Abstract
Stable mixed hematopoietic chimerism has been consistently established in dogs mildly immunosuppressed by 200 cGy of total body irradiation (TBI) before and given a brief course of immunosuppression with mycophenolate mofetil (28 days) and cyclosporine (35 days) after dog leukocyte antigen (DLA)-identical marrow transplantation. However, when TBI was reduced from 200 to 100 cGy, grafts were nearly uniformly rejected within 3 to 12 weeks. Here we asked whether stable engraftment could be accomplished after a suboptimal dose of 100 cGy TBI with host immunosuppression enhanced by donor-derived mesenchymal stromal cells (MSC) given after transplantation. MSC were cultured from marrow cells and evaluated in vitro for antigen expression. They showed profound immunosuppressive properties in mixed leukocyte reactions (MLR) in a cell dose-dependent manner not restricted by DLA. MSC and lymphocyte contact was not required, indicating immunosuppression was mediated by soluble factors. Prostaglandin E2 was increased in culture supernatant when MSC were co-cultured in MLR. Adding indomethacin restored lymphocyte proliferation in cultures containing MSC. MSC expressed CD10, CD13, CD29, CD44, CD73/SH-3, CD90/Thy-1, and CD106/VCAM-1. For in vivo studies, MSC were injected on the day of marrow grafting and on day 35, the day of discontinuation of postgrafting cyclosporine. MSC derived from the respective marrow donors failed to avert marrow graft rejection in 4 dogs given DLA-identical grafts after nonmyeloablative conditioning with 100 cGy in a time course not significantly different from control dogs not given MSC. While MSC displayed in vitro characteristics similar to those reported for MSC from other species, their immunosuppressive qualities failed to sustain stable marrow engraftment in vivo in this canine model.
Keywords: canine mesenchymal stromal cells, mixed lymphocyte reaction, TGF-beta, prostaglandin E2, immunosuppression, hematopoietic cell engraftment
INTRODUCTION
We showed consistent and sustained engraftment in dogs given a small, sublethal dose of 200 cGy total body irradiation (TBI) before, and immunosuppression consisting of mycophenolate mofetil (MMF) and cyclosporine (CSP) for 28 and 35 days, respectively, after dog leukocyte antigen (DLA)-identical marrow transplantation [1]. However, when TBI was reduced from 200 to 100 cGy, grafts were nearly uniformly rejected within 20 weeks, even when other immunosuppressive drugs were added to the postgrafting immunosuppression [2]. Very similar results were seen when rapamycin was substituted for MMF in this model [3]. The most likely role of pretransplant TBI was host immunosuppression and not creation of marrow space, as dogs conditioned with 450 cGy irradiation of the cervical, thoracic and upper abdominal lymph node chain in lieu of 200 cGy TBI engrafted [4]. Accordingly, other pretransplant immunosuppression has been evaluated along with 100 cGy TBI conditioning, and successful stable engraftment of DLA-identical marrow was achieved by blocking host T-cell costimulation with cytotoxic T lymphocyte antigen 4-immunoglobulin fusion protein (CTLA4-Ig) or antibody against CD154 along with donor lymphocytes [5,6]. Here, we used the DLA-identical marrow graft model to determine whether the immunomodulatory effects of marrow donor-derived mesenchymal stromal cells (MSC) could be substituted for T-cell costimulatory blockade in assuring sustained hematopoietic engraftment.
MSC have been described as multipotent nonhematopoietic progenitor cells that can differentiate into mature marrow stromal cells, osteoblasts, adipocytes, chondrocytes, fibrous tissue, neuroectoderm, and visceral mesoderm [7-9]. They grow in culture as adherent cells with spindle-shaped fibroblastic morphology and typically do not express hematopoietic markers on their surface but do express adhesion molecules, growth factors and cytokines, and integrins (for review see [10]). Cytokines and growth factors produced by MSC have been implicated in aspects of hematopoiesis. Pertinent to the current study, MSC display immunomodulatory effects. Human and mouse MSC suppress in vitro proliferation of lymphocytes induced by alloantigens or mitogens [11-13], prolong skin and cardiac allograft survival in mice [14] and are thought to be useful for enhancing hematopoietic engraftment [15] and treating GvHD in human patients [16,17]. The mechanisms underlying these effects of MSC have not been clearly identified, although most studies showed that soluble factors were involved [18].
Ex vivo expanded gene-marked MSC were previously evaluated in dogs for their ability to localize in the marrow [19]. Here, we phenotypically characterized canine MSC and demonstrated their ability to suppress mixed leukocyte reactions (MLR). Despite their profound in vitro immunosuppressive activity, marrow donor-derived MSC failed to show in vivo immunosuppressive properties in dogs as assessed by inability to sustain stable marrow engraftment after low-dose TBI exposure.
MATERIALS AND METHODS
Laboratory Animals
The Institutional Animal Care and Use Committee at the Fred Hutchinson Cancer Research Center (FHCRC), which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, approved this study. All dogs were enrolled in a veterinary preventive medicine program as described [1]. Cells from ten DLA-mismatched and unrelated dog pairs (20 dogs) were used for MLR studies. Four DLA-identical littermate pairs, 8-12 (median 10) months old, were used for marrow transplantation. DLA-identity was determined by matching for highly polymorphic DLA class 1 and 2 associated microsatellite markers [20]. In addition, specific DLA-DRB1 allele identity was confirmed by direct sequencing [21].
Bone Marrow Derived Stromal Cell and Cell Lines
Marrow (25mL) was obtained from the humerus by aspiration. This sample was hemolyzed with sterile ammonium chloride lysing solution (155mM ammonium chloride, 10mM sodium bicarbonate, 0.1mM EDTA) in a 37°C water bath for 5 min, centrifuged at 800 rpm for 5 min, and the pellet resuspended in 5 ml of Iscove's Modified Dulbecco's Medium (IMDM) plus 10% fetal bovine serum (FBS). Bone-marrow RBC lyses have been shown to be more efficient than Ficol separation for isolating MSC [22]. Marrow cells were plated at a density of 2 × 107 cells per 75cm2 culture flask (Costar, Cambridge MA, USA) in 20 mL of IMDM containing 10% FBS and 1% Penicillin/Streptomycin (100U/mL). Extra cells were frozen in medium containing 10% DMSO. Nonadherent cells were removed after 72 hours of culture with change of medium. Subsequently, half of the medium was changed twice a week. After 2-3 weeks, cultured cells were detached using 0.05% trypsin EDTA, washed by centrifugation, and expanded to three 75cm2 flasks. After reaching confluency (2-3 weeks), cells were detached using 0.05% trypsin EDTA and tested for MSC function in vitro. Frozen marrow cells were thawed and washed in IMDM, and treated in the same manner as fresh marrow cells.
Canine Fibroblasts
The canine fibroblast cell line (A-72) was purchased from American Type Culture Collection (ATCC, Manassas, VA). In addition, canine primary skin fibroblasts were obtained from skin punch biopsy, cut into several small pieces, and washed in phosphate buffered saline. Fibroblasts were transferred into 6-well, 25 cm2 culture flasks (Costar, Cambridge MA, USA) and cultured in RPMI medium containing 20% FBS and 1% penicillin/streptomycin (100 U/mL). Nonadherent cells were removed after 72 hours of culture by replacing the medium. Subsequently, they were cultured in the same manner as MSC.
Monoclonal Antibodies
Monoclonal antibodies (mAb) consisted of mouse anti-canine CD34 (2E9, IgG1) [23], MHC class II (H81.9F, IgG2a) [24], CD45 (CA12.10C12, IgG1), CD3 (CA17.6F9, IgG2b), and CD86 (CA24.3E4, IgG1). The last three mAb were a generous gift from Peter Moore (UC Davis, Davis CA). Anti-human CD14 (TUK4, IgG2a; Dako Cytomation, Denmark), anti-CD58 (L306.4, IgG2a; B-D, San Jose CA), anti-CD29 (MEM-101A, IgG1, Invitrogen, Carlsbad, CA), anti-CD90 (DH2A, IgM; Accurate Chemical Corp., Westbury, NY), and anti-CD105 (555690, IgG1; B.D. Pharmagen, San Jose, CA) were used because of high cross reactivity with canine cells. Mouse anti-canine CD44 mAb (S5, IgG1) was kindly provided from Dr. Brenda M. Sandmaier (FHCRC, Seattle WA, USA) [25]. MAb were either unconjugated or conjugated to fluorescein isothiocyanate (FITC). Isotype-matched control antibodies were used in parallel as negative controls (Dako Cytomation, Denmark). Isotype-specific FITC-conjugated antibodies were from Southern Biotech (Birmingham, AL). Flow cytometry was performed on a FACScan (Becton Dickinson, San Jose, CA) using standard methods.
Quantitative RT-PCR Analyses
RNA was extracted from MSC, primary skin fibroblast, peripheral blood mononuclear cells (PBMC), and fresh marrow cells using TRIzol (Invitrogen, Carlsbad CA, USA) and chloroform. Isopropanol and 75% ethanol were used to precipitate and resuspend the RNA. The RNA was dissolved in 100 μL of water. cDNA was prepared using standard methods and reagents (Invitrogen).
Measurement of Cytokines in MSC and Fibroblast Culture Supernatant by ELISA
When culture flasks were 90% confluent, culture medium was replaced and the FBS reduced to 0.5%. Twenty-four hours later, the supernatant was collected and concentrated 10-fold using Amicon 10K molecular weight cut-off filter (Millipore, Billerica USA). IMDM plus 0.5% FBS medium was the negative control. Prostaglandin E2 (PGE2) levels in the cell culture supernatants (not concentrated) were measured by competitive ELISA technique using a commercially available ELISA kit, KGE004 (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions. The other cytokines except for PGE2 were quantified using a sandwich type assay.
Capture/detection antibodies recognizing canine antigens were as follows: TNF-α: MAB610 / BAF210; IL-2: MAB602 / BAF202; SCF: MAB65 / BAF255; SDF1-α: MAB350 / BAF310; TGF-β: MAB240 / BAF240; VEGF: AF-293-NA / BAF293; IL-10: M-010 / M-011-B; IFN-γ: MAB781 / BAF781. All reagents were obtained through R & D Systems.
Mixed Lymphocyte Reactions
Allogeneic MLR were performed with lymphocytes from DLA-mismatched dogs in round-bottomed 96-well plates in 200 μl dog medium consisting of 42% Iscove's Modified Dulbecco's Medium (IMDM), 42% Waymouth's Medium, 10% heat-inactivated dog serum, 1% sodium pyruvate, 1% non-essential amino acids, 1% penicillin/streptomycin, and 2% glutamine. MSC were added at a stratified dose (1 × 104 to 1 × 103 cells per well) with responder and irradiated (2200 rad) stimulator lymphocytes from each dog at a concentration of 1 × 105 cells per well. In MLR (with or without MSC) cytokines, neutralizing antibodies, or biochemical antagonists were added at concentrations indicated. For MLR cultured with marrow cells, marrow was harvested from the humerus and used at the same stratified dose as MSC after hemolysis of the sample with ammonium chloride lysing solution. Indomethacin was used to block PGE2 synthesis at a concentration of 20 μM (Cayman Chemical, Ann Arbor, MI). For MLR containing MSC-conditioned medium, the 7-day culture supernatants were collected from 90% confluent MSC established after 4 weeks in culture. Lymphocyte proliferation was measured on day 7 after an 18-hour pulse with [3H]-Thymidine (1.04 Ci/well, Perkin Elmer, Boston, USA). 3H-TdR incorporation was measured by using a liquid scintillation counter (TopCount NXT, PerkinElmer, Waltham, MA, USA), expressed as cpm and represented the means of four separate replicates. Concanavalin A (Con A, Sigma-Aldrich, St. Louis, MO) was used as a control to induce lymphocyte proliferation.
Transwell Cultures
Transwell chambers with 0.4 μm pore size membrane (Millicell Cell Culture Insert, 12 mm, Millipore, Bedford, MA) were used to coculture PBMC with MSC. Responder lymphocytes (2 × 106cells/well) were cultured with 2200 rad-irradiated stimulator lymphocytes (2 × 106cells/well) in a lower chamber. MSC (1 × 106 cells/well) were cultured in an inner transwell chamber. After 6 days, the transwell chambers were removed, and [3H]-Thymidine was added at 10 μCi/well. Twenty-four hours later, the sample was transferred to a 96-well round-bottomed culture plate (200 μL/well), and the cells ware cultured for an additional 18 hrs.
Anti-CD3 and Anti-CD28 Antibody Induced Cell Stimulation
Round-bottomed 96-well culture plates were coated with anti-CD3 (17.6F9, 5 μg/mL) (Peter Moore, UCD, Davis, CA) and anti-CD28 with agonistic activity (5B8, 10 μg/mL) (Graves et al., manuscript submitted) to induce cell stimulation. Responder lymphocytes (1 × 105 cells/well) were co-cultured with 20 Gy-irradiated MSC for 3 or 5 days. [3H]-Thymidine was added to each well (10 μCi/well) and cultured for another 6 hours. [3H]-Thymidine incorporation was determined by a liquid scintillation counter.
HCT with Added Cultured Marrow-derived Stromal Cells
Marrow from each of the intended DLA-identical HCT donors was harvested on days -35 and -28 from the humeri (25 mL) for culture as described above. On day 0, marrow/MSC recipients were treated with 100 cGy TBI, at 7 cGy/min from a linear accelerator (Clinac 6, Varian, Palo Alto, CA). Three hours after TBI, MSC were injected intravenously at concentrations of 1.2–1.8 × 106 cells/kg. Marrow containing 1.19 to 6.11 (median, 4.14) × 108 total nucleated cells/kg of recipient body weight was injected intravenously 3 hours later. Postgrafting immunosuppression consisted of MMF, 10 mg/kg twice a day injected subcutaneously, from days 0 to 28, and CSP, 15 mg/kg twice a day orally from days -1 to 35. On day 35, a second infusion of marrow donor-derived MSC was given intravenously at concentrations of 1.1–1.3 × 106 cells/kg.
Donor chimerism levels among nucleated peripheral blood cells were assessed weekly by fluorescent variable number tandem repeat assays (VNTR-PCR) [26]. Neutrophil recovery after the postradiation nadir was defined as the first of 3 consecutive days with a neutrophil count exceeding 500 cells/μL. Platelet recovery was defined as the first of 5 consecutive days with an unsupported platelet count higher than 20,000/μL. Data from dogs in this study were compared with those from a historical group of eleven dogs given DLA-identical marrow grafts after 100cGy TBI and receiving postgrafting immunosuppression with CSP and MMF or CSP and rapamycin without MSC injections [1,3].
RESULTS
Morphology and Surface Determinants of MSC and Fibroblasts
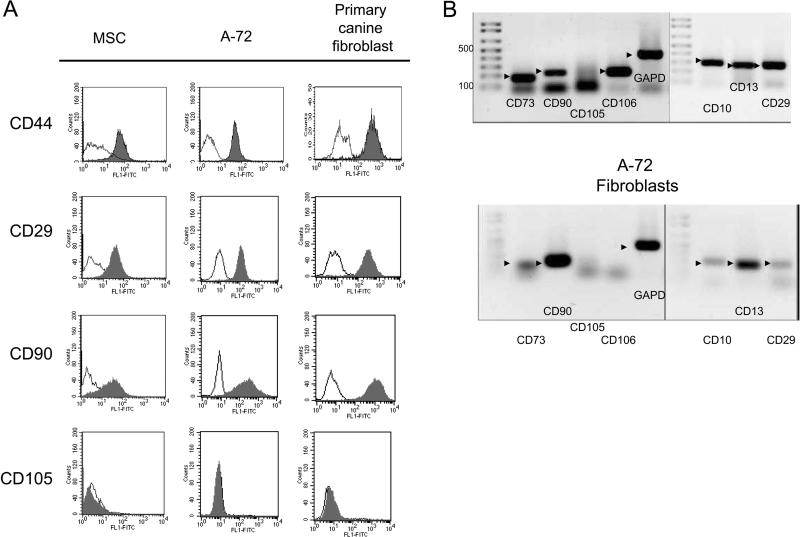
MSC derived from marrow cells, immortalized canine fibroblasts (A-72), and skin-derived primary canine fibroblasts all grew as spindle-like adherent cells (Figure 1). Expression of key cell surface determinants was assessed in part to phenotypically differentiate MSC from the fibroblast cell line (A-72) or primary skin fibroblasts. MSC, A-72 fibroblasts, and primary skin fibroblasts all stained positively with anti-CD29, anti-CD44 mAb, anti-CD90, but not CD105 (endoglin) (Figure 2A). All three cell types were negative for the hematopoietic cell surface antigens, CD3, CD14, CD34 and CD45 (data not shown). RT-PCR of cDNA prepared from MSC and the A-72 fibroblasts was used to confirm presence or absence of antibody immunoreactivity and evaluate expression of cell surface determinants for which mAb were not available. MSC expressed CD73, CD90, CD106, CD10, CD13, and CD29 (Figure 2B). All of these antigens were expressed by the fibroblast cell line A-72 with the exception of CD106. CD10 was expressed by MSC but not by marrow cells or PBMC (data not shown). All RT-PCR expression levels were normalized to GAPD.
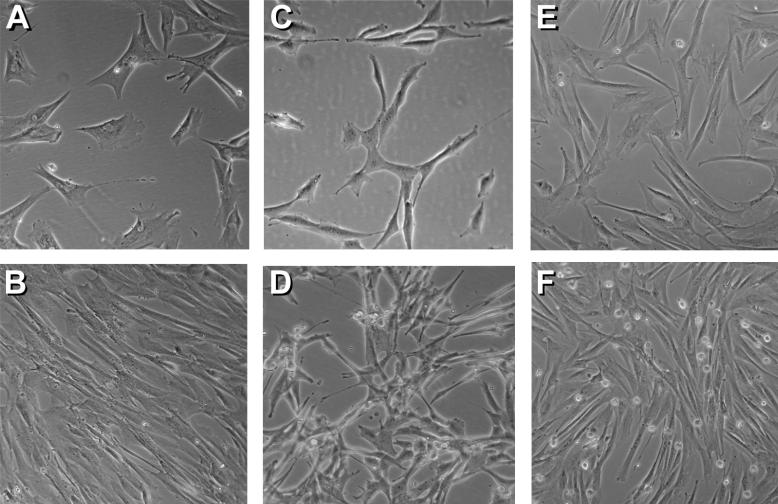
Figure 1.
Morphological features of marrow-derived MSC, canine fibroblasts (A-72) and primary skin canine fibroblasts. MSC, two weeks after marrow collection, were cultured to 40% confluence (A) and 100% confluence (B). A-72 cells were cultured to 30% confluence (C) and 80% confluence (D). Primary canine fibroblasts from skin biopsies were cultured to 50% confluence (E) and 90% confluence (F). Magnification for all photographs was 100x.
Figure 2. Expression of cell surface determinants on canine MSC and fibroblasts.
Cells were released from tissue culture plates using phosphate buffered saline/EDTA and treated with anti-CD29, anti-CD44, anti-CD90, or anti-CD105, washed by centrifugation and analyzed by flow cytometry. Filled regions indicate antibody binding and fine lines indicate binding by isotype controls (A). Quantitative RT PCR analysis was used to confirm presence or absence of expression of cell surface proteins shown in (A) and to characterize expression of proteins for which mAbs were not available (B). mRNA was obtained from cultured MSC or the canine fibroblast cell line A-72. Arrowheads indicate amplified DNA of the expected size. Molecular sizes were as follows: CD73, 182 bp; CD90/Thy-1, 218 bp; CD105/Endoglin , 237 bp; CD106/VCAM-1, 237 bp; CD10, 300 bp; CD13, 272 bo; CD29, 281 bp. GAPD was used as a standard. Ladder shown in 100 base pair increments to 500.
Cytokine Secretion for Canine MSC
Human and mouse MSC have been shown to produce several cytokines and growth factors, such as TGF-β, and biological mediators, such as PGE2, in vitro culture. In order to determine whether canine MSC produced these molecules, we measured the expression of SDF-1, TGFβ, and VEGF in supernatants of MSC and A-72 fibroblast cells by ELISA. MSC produced only TGF-beta (932 pg/ml, actual) and VEGF (11.8 ng/ml), while fibroblasts secreted VEGF (10.8 ng/ml), significantly less TGF-beta (176 ng/ml) than MSC and 10ng/ml of SDF-1. No TNF-α, IL-2, SCF, IL-10, IFN-γ, or PGE2 were detected in either MSC or fibroblast culture supernatants (data not shown). Subsequent experiments demonstrated that the levels of TGF-β detected in the supernatants of MSC were not sufficient to suppress MLR (data not shown).
MSC Suppress Proliferation of Lymphocytes
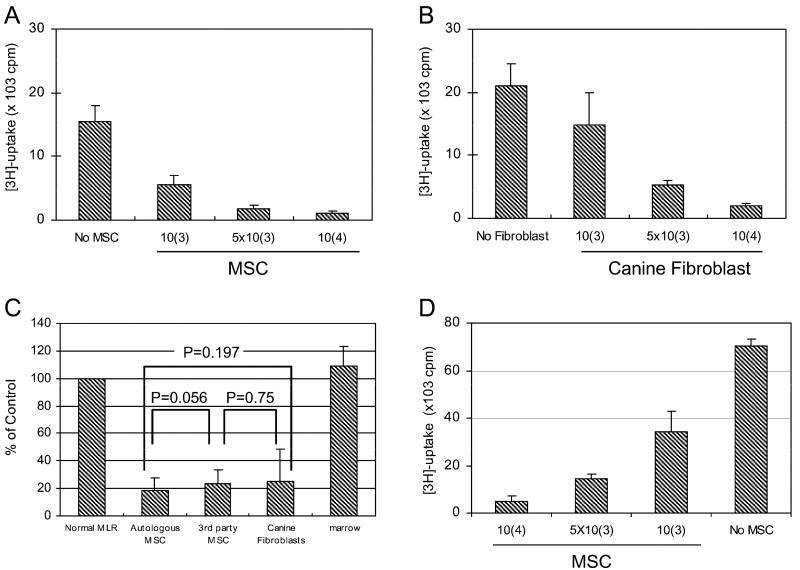
Human and murine MSC suppress lymphocyte proliferation in an MHC-unrestricted manner [27]. Both canine MSC and A-72 fibroblasts also suppressed MLR established with PBMC from two DLA-mismatched dogs in a dose-dependent fashion (Figure 3A,B).
Figure 3. Inhibition of MLR by canine MSC and fibroblasts (A-72).
Marrow derived MSC (A) or A-72 cells (B) were added to wells containing DLA-nonidentical responder and irradiated stimulator lymphocytes in 7-day MLR. Data are presented as total counts per minute of 3H-thymidine uptake. Cultured MSC from the dog supplying responder lymphocytes, cultured caMSC from an unrelated mismatched dog, or canine fibroblasts (A-72), added at (104 cells/well) in MLR did not differ significantly in their suppressive activity, while fresh marrow cells (caBM) at the same concentration failed to suppress the MLR (C). MSC, in dilutions starting at 1x 104 cells per well, suppressed lymphocyte proliferation in a 5-day culture assay of PBMC of a single dog stimulated with anti-canine CD3 and anti-canine CD8 antibodies (D).
Skin fibroblasts, from primary cultures, suppressed MLR similar to the fibroblast cell line A-72 (data not shown). Suppression was DLA-unrestricted as MSC from a third-party dog or A-72 fibroblasts suppressed MLR equally well as MSC isolated from the dog that supplied responder lymphocytes. Freshly isolated marrow cells failed to suppress MLR (Figure 3C).
Previous studies showed that human MSC suppressed lymphocyte proliferation induced by anti-CD3 and anti-CD28 mAb [13]. To determine whether stimulator cells were required for canine MSC-mediated immunosuppression, PBMC were cultured with canine-specific anti-CD3 and anti-CD28 mAb in the presence or absence of MSC. As shown in Figure 3D, MSC suppressed CD3/CD28-induced cell proliferation in a dose-dependent manner.
Cell-to-cell Contact not Required for MSC-mediated Suppression
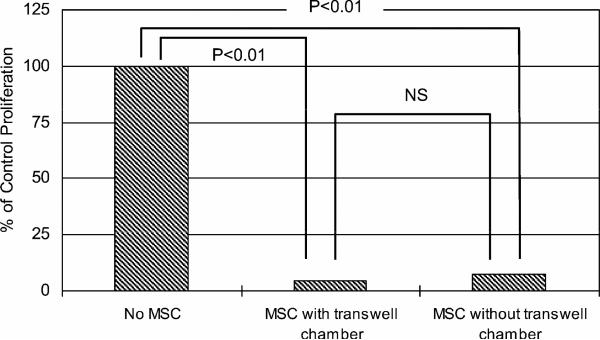
Supernatants collected from confluent cultures of MSC at concentrations as great as 25% of total volume of the MLR culture failed to suppress proliferation (data not shown). However, adding MSC to the inner chamber of a transwell culture system resulted in suppression of MLR, which was as strong as placing an equivalent number of MSC in direct contact with PBMC (Figure 4). This showed that direct cell-to-cell contact between MSC and PBMC was not required for MSC-mediated suppression.
Figure 4. MSC in transwell cultures suppress MLR.
MSC were placed in transwell culture chambers at 1 × 106 cells per well while PBMC from DLA-nonidentical dogs were placed in the outer chamber at 2 × 106 cells per well. After 6 days of culture, PBMC were transferred to 96 well format with 3H-thymidine for 18 hours. Suppression of lymphocyte proliferation was compared to PBMC incubated without caMSC or cultured in direct contact with caMSC.
PGE2 Plays a Key Role in the MSC-mediated Inhibition of MLR
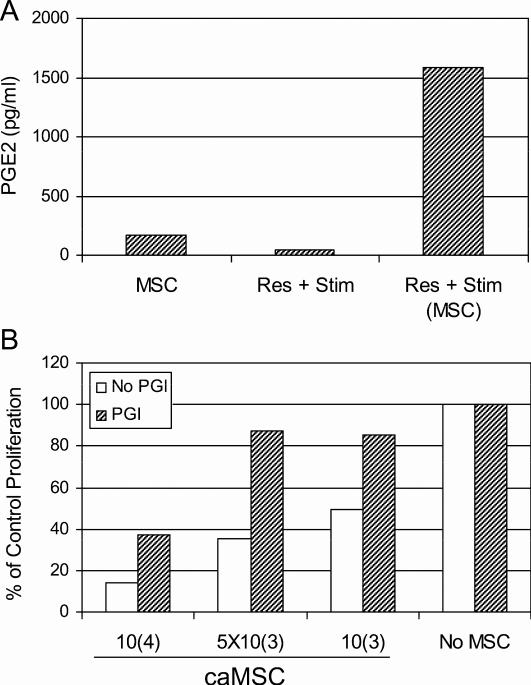
Results of the transwell assays suggested that soluble factor(s) induced after interaction between MSC and lymphocytes were responsible for the immunosuppressive effect of MSC. Human MSC secrete PGE2, thought to play an important role in MSC-mediated immunomodulatory effects [28]. Based on these data, we measured PGE2 in 4-day supernatants collected from MLR cultures containing MSC. Supernatants were also collected from plates containing MSC cultured alone for 4 days as control. As shown in Figure 5A, PGE2 production was dramatically increased upon co-culture of MSC plus responder and stimulator PBMC relative to either cell populations cultured alone.
Figure 5. Production of PGE2 by co-culture of MSC and PBMC.
PGE2 levels were measured by ELISA from 4-day culture supernatants from MSC, responder and stimulator PBMC (Res + Stim) and PBMC containing caMSC (A). Indomethacin, aprostaglandin inhibitor (PGI) (20 uM, crosshatched bars) or medium alone (open bars) was added to a 7-day MLR containing dilutions of MSC (B). Data are presented as percent inhibition relative to MLR containing no MSC.
In order to confirm that prostaglandin E2 played a major role in the MSC-mediated inhibition of MLR, indomethacin (20 μM), an inhibitor of PGE-2 production, was added to MLR containing MSC. Lymphocyte proliferation in the presence of indomethacin was restored in MLR containing MSC at 1 × 103, 5 × 103 and, to a lesser (50%) extent, 1 × 104 cells per well (Figure 5B).
Effect of MSC on HCT Following 100 cGy TBI
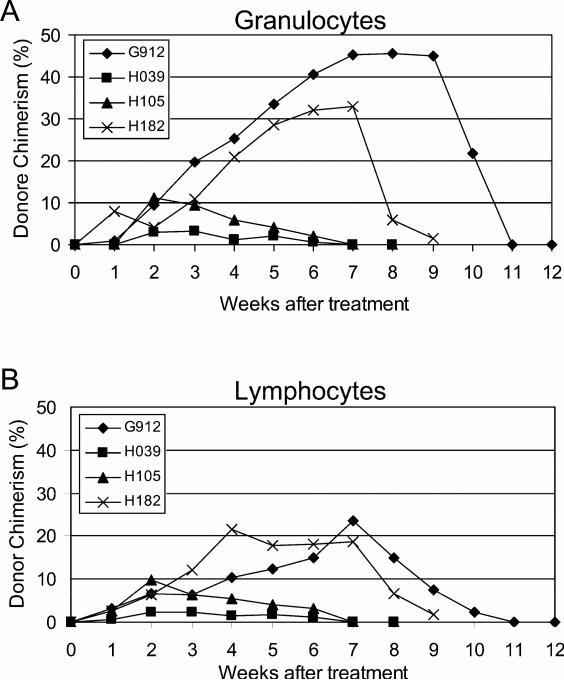
The potential in vivo immunosuppressive effects of MSC were tested in 4 dogs given DLA-identical HCT after conditioning with 100 cGy TBI followed by postgrafting MMF and CSP. Marrow donor-derived MSC were injected IV on the day of marrow transplantation and after completion of CSP administration (day 35). The suppressive activity of each lot of MSC was tested in vitro at the time of infusion and shown to be 31.7 to 91.5 (median, 71) % of control (Table 1). The kinetics of neutrophil and platelet count nadirs and recoveries in the four transplanted dogs did not differ from those of 11 historical controls (data not shown). Donor neutrophil and lymphocyte chimerism results are shown in Figure 6A and B, respectively. Initial donor cell engraftment was documented in all dogs, but all recipients eventually rejected their grafts after 7-11 (median, 8) weeks, a period of time not different from 11 control dogs given the same treatment but not MSC (Table 1). All survived with autologous hematopoietic recovery. Administering MSC after HCT failed to affect the duration of engraftment in dogs conditioned with an otherwise suboptimal dose of 1 Gy TBI. With 90% confidence, the addition of MSC did not result in an engraftment rate that was usefully high (50%).
Table 1.
Duration of donor chimerism in dogs given marrow grafts from DLA-identical donors after 100 cGy TBI and MMF/CSP or rapamycin/CSP without MSC (no MSC) or with two injections of MSC at days 0 and 35 (MSC).
| Dog No. | Nucleated Marrow Cells (×108 cells/kg) | MSCs (×106 cells/kg) |
Inhibition Index*(%) of MSCs in Pre-injection MLR |
Duration of Donor Chimerism, Among PBMC (weeks) | Final Status of Donor Marrow Cells | |||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 35 | Day 0 | Day 35 | |||||
| Yes | G912 | 6.1 | 1.2 | 1.2 | 89.3 | 91.5 | 11 | Rejected |
| H039 | 1.2 | 1.5 | 1.3 | 44.5 | 31.7 | 7 | Rejected | |
| H105 | 4.5 | 1.8 | 1.1 | 71.1 | 70.4 | 7 | Rejected | |
| H182 | 3.8 | 1.3 | 1.1 | 69.2 | 74.6 | 9 | Rejected | |
| No | E165 | 4.0 | – | – | – | – | 12 | Rejected |
| [1] | E166 | 4.0 | – | – | – | – | 10 | Rejected |
| E202 | 4.1 | – | – | – | – | 3 | Rejected | |
| E204 | 4.0 | – | – | – | – | 3 | Rejected | |
| E227 | 4.0 | – | – | – | – | 10 | Rejected | |
| E228 | 4.0 | – | – | – | – | 10 | Rejected | |
| No | G092 | 3.61 | – | – | – | – | 9 | Rejected |
| [3] | G111 | 6.27 | – | – | – | – | 11 | Rejected |
| G151 | 3.69 | – | – | – | – | 3 | Rejected | |
| G156 | 3.8 | – | – | – | – | 9 | Rejected | |
| G167 | 4.18 | – | – | – | – | 9 | Rejected | |
Inhibition index: percent of control proliferation in MLR wells containing 104 MSC /well.
Figure 6. Duration of engraftment of donor marrow after 100 cGy TBI and immunosuppression with MMF/CSP and two doses of MSC.
Four dogs received HCT from DLA-identical littermates after 100 cGy TBI. Postgrafting immunosuppression consisted of two injections of MSC (days 0 and 35) and twice daily injections of MMF (days 0 to 28) and CSP (days -1 to 35). Percent donor chimerism was evaluated weekly from the peripheral blood of the recipients by VNTR-PCR for granulocytes (A) and lymphocytes (B).
DISCUSSION
Numerous studies have indicated that marrow-derived murine or human MSC possess immunosuppressive activities [13,27-31]. These characteristics have prompted clinical studies evaluating MSC for the enhancement of hematopoietic engraftment [15,32] and for the control of graft-versus-host disease (GvHD) following HCT [33]; the latter report on cure of steroid-refractory GVHD utilizing infusion of unrelated MSC resulted in wide-spread interest in clinical use of these cells. Subsequent reports have been inconclusive as to the efficacy of these cells for treating patients with GVHD and generally not informative as to mechanisms. Because studies in dogs have been instrumental in developing and understanding clinically relevant HCT regimens, we evaluated the immunosuppressive properties of MSC in that model.
Our past experience with MSC in dogs has been limited to attempting to identify localization of ex vivo expanded green fluorescent protein transduced MSC within the marrow space 6 months after intravenous injection [19]. In that report localization of MSC was inferred by PCR detection of transgene sequences. Anecdotally, we described bilateral diffuse pulmonary ectopic ossification including myeloid marrow after a DLA-matched marrow graft, presumably resulting from the presence of MSC in the graft [34]. In another study (Mielcarek et al., unpublished), in vivo distribution of 111Indium-labeled MSC was studied in dogs. Immediately after infusion, MSC were found in lung, but within 24 hours, labeled cells were seen in liver and spleen and, to a lesser extent, in bone marrow and gut. Residual labeling in liver and spleen could be detected as long as 9 days.
Our current goal was to produce and characterize canine MSC relative to what has been described for mouse and human MSC. We successfully cultured MSC from marrow and found them to be morphologically similar to human and mouse MSC [10,35]. MSC showed characteristics of spindle-shaped fibroblastic cells, adherence to plastic surfaces, and antiproliferative activity, features that were similar to the fibroblast cell line, A-72 and skin-derived primary canine fibroblasts. Human MSC and fibroblasts also possess morphological similarities and the ability to suppress MLR [36].
It has been reported that MSC expressed several surface markers, such as CD13, CD29, CD44, CD73, CD90, CD106, and CD166 [37,38]. However, no single specific marker for MSC has been identified [39]. Canine MSC shared cell surface expression profiles with human MSC except for CD105 as determined both by antibody staining and PCR methods. Antigen expression by canine fibroblasts differed from MSC with respect to CD10, CD29, CD73, and CD106.
Of interest was the dose-dependent immunosuppressive effect of canine MSC against lymphocytes, which was not restricted by DLA. MHC-restriction by MSC is controversial. MHC restriction has been reported absent in certain human and mouse studies [27,31]. However, others have shown preferential expansion of human umbilical cord blood-derived CD34+ cells cultured on MHC-matched amnion derived MSC [40]. Similarly, in studies using B10 congenic strains of mice, hematopoietic stem cell cobblestone colony formation was superior on MHC-matched MSC compared to mismatched MSC [41].
While cell culture supernatants of MSC contained measurable levels of TGF-β, these were not sufficient to account for immunosuppression, suggesting that other factor(s) might be involved. Similarly, Rasmusson et al. [30] reported that, even when increasing concentrations of MSC were added to MLR, levels of TGF-β in the medium did not change, consistent with the notion that the immunosuppressive effects were not solely due to TGF-β. Although MSC culture supernatants failed to suppress lymphocyte proliferation in MLR, they suppressed MLR in a transwell system as effectively as MSC added directly to the MLR, indicating that inhibitory soluble factors were involved that required communication between the PBMC and MSC for triggering the release of these factors from MSC. The addition of indomethacin, an inhibitor of PGE2 biosynthesis, restored lymphocyte proliferation in MLR. PGE2 has been known to inhibit both T-cell proliferation and cytokine production [42]. In addition, the suppression of lymphoproliferation might have been mediated by release of indoleamine 2,3, dioxygenase (IDO) from MSC [43]. IDO release has also been shown to be sensitive to inhibitors of prostaglandin biosynthesis [44].
Given the reported benefits of MSC on engraftment of marrow [15,45,46], we investigated the in vivo immunosuppressive qualities of MSC in a well-established model of DLA-identical marrow transplantation. To this end, we conditioned recipient dogs with 100 cGy TBI, which is just below the bar of 200 cGy TBI at which consistent, sustained engraftment was seen [1]. Extensive immunologic studies in dogs not given marrow grafts had shown that, while 100 cGy TBI was immunosuppressive, suppression was less profound than 200 cGy in almost all parameters studied [2]. For example, 100 cGy decreased the lymph node CD3 cell contents on day 7 by 50% compared to 78% with 200 cGy. On the other hand, MLR responses were absent during the first two weeks and on day 35 after TBI both in dogs given 100 cGy and 200 cGy. Above its immunosuppressive properties, 100 cGy TBI caused significant marrow ablation resulting in 50–80% declines of peripheral blood, neutrophil, lymphocyte, and platelet counts [5]. Therefore, there should have been marrow “space” for MSC to engraft. We were further encouraged to use 100 cGy in this model because previous studies had shown that inducing host-vs.-graft immune hyporesponsiveness by T-cell costimulatory blockade in addition to 100 cGy TBI resulted in extended and stable donor cell engraftment [5,6]. The in vitro functional activity of MSC was confirmed in MLR at the time of injection. All four dogs engrafted but eventually rejected their grafts. The duration of donor engraftment in MSC-treated dogs did not differ significantly from that among 11 historical control dogs not given MSC. The complete absence of stable engraftment following two injections of marrow donor-derived MSC was unexpected but suggests absence of powerful in vivo immunosuppressive effects of these cells in this setting.
Similar results were obtained in a murine parental into F1 model of GVHD [47], although the authors demonstrated potent in vitro immunosuppressive properties of MSC derived from marrow, placenta or umbilical cord tissues, multiple injections of the maximum allowable numbers of MSC failed to prevent GVHD in marrow recipients. Localized injection of MSC under the kidney capsule did, however, result in ectopic bone production, suggesting one in vivo function of MSC was maintained.
Conversely, other studies in mice [48] and rats [49] did demonstrate that repeated IV injections of recipient adipocyte-derived MSC could reverse mild GVHD. Collectively, our results and those obtained in other models suggest that the correct paradigm for in vivo MSC immunosuppression has yet to be fully defined, perhaps by route of injection or cell numbers. Although the doses of MSC (mean 1.2 × 106 cells/kg) in the present study were consistent with those that others have used [15-17,45,46,50,51] it cannot be ruled out that higher doses or more frequent administration of MSC could improve success in our model.
In conclusion, canine MSC have the same cytological features and in vitro immunosuppressive and immunomodulatory effects as reported for humans and mice. However, MSC failed to promote sustained hematopoietic engraftment in dogs given conditioning with low dose TBI.
ACKNOWLEDGMENTS
The authors are grateful to Michele Spector, DVM, Alix Joslyn, Brian Steinmetz and the Fred Hutchinson Research Animal Research Technicians for assistance in animal care; Stacy Zellmer and Patrice Stroup for DLA typing; Drs. Beard, Wang, Enslee, Rotta, Nash, Georges, Parker, Thakar, Gyurkocza, Leskinova, and Mathes, who participated in weekend treatments; and Bonnie Larson and Helen Crawford for manuscript preparation. The authors are also grateful for the gift of cyclosporine from Novartis.
Grant support: This work was supported by National Institutes of Health grants CA78902, CA15704, DK56465 and AI067770. Support was also provided by the Inje Research and Scholarship Foundation, 2008 (to W.S.L.), and awards from the Joseph Steiner Krebsstifung, Bern, Switzerland and Lupin Foundation, Metairie, Louisiana (both to R.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Current Addresses: Dr. W.S. Lee: Department of Internal Medicine, Inje University School of Medicine, Busan, Korea; Drs. Y. Suzuki and S. Ikehara: First Department of Pathology, Kansai Medical University, Osaka, Japan.
Conflict of interest statement: The authors have no conflicts of interest regarding financial relationships to declare.
REFERENCES
- 1.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 2.Sorror ML, Leisenring W, Mielcarek M, et al. Intensified postgrafting immunosuppression failed to assure long-term engraftment of dog leukocyte antigen-identical canine marrow grafts after 1 gray total body irradiation. Transplantation. 2008;85:1023–1029. doi: 10.1097/TP.0b013e318169be24. [DOI] [PubMed] [Google Scholar]
- 3.Hogan WJ, Little M- T, Zellmer E, et al. Postgrafting immunosuppression with sirolimus and cyclosporine facilitates stable mixed hematopoietic chimerism in dogs given sublethal total body irradiation before marrow transplantation from DLA-identical littermates. Biol Blood Marrow Transplant. 2003;9:489–495. doi: 10.1016/s1083-8791(03)00148-4. [DOI] [PubMed] [Google Scholar]
- 4.Storb R, Yu C, Barnett T, et al. Stable mixed hematopoietic chimerism in dog leukocyte antigen-identical littermate dogs given lymph node irradiation before and pharmacologic immunosuppression after marrow transplantation. Blood. 1999;94:1131–1136. [PubMed] [Google Scholar]
- 5.Storb R, Yu C, Zaucha JM, et al. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant. Blood. 1999;94:2523–2529. [PubMed] [Google Scholar]
- 6.Jochum C, Beste M, Zellmer E, Graves SS, Storb R. CD154 blockade and donor-specific transfusions in DLA-identical marrow transplantation in dogs conditioned with 1-Gy total body irradiation. Biol Blood Marrow Transplant. 2007;13:164–171. doi: 10.1016/j.bbmt.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Bianco P, Gehron RP. Marrow stromal stem cells. J Clin Invest. 2000;105:1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 10.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses (Review). Exp Hematol. 2000;28:875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 11.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 12.Sun S, Guo Z, Xiao X, et al. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells. 2003;21:527–535. doi: 10.1634/stemcells.21-5-527. [DOI] [PubMed] [Google Scholar]
- 13.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S, Wehner R, Bornhäuser M, Wassmuth R, Bachmann M, Schmitz M. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells and Development. 2009 October; doi: 10.1089/scd.2009.0345. doi:10.1089/scd.2009.0345. [DOI] [PubMed] [Google Scholar]
- 15.Ball LM, Bernardo ME, Roelofs H, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764–2767. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 16.Lazarus HM, Koc ON, Devine SM, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Ringdén O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz EM, Dominici M. How do mesenchymal stromal cells exert their therapeutic benefit? (Review). Cytotherapy. 2008;10:771–774. doi: 10.1080/14653240802618085. [DOI] [PubMed] [Google Scholar]
- 19.Mosca JD, Hendricks JK, Buyaner D, et al. Mesenchymal stem cells as vehicles for gene delivery. Clinical Orthopaedics and Related Research. 2000;379S:S71–S90. doi: 10.1097/00003086-200010001-00011. [DOI] [PubMed] [Google Scholar]
- 20.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 21.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication). Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 22.Horn P, Bork S, Diehlmann A, et al. Isolation of human mesenchymal stromal cells is more efficient by red blood cell lysis. Cytotherapy. 2008;10:676–685. doi: 10.1080/14653240802398845. [DOI] [PubMed] [Google Scholar]
- 23.McSweeney PA, Rouleau KA, Wallace PM, et al. Characterization of monoclonal antibodies that recognize canine CD34. Blood. 1998;91:1977–1986. [PubMed] [Google Scholar]
- 24.Huss R, Beckham C, Storb R, Deeg HJ. Major histocompatibility complex class II expression is required for posttransplant immunological but not for hemopoietic reconstitution in mice. Transplantation. 1994;58:1366–1371. [PubMed] [Google Scholar]
- 25.Sandmaier BM, Storb R, Bennett KL, Appelbaum FR, Santos EB. Epitope specificity of CD44 for monoclonal antibody dependent facilitation of marrow engraftment in a canine model. Blood. 1998;91:3494–3502. [PubMed] [Google Scholar]
- 26.Graves SS, Hogan W, Kuhr CS, et al. Stable trichimerism after marrow grafting from 2 DLA-identical canine donors and nonmyeloablative conditioning. Blood. 2007;110:418–423. doi: 10.1182/blood-2007-02-071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 29.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 30.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 32.Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11:503–515. doi: 10.1080/14653240903193806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Bonin M, Stolzel F, Goedecke A, et al. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant. 2009;43:245–251. doi: 10.1038/bmt.2008.316. [DOI] [PubMed] [Google Scholar]
- 34.Sale GE, Storb R. Bilateral diffuse pulmonary ectopic ossification after marrow allograft in a dog. Evidence for allotransplantation of hemopoietic and mesenchymal stem cells. Exp Hematol. 1983;11:961–966. [PubMed] [Google Scholar]
- 35.Tropel P, Noel D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 36.Haniffa MA, Collin MP, Buckley CD, Dazzi F. Mesenchymal stem cells: the fibroblasts’ new clothes? (Review). Haematologica. 2009;94:258–263. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mareschi K, Ferrero I, Rustichelli D, et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006;97:744–754. doi: 10.1002/jcb.20681. [DOI] [PubMed] [Google Scholar]
- 38.Buhring HJ, Treml S, Cerabona F, de Zwart P, Kanz L, Sobiesiak M. Phenotypic characterization of distinct human bone marrow-derived MSC subsets. Ann NY Acad Sci. 2009;1176:124–134. doi: 10.1111/j.1749-6632.2009.04564.x. [DOI] [PubMed] [Google Scholar]
- 39.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells (Review). Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 40.Mizokami T, Hisha H, Okazaki S, et al. Preferential expansion of human umbilical cord blood-derived CD34-positive cells on major histocompatibility complex-matched amnion-derived mesenchymal stem cells. Haematologica. 2009;94:618–628. doi: 10.3324/haematol.2008.004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugiura K, Hisha H, Ishikawa J, et al. Major histocompatibility complex restriction between hematopoietic stem cells and stromal cells in vitro. Stem Cells. 2001;19:46–58. doi: 10.1634/stemcells.19-1-46. [DOI] [PubMed] [Google Scholar]
- 42.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity (Review). Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 43.Yang SH, Park MJ, Yoon IH, et al. Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Experimental and Molecular Medicine. 2009;41:315–324. doi: 10.3858/emm.2009.41.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sayama S, Yoshida R, Oku T, Imanishi J, Kishida T, Hayaishi O. Inhibition of interferon-mediated induction of indoleamine 2,3-dioxygenase in mouse lung by inhibitors of prostaglandin biosynthesis. PNAS. 1981;78:7327–7330. doi: 10.1073/pnas.78.12.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masuda S, Ageyama N, Shibata H, et al. Cotransplantation with MSCs improves engraftment of HSCs after autologous intra-bone marrow transplantation in nonhuman primates. Exp Hematol. 2009;37:1250–1257. doi: 10.1016/j.exphem.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Kim DW, Chung YJ, Kim TG, Kim YL, Oh IH. Cotransplantation of third-party mesenchymal stromal cells can alleviate single-donor predominance and increase engraftment from double cord transplantation. Blood. 2004;103:1941–1948. doi: 10.1182/blood-2003-05-1601. [DOI] [PubMed] [Google Scholar]
- 47.Prigozhina TB, Khitrin S, Elkin G, Eizik O, Morecki S, Slavin S. Mesenchymal stromal cells lose their immunosuppressive potential after allotransplantation. Exp Hematol. 2008;36:1370–1376. doi: 10.1016/j.exphem.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 48.Yanez R, Lamana ML, Garcia-Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 49.Aksu AE, Horibe E, Sacks J, et al. Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clinical Immunology. 2008;127:348–358. doi: 10.1016/j.clim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 51.Ringden O, Uzunel M, Sundberg B, et al. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia. 2007;21:2271–2276. doi: 10.1038/sj.leu.2404833. [DOI] [PubMed] [Google Scholar]