a;51:1133–1135. [PubMed] [Google Scholar]
- Possible West Nile virus transmission to an infant through breast-feeding--Michigan, 2002. MMWR Morb Mortal Wkly Rep. 2002b;51:877–878. [PubMed] [Google Scholar]
- Alpert SG, Fergerson J, Noel LP. Intrauterine West Nile virus: ocular and systemic findings. Am J Ophthalmol. 2003;136:733–735. doi: 10.1016/s0002-9394(03)00452-5. [DOI] [PubMed] [Google Scholar]
- Anderson JF, Rahal JJ. Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro. Emerg Infect Dis. 2002;8:107–108. doi: 10.3201/eid0801.010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjona A, Foellmer HG, Town T, Leng L, McDonald C, Wang T, Wong SJ, Montgomery RR, Fikrig E, Bucala R. Abrogation of macrophage migration inhibitory factor decreases West Nile virus lethality by limiting viral neuroinvasion. J Clin Invest. 2007;117:3059–3066. doi: 10.1172/JCI32218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Town T, Qian F, Wang P, Kamanaka M, Connolly TM, Gate D, Montgomery RR, Flavell RA, Fikrig E. IL-10 signaling blockade controls murine West Nile virus infection. PLoS Pathog. 2009 doi: 10.1371/journal.ppat.1000610. e1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank I, DePinho RA, Brenner MB, Cassimeris J, Alt FW, Chess L. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature. 1986;322:179–181. doi: 10.1038/322179a0. [DOI] [PubMed] [Google Scholar]
- Ben-Nathan D, Huitinga I, Lustig S, van Rooijen N, Kobiler D. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch Virol. 1996;141:459–469. doi: 10.1007/BF01718310. [DOI] [PubMed] [Google Scholar]
- Brehin AC, Mouries J, Frenkiel MP, Dadaglio G, Despres P, Lafon M, Couderc T. Dynamics of immune cell recruitment during West Nile encephalitis and identification of a new CD19+B220-BST-2+ leukocyte population. J Immunol. 2008;180:6760–6767. doi: 10.4049/jimmunol.180.10.6760. [DOI] [PubMed] [Google Scholar]
- Byrne SN, Halliday GM, Johnston LJ, King NJ. Interleukin-1beta but not tumor necrosis factor is involved in West Nile virus-induced Langerhans cell migration from the skin in C57BL/6 mice. J Invest Dermatol. 2001;117:702–709. doi: 10.1046/j.0022-202x.2001.01454.x. [DOI] [PubMed] [Google Scholar]
- Cai JL, Tucker PW. Gammadelta T cells: immunoregulatory functions and immunoprotection. Chem Immunol. 2001;79:99–138. doi: 10.1159/000058827. [DOI] [PubMed] [Google Scholar]
- Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2:519–529. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- Chang J, Braciale TJ. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat Med. 2002;8:54–60. doi: 10.1038/nm0102-54. [DOI] [PubMed] [Google Scholar]
- Charatan F. Organ transplants and blood transfusions may transmit West Nile virus. Bmj. 2002;325:566. doi: 10.1136/bmj.325.7364.566/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C, Wolfe J, Roessner K, Shi C, Sigal LH, Budd RC. Lyme arthritis synovial gammadelta T cells instruct dendritic cells via fas ligand. J Immunol. 2005;175:5656–5665. doi: 10.4049/jimmunol.175.9.5656. [DOI] [PubMed] [Google Scholar]
- Daffis S, Samuel MA, Suthar MS, Gale M, Jr., Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol. 2008;82:10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton JE, Howell G, Pearson J, Scott P, Carding SR. Fas-Fas ligand interactions are essential for the binding to and killing of activated macrophages by gamma delta T cells. J Immunol. 2004;173:3660–3667. doi: 10.4049/jimmunol.173.6.3660. [DOI] [PubMed] [Google Scholar]
- Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, Bowen R, Bunning ML. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75:4040–4047. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 2003a;16:259–278. doi: 10.1089/088282403322396082. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med. 2003b;198:1853–1862. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich JB. The adhesion molecule ICAM-1 and its regulation in relation with the blood-brain barrier. J Neuroimmunol. 2001;128:58–68. doi: 10.1016/s0165-5728(02)00114-5. [DOI] [PubMed] [Google Scholar]
- Eberl M, Engel R, Aberle S, Fisch P, Jomaa H, Pircher H. Human Vgamma9/Vdelta2 effector memory T cells express the killer cell lectin-like receptor G1 (KLRG1) J Leukoc Biol. 2005;77:67–70. doi: 10.1189/jlb.0204096. [DOI] [PubMed] [Google Scholar]
- Fang H, Welte T, Zheng X, Chang GJ, Holbrook MR, Soong L, Wang T. gammadelta T cells promote the maturation of dendritic cells during West Nile virus infection. FEMS Immunol Med Microbiol. 2010 doi: 10.1111/j.1574-695X.2010.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ferrara JL, Ward PA. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008;22:2198–2205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- Fredericksen BL, Gale M., Jr. West Nile virus evades activation of interferon regulatory factor 3 through RIG-I-dependent and -independent pathways without antagonizing host defense signaling. J Virol. 2006;80:2913–2923. doi: 10.1128/JVI.80.6.2913-2923.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericksen BL, Keller BC, Fornek J, Katze MG, Gale M., Jr. Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J Virol. 2008;82:609–616. doi: 10.1128/JVI.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- Hahn YS, Ji XY, Woo SI, Choi YK, Song MS, Shin KS, Jin N, O'Brien RL, Born WK. Vgamma1+ gammadelta T cells reduce IL-10-producing CD4+CD25+ T cells in the lung of ovalbumin-sensitized and challenged mice. Immunol Lett. 2008;121:87–92. doi: 10.1016/j.imlet.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy M, Akov Y, Ben-Nathan D, Kobiler D, Lachmi B, Lustig S. Loss of active neuroinvasiveness in attenuated strains of West Nile virus: pathogenicity in immunocompetent and SCID mice. Arch Virol. 1994;137:355–370. doi: 10.1007/BF01309481. [DOI] [PubMed] [Google Scholar]
- Hayday AC. Gammadelta cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- Huber SA, Graveline D, Newell MK, Born WK, O'Brien RL. V gamma 1 + T cells suppress and V gamma 4 + T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- Ismaili J, Olislagers V, Poupot R, Fournie JJ, Goldman M. Human gamma delta T cells induce dendritic cell maturation. Clin Immunol. 2002;103:296–302. doi: 10.1006/clim.2002.5218. [DOI] [PubMed] [Google Scholar]
- Johnston LJ, Halliday GM, King NJ. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J Invest Dermatol. 2000;114:560–568. doi: 10.1046/j.1523-1747.2000.00904.x. [DOI] [PubMed] [Google Scholar]
- Katze MG, He Y, Gale M., Jr. Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- Kesson AM, Blanden RV, Mullbacher A. The primary in vivo murine cytotoxic T cell response to the flavivirus, West Nile. J Gen Virol. 1987;68:2001–2006. doi: 10.1099/0022-1317-68-7-2001. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Bernard KA. West Nile virus infection in birds and mammals. Ann N Y Acad Sci. 2001;951:84–93. doi: 10.1111/j.1749-6632.2001.tb02687.x. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Mullbacher A, Blanden RV. Functional analysis of macrophages, B cells and splenic dendritic cells as antigen-presenting cells in West Nile virus-specific murine T lymphocyte proliferation. Immunol Cell Biol. 1991;69(Pt 2):71–80. doi: 10.1038/icb.1991.12. [DOI] [PubMed] [Google Scholar]
- Kunzmann V, Kretzschmar E, Herrmann T, Wilhelm M. Polyinosinic-polycytidylic acid-mediated stimulation of human gammadelta T cells via CD11c dendritic cell-derived type I interferons. Immunology. 2004;112:369–377. doi: 10.1111/j.1365-2567.2004.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie DS, Vincent MS, Spada FM, Das H, Sugita M, Morita CT, Brenner MB. CD1-mediated gamma/delta T cell maturation of dendritic cells. J Exp Med. 2002;196:1575–1584. doi: 10.1084/jem.20021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Blanden RV, Mullbacher A. Identification of cytolytic lymphocytes in West Nile virus-infected murine central nervous system. J Gen Virol. 1989;70:565–573. doi: 10.1099/0022-1317-70-3-565. [DOI] [PubMed] [Google Scholar]
- Lucas M, Mashimo T, Frenkiel MP, Simon-Chazottes D, Montagutelli X, Ceccaldi PE, Guenet JL, Despres P. Infection of mouse neurones by West Nile virus is modulated by the interferon-inducible 2'–5' oligoadenylate synthetase 1b protein. Immunol Cell Biol. 2003;81:230–236. doi: 10.1046/j.1440-1711.2003.01166.x. [DOI] [PubMed] [Google Scholar]
- Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya T, Takimoto H, Matsuzaki G, Hamano S, Yoshida H, Yoshikai Y, Kimura G, Nomoto K. Vgamma1+ gammadelta T cells play protective roles at an early phase of murine cytomegalovirus infection through production of interferon-gamma. Immunology. 2000;99:187–194. doi: 10.1046/j.1365-2567.2000.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RL, Yin X, Huber SA, Ikuta K, Born WK. Depletion of a gamma delta T cell subset can increase host resistance to a bacterial infection. J Immunol. 2000;165:6472–6479. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- Paessler S, Yun NE, Judy BM, Dziuba N, Zacks MA, Grund AH, Frolov I, Campbell GA, Weaver SC, Estes DM. Alpha-beta T cells provide protection against lethal encephalitis in the murine model of VEEV infection. Virology. 2007;367:307–323. doi: 10.1016/j.virol.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnev AG, Swayne DE, Speicher J, Rumyantsev AA, Murphy BR. Chimeric West Nile/dengue virus vaccine candidate: Preclinical evaluation in mice, geese and monkeys for safety and immunogenicity. Vaccine. 2006;24:6392–6404. doi: 10.1016/j.vaccine.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Dittel BN. Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J Immunol. 2005;174:4678–4687. doi: 10.4049/jimmunol.174.8.4678. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Novikova M, Yassai M, Szczepanik M, Gorski J, Dittel BN. Gamma delta T cell regulation of IFN-gamma production by central nervous system-infiltrating encephalitogenic T cells: correlation with recovery from experimental autoimmune encephalomyelitis. J Immunol. 2004;173:1587–1595. doi: 10.4049/jimmunol.173.3.1587. [DOI] [PubMed] [Google Scholar]
- Ratterree MS, Gutierrez RA, Travassos da Rosa AP, Dille BJ, Beasley DW, Bohm RP, Desai SM, Didier PJ, Bikenmeyer LG, Dawson GJ, Leary TP, Schochetman G, Phillippi-Falkenstein K, Arroyo J, Barrett AD, Tesh RB. Experimental infection of rhesus macaques with West Nile virus: level and duration of viremia and kinetics of the antibody response after infection. J Infect Dis. 2004;189:669–676. doi: 10.1086/381461. [DOI] [PubMed] [Google Scholar]
- Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of Collagen-Induced Arthritis by Oligoclonal, IL-17-Producing {gamma}{delta} T Cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrig JT, Staudinger LA, Hunt AR, Mathews JH, Blair CD. Antibody prophylaxis and therapy for flavivirus encephalitis infections. Ann N Y Acad Sci. 2001;951:286–297. doi: 10.1111/j.1749-6632.2001.tb02704.x. [DOI] [PubMed] [Google Scholar]
- Rowell JF, Griffin DE. Contribution of T cells to mortality in neurovirulent Sindbis virus encephalomyelitis. J Neuroimmunol. 2002;127:106–114. doi: 10.1016/s0165-5728(02)00108-x. [DOI] [PubMed] [Google Scholar]
- Sampson BA, Armbrustmacher V. West Nile encephalitis: the neuropathology of four fatalities. Ann N Y Acad Sci. 2001;951:172–178. [PubMed] [Google Scholar]
- Samuel MA, Diamond MS. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J Virol. 2005;79:13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BS, McGee CE, Jordan JM, Stevenson HL, Soong L, Higgs S. Prior exposure to uninfected mosquitoes enhances mortality in naturally-transmitted West Nile virus infection. PLoS One. 2007;2:e1171. doi: 10.1371/journal.pone.0001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciammas R, Bluestone JA. TCRgammadelta cells and viruses. Microbes Infect. 1999;1:203–212. doi: 10.1016/s1286-4579(99)80035-5. [DOI] [PubMed] [Google Scholar]
- Sciammas R, Kodukula P, Tang Q, Hendricks RL, Bluestone JA. T cell receptor gammadelta cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selin LK, Santolucito PA, Pinto AK, Szomolanyi-Tsuda E, Welsh RM. Innate immunity to viruses: control of vaccinia virus infection by gamma delta T cells. J Immunol. 2001;166:6784–6794. doi: 10.4049/jimmunol.166.11.6784. [DOI] [PubMed] [Google Scholar]
- Shahar A, Lustig S, Akov Y, David Y, Schneider P, Levin R. Different pathogenicity of encephalitic togaviruses in organotypic cultures of spinal cord slices. J Neurosci Res. 1990;25:345–352. doi: 10.1002/jnr.490250311. [DOI] [PubMed] [Google Scholar]
- Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Gottlieb D, Diamond MS. Infection and injury of neurons by West Nile encephalitis virus. J Virol. 2003;77:13203–13213. doi: 10.1128/JVI.77.24.13203-13213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Wang T, Samuel MA, Whitby K, Craft J, Fikrig E, Diamond MS. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J Virol. 2006;80:5338–5348. doi: 10.1128/JVI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitati EM, Diamond MS. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J Virol. 2006;80:12060–12069. doi: 10.1128/JVI.01650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Bai F, Wang T, Kaplan AT, Qian F, Montgomery RR, Anderson JF, Flavell RA, Fikrig E. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009;30:242–253. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–49. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Kamath A, Das H, Li L, Bukowski JF. Antibacterial effect of human V gamma 2V delta 2 T cells in vivo. J Clin Invest. 2001;108:1349–1357. doi: 10.1172/JCI13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Welte T, Fang H, Chang GJ, Born WK, O'Brien RL, Sun B, Fujii H, Kosuna K, Wang T. Oral administration of active hexose correlated compound enhances host resistance to West Nile encephalitis in mice. J Nutr. 2009;139:598–602. doi: 10.3945/jn.108.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Welte T, McGargill M, Town T, Thompson J, Anderson JF, Flavell RA, Fikrig E, Hedrick SM, Wang T. Drak2 contributes to West Nile virus entry into the brain and lethal encephalitis. J Immunol. 2008;181:2084–2091. doi: 10.4049/jimmunol.181.3.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Gao Y, Scully E, Davis CT, Anderson JF, Welte T, Ledizet M, Koski R, Madri JA, Barrett A, Yin Z, Craft J, Fikrig E. Gammadelta T cells facilitate adaptive immunity against West Nile virus infection in mice. J Immunol. 2006;177:1825–1832. doi: 10.4049/jimmunol.177.3.1825. [DOI] [PubMed] [Google Scholar]
- Wang T, Scully E, Yin Z, Kim JH, Wang S, Yan J, Mamula M, Anderson JF, Craft J, Fikrig E. IFN-gamma-producing gammadelta T cells help control murine West Nile virus infection. J Immunol. 2003a;171:2524–2531. doi: 10.4049/jimmunol.171.5.2524. [DOI] [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lobigs M, Lee E, Mullbacher A. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J Virol. 2003b;77:13323–13334. doi: 10.1128/JVI.77.24.13323-13334.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte T, Aronson J, Gong B, Rachamallu A, Mendell N, Tesh R, Paessler S, Born W, O'Brien R, Wang T. Vγ4+ T Cells Regulate Host Immune Response to West Nile Virus Infection FEMS. Immunol Med Microbiol. 2011 doi: 10.1111/j.1574-695X.2011.00840.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte T, Lamb J, Anderson JF, Born WK, O'Brien RL, Wang T. Role of two distinct gammadelta T cell subsets during West Nile virus infection. FEMS Immunol Med Microbiol. 2008;53:275–283. doi: 10.1111/j.1574-695X.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao SY, Guzman H, Zhang H, Travassos da Rosa AP, Tesh RB. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg Infect Dis. 2001;7:714–721. doi: 10.3201/eid0704.010420. [DOI] [PMC free article] [PubMed] [Google Scholar]