Abstract
Purpose:
To evaluate the feasibility, efficacy, and side effects of dose escalation in hypofractionated stereotactic radiotherapy (hfSRT) for intrapulmonary tumors with the Novalis™ system (BrainLAB AG, Heimstetten, Germany).
Patients and Methods:
From 07/2003 to 01/2005, 21 patients/39 tumors were treated with 5 × 7 Gy (n = 21; total dose 35 Gy) or 5 × 8 Gy (n = 18; total dose 40 Gy). There were three cases of primary lung cancer, the remainder were metastases. Median gross tumor volume (GTV) and planning target volume (PTV) were 2.89 cm3 (range, 0.15–67.94 cm3) and 25.75 cm3 (range, 7.18–124.04 cm3), respectively.
Results:
Rates of complete remission, partial remission, no change, and progressive disease were 51%, 33%, 3%, and 13%, respectively. No grade 4 toxicity occurred, nearly all patients had grade 1 initially. One grade 3 toxicity, i.e., dyspnea, was documented for a period of 6 months after therapy. Radiosurgery quality assurance guidelines could be met.
Conclusion:
hfSRT of primary and secondary lung tumors using a schedule of five fractions at 7–8 Gy each was well tolerated. Further dose escalation is planned.
Key Words: Hypofractionated stereotactic radiotherapy, Lung tumors, Novalis™ system
Schlüsselwörter: Hypofraktionierte stereotaktische Radiotherapie, Lungentumoren, Novalis™-System
Abstract
Ziel:
Auswertung der Durchführbarkeit, Wirksamkeit und Nebenwirkungen einer Phase-I/II-Studie zur Dosiseskalation bei hypofraktionierter stereotaktischer Radiotherapie (hfSRT) von Lungentumoren mit dem Novalis™-System (BrainLAB AG, Heimstetten).
Patienten und Methodik:
21 Patienten/39 Tumoren wurden von Juli 2003 bis Januar 2005 mit 5 × 7 Gy (n = 21; Gesamtdosis [GD] 35 Gy) oder 5 × 8 Gy (n = 18; GD 40 Gy) bestrahlt. Drei Patienten hatten ein primäres Lungenkarzinom, die übrigen Metastasen. Das mediane „gross tumor volume”(GTV) und Planungszielvolumen (PTV) betrugen 2,89 cm3 (0,15–67,94 cm3) und 25,75 cm3 (7,18–124,04 cm3).
Ergebnisse:
Eine komplette Remission, partielle Remission, keine Änderung und Progression fanden sich bei 51%, 33%, 3% und 13%. Nach initialer Grad-1-Toxizitat in fast allen Fälle trat keine Grad-4-Toxizitat auf. Eine Patientin erlitt eine Grad-3-Toxizität. Die RTOG-Qualitatskriterien für die Radiochirurgie wurden bei allen Patienten erfüllt.
Schlussfolgerung:
Die hfSRT mit 5 × 7 Gy und 5 × 8 Gy wurde gut vertragen. Die Dosiseskalation wird fortgeführt.
Introduction
Stereotactic radiotherapy and radiosurgery (SRS) is well established for the treatment of brain tumors [5, 13, 15]. Given the ability to perform stereotactic radiosurgery and fractionated stereotactic treatment with the Novalis™ system (Brain-LAB AG, Heimstetten, Germany), we decided to translate the technique into body stereotactic treatment. Extracranial stereotactic radiotherapy (ESRT) has demonstrated high efficacy and a low rate of side effects [4, 32, 36, 38]. Hypofractionation, from a radiobiological point of view, may yield significant benefits over using a single high-dose radiosurgery by opening up a therapeutic window between tumor control and late effects. This paradigm holds especially true for malignant tumors [10, 26]. However, the optimal single and total dose have yet to be defined. In this paper, early results including toxicity of a phase I/II study of hypofractionated stereotactic radiotherapy (hfSRT) for intrapulmonary tumors are given.
Patients and Methods
Eligibility and Process of Dose Escalation
Treatment protocol and consent form were approved by an institutional review board and ethic’s committee of the University of Erlangen, Germany. All patients were required to be medically inoperable or not eligible for surgery due to unfavorable tumor location as indicated by an experienced thoracic surgeon. Patients with stage I non-small cell lung cancer (NSCLC), i.e., T1 or T2 N0 M0, were accepted, if the gross tumor volume (GTV) had a diameter of ≤7 cm (existing data for hfSRT of lung cancer [31]). Second indication were patients with oligometastatic disease (up to four metastases, each of them not > 4 cm, one single metastasis not > 7 cm in diameter). No exclusion criteria for FEV1 (forced expiratory volume in 1 s) were defined, because hfSRT was the only alternative treatment to surgery. First dose level was 5 × 7 Gy (90% isodose). Patient groups in the subsequent levels received an additional 1 Gy per fraction. Fractions were separated by an interval of 2 days. Overall treatment time was 10 days, including the weekend pause. A minimum of three patients should be assigned to each dose level. Toxicity was graded according to the Common Toxicity Criteria (CTC) [27]. Dose-limiting toxicity (DLT) was defined as any grade 3/4 pulmonary, esophageal, cardiac, or spinal toxicity. If two or more patients experienced DLT, the maximum tolerable dose (MTD) would be reached. If DLT occurred in one patient, another two for the same dose level would be enrolled. Proceeding to the next dose level without DLT in these two patients would be possible after a minimum observation period of 12 weeks after treatment.
Patient and Tumor Characteristics
21 patients were entered (07/2003–01/2005), median age 54 years (range, 18–75 years), Karnofsky performance status 80 (range, 70–100). Further characteristics are given in Table 1. FEV1 < 40% was present in three patients (one third required home oxygen therapy before hfSRT).
Table 1.
Patient and tumor characteristics. GTV: gross tumor volume; PTV: planning target volume.
| Patients (n) | |
|---|---|
| Age (years) | |
| Median | 54 |
| Range | 18–75 |
| Gender | |
| Male | 8 |
| Female | 13 |
| Histology (39 tumors) | |
| Lung cancer | |
| • Adenocarcinoma | 8 |
| • Squamous cell carcinoma | 1 |
| Thyroid cancer | |
| • Follicular | 12 |
| Breast cancer | 4 |
| Rectal cancer | 8 |
| Cervical carcinoma | 2 |
| Ewing’s sarcoma | 3 |
| Leiomyosarcoma | 1 |
| Tumor side | |
| Right lung | 18 |
| Left lung | 21 |
| Tumor location | |
| With ≤ 2 cm distance to basal pleura | 4 |
| With ≤ 2 cm distance to medial pleura | 13 |
| With ≤ 2 cm distance to lateral pleura | 14 |
| Central | 8 |
| Treatment volumes [cm3, median (range)] | |
| GTV | 2.89 (0.15–67.94) |
| PTV | 25.75 (7.18–124.04) |
| Dose level | |
| 5 × 7 Gy | 21 lesions |
| 5 × 8 Gy | 18 lesions |
Treatment Planning, Immobilization, and Radiation Delivery
All patients were immobilized in supine position, with a self-constructed abdominal press with three plungers, one anterior and two on each flank (Figure 1). Helical CT images (3 mm, SomatomPlus4, Siemens, Erlangen, Germany) were obtained in deep inspiration breath hold (room lasers marked on the skin and three fiducial markers positioned). Solid tumor with blurred margin was considered to be GTV. Clinical target volume (CTV) was GTV without margin. Planning target volume (PTV), as to include setup inaccuracies and potential tumor movement, was the expansion of GTV plus 5, 5, and 10 mm in x-, y-, and z-directions, respectively. Planning was performed with Novalis™ Brain Scan treatment- planning system (Version 5.31, BrainLAB AG). All isocenters were marked by the use of laser lines on the skin at our treatment simulation X-ray unit (Simulix-HQ, Nucletron, Veneridaal, The Netherlands). Radiation was delivered using a median number of three beams (range, one to six beams). In 38 cases, dynamic conformal arc technique was performed, in one case static conformal beams were used. Dose calculation was done by pencil beam algorithm. The treatment delivery Novalis™/ExacTrac™ system (BrainLAB AG) has been described before [7]. ExacTrac™ is intended to place patients at the isocenter of a linear accelerator. It uses stereoscopic X-ray registration of two radiographs, X-ray fusion with the DRR (digitally reconstructed radiograph), and automatic positioning correction.
Figures 1a and 1b.
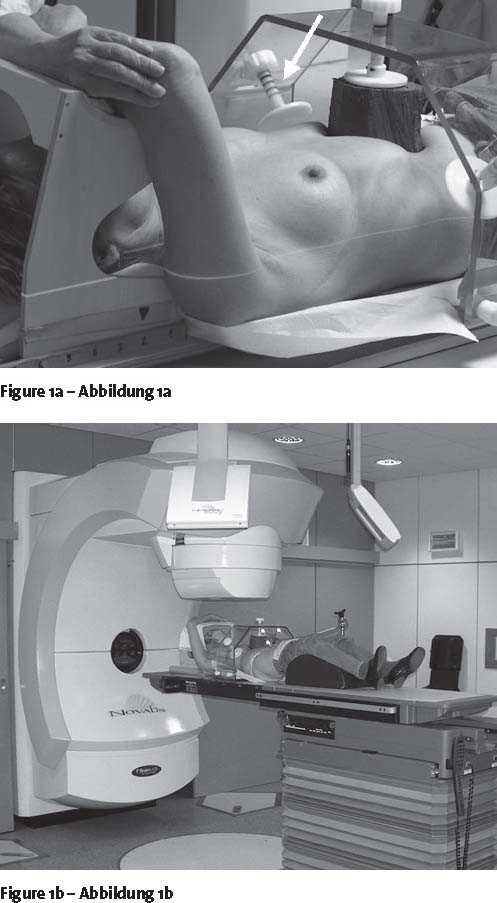
Patient setup with abdominal press. Treatment planning with CT scan (a), setup at the linear accelerator (b). Arrow showing the individual and marked impression by the abdominal press.
Quality Criteria and Evaluation
According to the RTOG guidelines [28] for radiosurgery dose homogeneity, conformation and 90% isodose coverage for 90% of the PTV were required. Dmin and Dmax were documented.
Follow-up and Statistics
We evaluated the quality of hfSRT using the aforementioned quality criteria and response after hfSRT with CT imaging 8 weeks after the end of hfSRT and every 3 months thereafter. Treatment-related side effects were documented according to the CTC scoring system.
Results
Local Tumor Control and Survival
Complete response (CR), partial response (PR), no change (NC), and progressive disease (PD) were seen in 51%, 33%, 3%, and 13%, respectively. This resulted in an overall response rate of 87% (no statistically significant difference between dose levels). Median follow-up was 6.3 months (range, 1–21 months). Four local relapses occurred after 8, 9 (two lesions), and 13 months. Eight patients (38%) live with no evidence of disease, three are alive with progressive disease, six with new lung metastases, one with local control and extrapulmonary progressive disease, three died of local progressive disease (follow-up 1.4 months), extrapulmonary disease (follow-up 9 months) and other reasons (follow-up 2.3 months). Follow-up for patients without progression was 1–21 months (median 6.4 months). Local control was not associated with histology.
Typical Follow-up Imaging Study
The typical appearance of a treated tumor showing minimal change of normal lung tissue together with tumor shrinkage is demonstrated in Figure 2.
Figures 2a to 2e.
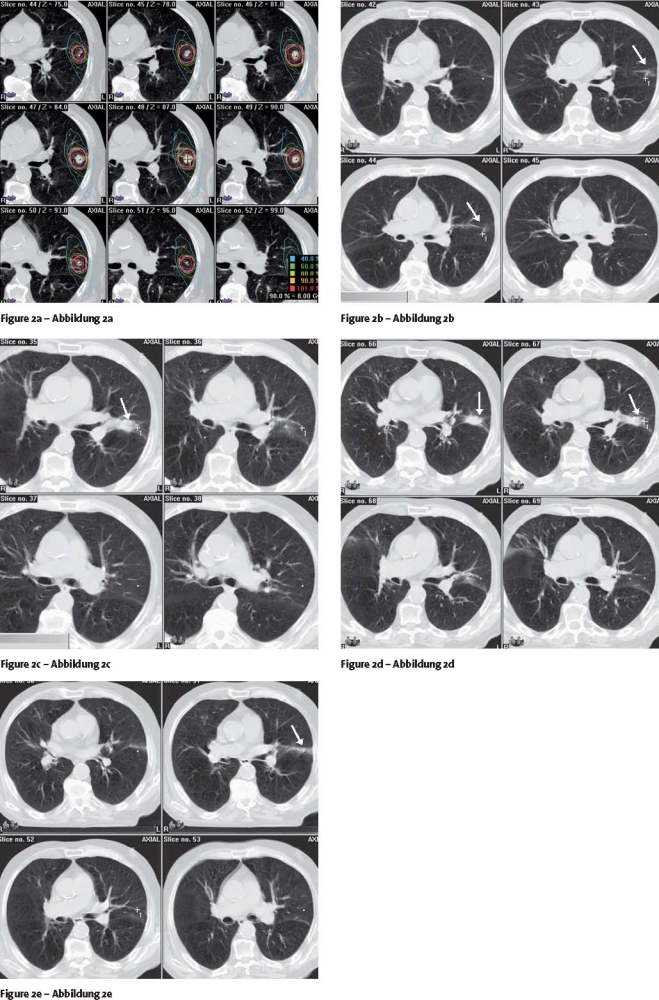
Case follow-up study. Treatment planning (a), three dynamic conformal arcs; follow-up at 6 months (b), 9 months (c), 12 months (d) and 15 months (e). Arrows showing the residual tumor (b) and the normal-tissue reactions (e) Case follow-up study. Follow-up at 15 months (e). Case follow-up study. Follow-up at 15 months (e).
Quality Criteria
With the Novalis™ system, all of the RTOG quality criteria were met: median homogeneity index 1.16 (range, 1.02–1.36), median conformity index 1.29 (range, 1.12–1.98), and median coverage 97.8% (range, 86.6–100%). Median maximum dose was 115% (range, 102–136%), and medium minimum dose 88% (range, 70–100%).
Organs at Risk and Clinical Outcome
Follow-up pulmonary function tests (PFTs) were only performed in the three patients with FEV1 < 40%. PFTs remained stable throughout the observation period. Grade 1 clinical side effects occurred in nearly all patients for up to 0.5 years, radiologic side effects for up to 1 year after treatment. No grade 2 pulmonary toxicity was seen. One patient (dose level 1) experienced grade 3 toxicity (dyspnea at rest, Table 2). This resolved with steroids after 0.5 years. Dose-volume histograms (DVHs) of normal lung tissue were documented. With 18 right-sided and 21 left-sided tumors, the following values were drawn from the DVHs: left lung: a median total volume of 180 cm3 (range, 0–730 cm3) was irradiated with > 2 Gy, 77.5 cm3 (range, 0–250 cm3) with > 4 Gy, and 42.5 cm3 (range, 0–140 cm3) with > 6 Gy, respectively; right lung: a median total volume of 110 cm3 (range, 0–670 cm3) was irradiated with > 2 Gy, 30 cm3 (range, 0–305 cm3) with > 4 Gy, and 20 cm3 (range, 0–180 cm3) with > 6 Gy, respectively.
Table 2.
Toxicity (CTC [Common Toxicity Criteria] Score).
| Follow-up time (months) | 2 | 5 | 8 | 11 | 14 | 17 |
| At risk | 39 | 32 | 13 | 11 | 8 | 8 |
| Grade 1 | 30 | 32 | 7 | 2 | 0 | 0 |
| Grade 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade 3 | 1 | 1 | 0 | 0 | 0 | 0 |
| Grade 4 | 0 | 0 | 0 | 0 | 0 | 0 |
Discussion
Rationale for Extracranial Stereotactic Radiotherapy (ESRT)
Surgical resection remains the treatment of choice for patients with NSCLC stage I. However, there exists a large medically inoperable subgroup. Older studies revealed that 15% of these patients are long-term survivors, about 25% die of intercurrent disease, 30% of distant metastatic disease, and a significant percentage of 30% die after local failure only, respectively [29]. Regional failure only occurs in not more than about 7% of all stage I NSCLC patients [30]. Thus, elective node irradiation is not necessary. Patients with their primary controlled had a cause-specific survival at 5 years four times higher than those with uncontrolled primary (46% vs. 12%; p = 0.03) [30]. Retrospective data showed a trend toward improved cause-specific survival with higher radiotherapy doses. This emphasizes the need for dose-escalation studies. Belderbos et al. achieved nearly 90% overall response rate (CR and PR) in 50 patients treated with a total dose of 74.3 or 81.0 Gy with 2.25 Gy per fraction. DLT was not reached at the last dose level [2]. However, until now, complete results of toxicity except esophagitis have not been published yet [1]. SRS and hfSRT today have been expanded to extracranial targets [3, 22, 33, 36]. Tumor control rates up to 85–97% have been reached which can compete with the best surgical series (Table 3) [21]. Systematic lymph node dissection in T1 and T2 tumors may no longer be essential due to staging with positron emission tomography. So, for the future, both modalities should be considered also with regard to low side effects after ESRT and low costs. Besides stage I NSCLC patients, a large proportion of our patient group were those with a finite number (one to four) of metastases (oligometastatic disease). From literature reviews, we know that these patients may experience improved survival by resection of their metastases and the primary site [6, 12].
Table 3.
Review of literature. LC: local control; NSCLC: non-small cell lung cancer.
| Authors/year | Indications | Dose concept (dose specification) | LC (NSCLC) | LC (metastases) |
|---|---|---|---|---|
| Blomgren et al. 1995 [3] | Metastases NSCLC | 3 × 10 − 2 × 15 Gy, 65% isodose | 3/3 100% | 13/14 93% |
| Uematsu et al. 1998 [33] | Metastases NSCLC | 5–15 fx, 30–76 Gy, 80% isodose | 22/23 96% | 42/43 98% |
| Uematsu et al. 2001 [34] | NSCLC | 5–10 fx, 50–60 Gy, 100% isodose, 80% coverage | 47/50 96% | – 2_ |
| Nagata et al. 2002 [23] | Metastases NSCLC | 4 × 10–12 Gy, 100% isodose | 31/33 94% | 31/33 94% |
| Onimaru et al. 2003 [25] | Metastases NSCLC | 8 fx, 40–60 Gy, 80–100% isodose | 20/25 80% | 18/20 90% |
| Lee et al. 2003 [20] | Metastases NSCLC | 3–4 × 10 Gy, 90% isodose | 8/9 89% | 23/25 92% |
| Timmerman et al. 2003 [31] | NSCLC | 3 × 8 – 3 × 20 Gy, 80% isodose | 31/37 84%, no relapse ≥ 18 Gy | |
| This study | NSCLC | 5 × 7–8 Gy, 90% isodose | 3/3 100% | 31/36 86% |
Patient Setup Accuracy
The following prerequisites have to be met for high setup accuracy: reliable immobilization, reduction of organ motion, and quick radiation delivery. Different fixation methods for patients are used. A system using a stereotactic body frame with integrated vacuum pillow revealed positioning errors in all directions of up to 5 mm and target setup deviations of up to 10 mm [14, 19, 24]. Since the Novalis™ system allows for the control of deviations as related to bony structures in all six planes, i.e., translational and rotational directions, there is no necessity of correctly positioning a frame around the patient as the bony landmarks themselves are positioned. X-ray verification with ExacTrac™ revealed a setup accuracy within 1 mm in all directions for all patients. In addition, abdominal pressure devices may significantly reduce organ movements [14, 19, 33]. Therefore, a home-built abdominal press was implemented into our system (Figure 1). We applied as much pressure as could be tolerated without any side effects. However, even with abdominal pressure, breathing mobility remains the major factor for setup inaccuracy, especially for lesions close to the diaphragm [16].
Dose Prescription, Planning Algorithm, and Radiation Dose Delivery
According to literature data, using SRS techniques, lung tumors are being treated with two to eight fractions of 5–20 Gy each. Although overall response rates varied in an only small range between 80% and 100% [3, 20, 23, 25, 31, 33], a comparison of the results remains difficult because of different total dose and variations in dose prescription. According to the existing data, dose was being prescribed to the 65–100% isodose and there is no evidence, that an increased inhomogeneity inside the target volume may be followed by higher response rates (Table 3). As an admission to the guidelines for cranial radiosurgery, we would recommend to meet the requirements for homogeneity, coverage and conformity [28] in order to better compare the future results.
Another problem is the lack of detailed information on the calculation models that are used. It is well known for low-density lung tissue, that simple calculation models like pencil beam algorithm in contrast to collapsed cone and Monte Carlo algorithm may overestimate the amount of absorbed dose up to 20% at the interface between tumor and lung tissue. Several studies have proven this effect especially for prescription of the dose to the edge of the target, for small targets with a PTV ≤ 100 cm3 and the use of high-energy, i.e., 18-MeV, photons [9, 17, 18] as compared to low-energy photons. Consequently, the more reliable collapsed cone algorithm should be used in future trials.
The majority of our patients were treated by dynamic conformal arcs guaranteeing a maximum of dose conformity. Furthermore, it is reasonable to disperse the dose outside the target volume over large areas and reduce the lung volumes receiving high doses. Willner et al. [35] showed that reducing the high-dose volume will result in a lower pneumonitis rate as compared to a reduction of lung volumes receiving low dose.
Response Rates and Toxicity
Until now, a number of well-conducted studies revealed remarkably good results following fractionated stereotactic treatment of early lung cancer (Table 3). Few data, however, exist on toxicity after hfSRT of lung tumors: acute grade 1 toxicity was documented in 5–9% of patients [33], and grade 2 toxicity in 4% of patients [11, 25], respectively. Severe side effects were only reported occasionally [3, 25]. Our data compare very favorably with these results. Nevertheless, four patients relapsed in this series up to 13 months after treatment, even after 5 × 8 Gy. Timmerman et al. [31] saw no recurrences when treating patients with doses > 3 × 18 Gy. Using the simple calculation model given by Fowler [8] assuming an α/β-value of 10 Gy for malignant tumors, the two aforementioned schedules translate into an equivalent total dose of 126 Gy2 and 60 Gy2, respectively. An escalation to 5 × 9 Gy would gain another 11.25 Gy. Using another model described by Yaes s Maruyama [37], keeping the same α/β-value of 10 Gy, the biologically effective doses of 151.2 Gy2 versus 72 Gy2 would result, respectively.
Conclusion
hfSRT of lung tumors using a dose-escalating schedule of five fractions with 7 Gy and 8 Gy was well tolerated. Dose escalation will be continued.
References
- 1.Belderbos J., Heemsbergen W., Hoogeman M., et al. Acute esophageal toxicity in non-small cell lung cancer patients after high-dose conformal radiotherapy. Radiother Oncol. 2005;75:157–164. doi: 10.1016/j.radonc.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Belderbos J.S., Jaeger K., Heemsbergen W.D., et al. First results of a phase I/II dose escalation trial in non-small cell lung cancer using three-dimensional conformal radiotherapy. Radiother Oncol. 2003;66:119–126. doi: 10.1016/S0167-8140(02)00377-8. [DOI] [PubMed] [Google Scholar]
- 3.Blomgren H., Lax I., Naslund I., et al. Stereotactic high-dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 4.Boda-Heggemann J., Walter C., Mai S., et al. Frameless stereotactic radiosurgery of a solitary liver metastasis using active breathing control and stereotactic ultrasound. Strahlenther Onkol. 2006;182:216–221. doi: 10.1007/s00066-006-1453-8. [DOI] [PubMed] [Google Scholar]
- 5.Combs S.E., Gutwein S., Thilmann C., et al. Reirradiation of recurrent WHO grade III astrocytomas using fractionated stereotactic radiotherapy (FSRT) Strahlenther Onkol. 2005;181:768–773. doi: 10.1007/s00066-005-1415-6. [DOI] [PubMed] [Google Scholar]
- 6.Downey R.J., Ng K. The management of non-small-cell lung cancer with oligometastases. Chest Surg Clin N Am. 2001;11:121–132. [PubMed] [Google Scholar]
- 7.Ernst-Stecken A., Lambrecht U., Ganslandt O., et al. Radiosurgery of small skull-base lesions. No advantage for intensity-modulated stereotactic radiosurgery versus conformal arc technique. Strahlenther Onkol. 2005;181:336–344. doi: 10.1007/s00066-005-1371-1. [DOI] [PubMed] [Google Scholar]
- 8.Fowler J. Non-standard fractionation in radiotherapy. Int J Radiat Oncol Biol Phys. 1984;10:755–759. doi: 10.1016/0360-3016(84)90308-0. [DOI] [PubMed] [Google Scholar]
- 9.Haedinger U., Flentje M., Wulf J., et al. Influence of calculation model on dose distribution in stereotactic radiotherapy for pulmonary targets. Int J Radiat Oncol Biol Phys. 2005;61:239–249. doi: 10.1016/j.ijrobp.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Hall E.J., Brenner D. The radiobiology of radiosurgery: rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol Biol Phys. 1993;25:381–385. doi: 10.1016/0360-3016(93)90367-5. [DOI] [PubMed] [Google Scholar]
- 11.Hara R., Kondo T., Aruga T., et al. Stereotactic single high dose irradiation of lung tumors under respiratory gating. Radiother Oncol. 2002;63:159–163. doi: 10.1016/S0167-8140(02)00063-4. [DOI] [PubMed] [Google Scholar]
- 12.Hellman S., Weichselbaum R. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 13.Henzel M., Gross M., Hamm K., et al. Stereotactic radiotherapy of meningiomas. Symptomatology, acute and late toxicity. Strahlenther Onkol. 2006;182:382–388. doi: 10.1007/s00066-006-1535-7. [DOI] [PubMed] [Google Scholar]
- 14.Herfarth K.K., Lohr F., Bahner M.L., et al. Extracranial stereotactic radiation therapy: set-up accuracy of patients treated for liver metastases. Int J Radiat Oncol Biol Phys. 2000;46:329–335. doi: 10.1016/S0360-3016(99)00413-7. [DOI] [PubMed] [Google Scholar]
- 15.Hocht S., Stark R., Seiler F., et al. Proton or stereotactic photon irradiation for posterior uveal melanoma? A planning intercomparison. Strahlenther Onkol. 2005;181:783–788. doi: 10.1007/s00066-005-1395-6. [DOI] [PubMed] [Google Scholar]
- 16.Hof H., Munter M., Hoess A., et al. Stereotactic single-dose radiotherapy of stage I non-small-cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 2003;56:335–341. doi: 10.1016/S0360-3016(02)04504-2. [DOI] [PubMed] [Google Scholar]
- 17.Koelbl O., Haedinger U., Sauer O., et al. Influence of calculation algorithm on dose distribution in irradiation of non-small cell lung cancer (NSCLC). Collapsed cone versus pencil beam. Strahlenther Onkol. 2004;180:783–788. doi: 10.1007/s00066-004-1268-4. [DOI] [PubMed] [Google Scholar]
- 18.Krieger T. Monte Carlo- versus pencil-beam-/collapsed-cone-dose calculation in a heterogeneous multi-layer phantom. Phys Med Biol. 2005;50:859–868. doi: 10.1088/0031-9155/50/5/010. [DOI] [PubMed] [Google Scholar]
- 19.Lax I., Blomgren H., Naslund I., et al. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol. 1994;33:677–683. doi: 10.3109/02841869409121782. [DOI] [PubMed] [Google Scholar]
- 20.Lee S.W., Park H.J., Ahn S.D., et al. Stereotactic body frame based fractionated radiosurgery on consecutive days for primary or metastatic tumors in the lung. Lung Cancer. 2003;40:309–315. doi: 10.1016/s0169-5002(03)00040-0. [DOI] [PubMed] [Google Scholar]
- 21.Martini N., Burt M., Zakowski M.F., et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109:120–129. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 22.Munter M.W., Nill S., Thilmann C., et al. Stereotactic intensity-modulated radiation therapy (IMRT) and inverse treatment planning for advanced pleural mesothelioma. Feasibility and initial results. Strahlenther Onkol. 2003;179:535–541. doi: 10.1007/s00066-003-1055-7. [DOI] [PubMed] [Google Scholar]
- 23.Nagata Y., Aoki T., Mizowaki T., et al. Clinical outcomes of 3D conformal hypofractionated single high-dose radiotherapy for one or two lung tumors using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2002;52:1041–1046. doi: 10.1016/s0360-3016(01)02731-6. [DOI] [PubMed] [Google Scholar]
- 24.Nevinny-Stickel M., Sweeney R., Bale R.J. Reproducibility of patient positioning for fractionated extracranial stereotactic radiotherapy using a double-vacuum technique. Strahlenther Onkol. 2004;180:117–122. doi: 10.1007/s00066-004-1146-0. [DOI] [PubMed] [Google Scholar]
- 25.Onimaru R., Shimizu S., Kitamura K., et al. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys. 2003;56:126–135. doi: 10.1016/s0360-3016(03)00095-6. [DOI] [PubMed] [Google Scholar]
- 26.Ruggieri L. Hypofractionation in non-small cell lung cancer (NSCLC): suggestions from modelling both acute and chronic hypoxia. Phys Med Biol. 2004;49:4811–4823. doi: 10.1088/0031-9155/49/20/011. [DOI] [PubMed] [Google Scholar]
- 27.Seegenschmiedt M.H. Interdisciplinary documentation of treatment side effects in oncology. Present status and perspectives. Strahlenther Onkol. 1998;174(Suppl3):25–29. [PubMed] [Google Scholar]
- 28.Shaw E., Gillin M., Souhami L., et al. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993;27:1231–1239. doi: 10.1016/0360-3016(93)90548-a. [DOI] [PubMed] [Google Scholar]
- 29.Sibley G.S. Radiotherapy for patients with medically inoperable stage I nonsmall cell lung carcinoma: smaller volumes and higher doses — a review. Cancer. 1998;82:433–438. doi: 10.1002/(SICI)1097-0142(19980201)82:3<433::AID-CNCR2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 30.Sibley G.S., Jamieson T., Marks L.B., et al. Radiotherapy alone for medically inoperable stage I non-small-cell lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys. 1998;40:149–154. doi: 10.1016/S0360-3016(97)00589-0. [DOI] [PubMed] [Google Scholar]
- 31.Timmerman R., Papiez L., McGarry R., et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946–1955. doi: 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 32.Timmerman R., Papiez L., Suntharalingam M. Extracranial stereotactic radiation delivery: expansion of technology beyond the brain. Technol Cancer Res Treat. 2003;2:153–160. doi: 10.1177/153303460300200212. [DOI] [PubMed] [Google Scholar]
- 33.Uematsu M., Shioda A., Tahara K., et al. Focal, high dose, and fractionated modified stereotactic radiation therapy for lung carcinoma patients: a preliminary experience. Cancer. 1998;82:1062–1070. doi: 10.1002/(SICI)1097-0142(19980315)82:6<1062::AID-CNCR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 34.Uematsu M., Shioda A., Suda A., et al. Computed tomography-guided stereotactic radiotherapy for stage I non-small cell lung cancer: a 5-year experience. Int J Radiat Oncol Biol Phys. 2001;51:666–670. doi: 10.1016/s0360-3016(01)01703-5. [DOI] [PubMed] [Google Scholar]
- 35.Willner J., Baier K., Flentje M. A little to a lot or a lot to a little? An analysis of pneumonitis risk from dose-volume histogram parameters of the lung in patients with lung cancer treated with 3-D conformal radiotherapy. Strahlenther Onkol. 2003;179:548–556. doi: 10.1007/s00066-003-1078-0. [DOI] [PubMed] [Google Scholar]
- 36.Wulf J., Haedinger U., Oppitz U., et al. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol. 2001;177:645–655. doi: 10.1007/PL00002379. [DOI] [PubMed] [Google Scholar]
- 37.Yaes R.J., Maruyama Y. On using the linear-quadratic model in daily clinical practice. Int J Radiat Oncol Biol Phys. 1991;20:1353–1362. doi: 10.1016/0360-3016(91)90249-4. [DOI] [PubMed] [Google Scholar]
- 38.Zimmermann F.B., Geinitz H., Schill S., et al. Stereotactic hypofractionated radiation therapy for stage I non-small cell lung cancer. Lung Cancer. 2005;48:107–114. doi: 10.1016/j.lungcan.2004.10.015. [DOI] [PubMed] [Google Scholar]


