Abstract
In the normal mammalian brain, neuronal synchrony occurs on a spatial scale of submillimeters to centimeters and temporal scale of submilliseconds to seconds that is reflected in the occurrence of high-frequency oscillations, physiological sharp waves and slow wave sleep oscillations referred to as Up–Down states. In the epileptic brain, the well-studied pathologic counterparts to these physiological events are pathological high-frequency oscillations and interictal spikes that could be electrophysiological biomarkers of epilepsy. Establishing these abnormal events as biomarkers of epilepsy will largely depend on a better understanding of the mechanisms underlying their generation, which will not only help distinguish pathological from physiological events, but will also determine what roles these pathological events play in epileptogenesis and epileptogenicity. This article focuses on the properties and neuronal mechanisms supporting the generation of high-frequency oscillations and interictal spikes, and introduces a new phenomenon called Up-spikes.
Keywords: epileptogenesis, epileptogenicity, fast ripple, interictal electroencephalographic spike, ripple, Up-spike
High-frequency oscillations
Ripples in normal hippocampus
High-frequency oscillations (HFOs) are local oscillatory field potentials that contain spectral power greater than 100 Hz and are typically tens of milliseconds in duration. In the normal rat hippocampus, one type of HFO is the ripple oscillation found in CA1 that contains spectral frequencies between 100 and 200 Hz and occurs with greatest probability during episodes of awake immobility and slow-wave sleep (SWS) [1]. In addition to CA1, ripples exist in CA3, subiculum and the entorhinal cortex [2,3], while other studies have found ripples in other nonprimates as well as nonhuman primates and humans that have spectral frequency and state-dependent characteristics similar to those in rats [4–7].
In CA1 of behaving rats, ripples are typically largest in amplitude in the pyramidal cell layer and superimposed on large amplitude slow waves called sharp waves [1]. Physiological sharp waves are triggered by CA3 population bursts resulting from a transient reduction in inhibition, and reflect large postsynaptic potentials in CA1 and subicular pyramidal cells induced via Schaffer collaterals, and in dentate gyrus granule cells through hilar associational pathways [8]. During sharp-wave ripple bursts recorded in CA1 stratum radiatum, pyramidal cells tend to fire infrequently and do so during the individual troughs of the ripple oscillation [3]. Parvalbumin- and cholecystokinin-expressing basket cells tend to increase firing during ripple oscillations with most spikes consistently phase aligned with respect to the ripple [9,10]. Intracellular recordings from pyramidal cells reveal a ripple frequency potential that reverses in polarity relative to the extra-cellular ripple at membrane potentials more negative than −80 mV [11]. These data suggest the fast intracellular potential is mediated by chloride ions due to the activation of GABA A receptors by GABAergic interneurons. Ripples in CA1, and possibly other hippocampal areas, could reflect phasic activation of interneurons by external input, produce rhythmic inhibitory postsynaptic potentials that strongly regulate pyramidal cell discharges [11]. This synchronous interaction between interneurons and pyramidal cells is thought to generate active inward and outward extracellular currents that contribute to the ripple field potential.
It is believed that ripples facilitate information transfer during sleep between hippocampal and cortical structures [12]. Evidence to support this hypothesis are derived from animal studies, which show that during SWS ripples are temporally coupled with forebrain spindle oscillations [13]. In addition, prefrontal cortex neurons increase their firing within 100 ms after hippocampal sharp wave ripple bursts [14]. Coordinated discharges along hippocampal and neocortical pathways could be related to processes associated with memory consolidation when short-term hippocampal memory traces are transferred to long-term neocortical stores.
Neocortical HFOs
Spontaneous ripple frequency HFOs occur in normal neocortex. In cats, neocortical HFOs can be recorded most frequently during episodes of SWS and ketamine anesthesia-induced sleep-like states [15]. Neocortical HFOs are often associated with the EEG depth-negative component or Up-phase of the neocortical slow-wave oscillation. Coherence among neo-cortical HFOs is strong over distances of up to 10 mm within individual gyri, but coherence weakens between HFOs in different gyri [15]. Neocortical HFOs exists in isolated cortical tissue, which suggests that intracortical circuits can support their generation.
In rat and human neocortex, somatosensory evoked potentials are associated with HFOs that contain spectral frequencies between 200 and 600 Hz. In rats, rapid mechanical stimulation of the vibrissae or electrical stimulation of the thalamic ventrobasal nuclei can evoke HFOs in somatosensory barrel cortex [16–19]. In humans, peripheral nerve stimulation elicits somatosensory evoked potentials that contain HFOs with different components of the HFO arising from thalamic and cortical circuits [20,21]. Sensory-evoked neocortical HFOs are typically superimposed on the earliest components of the biphasic positive–negative somatosensory evoked potential, and in rat barrel cortex can propagate in-phase over several millimeters [17]. Simultaneous stimulation of individual vibrissa evokes HFOs that propagate across barrel cortex and can constructively interact [22]. This interaction results in a supralinear summation of HFOs that can be abolished with an interruption of intracortical pathways [23]. These results suggest that the locally facilitated evoked HFO could reflect a recruitment of additional neurons that discharge due to the in-phase interactions between propagating HFOs.
Excitatory and inhibitory neurons have been implicated in the generation of neocortical HFOs. Fast spiking cells, presumably GABA-containing neurons, discharge bursts of action potentials at intraburst intervals that correspond with the frequency of the extracellular HFO [15]. Fast spiking cell discharges typically precede those from regular spiking cells, which occur during the trough of the extra-cellular HFO [24]. Similar to the pattern of firing between pyramidal cells and interneurons during hippocampal ripples, neocortical HFOs could reflect inhibitory postsynaptic potentials from fast spiking cells that regulate the timing and firing of regular spiking cells.
HFOs in the epileptic brain
In chronic models of acquired epilepsy, interictal HFOs with spectral frequencies between 250 and 600 Hz, termed fast ripples (FR) (Figure 1A), are found in brain areas capable of generating spontaneous seizures, including dentate gyrus, hippocampus, subiculum and entorhinal cortex [25]. FR are found in rats that exhibit recurrent spontaneous seizures, but not in rats that have been subjected to an epileptogenic insult, for example status epilepticus (SE), which do not exhibit spontaneous seizures. Subsequent studies in this same chronic model also detected ripple frequency HFOs in the epileptogenic dentate gyrus [26], which was an important finding for several reasons. First, there is very little evidence 100–200 Hz ripples occur in normal dentate gyrus and indicate HFOs in this structure are abnormal. Second, these data suggest that abnormal ripple frequency HFOs occur in CA1 or CA3, but it is not yet possible to distinguish abnormal from normal HFOs in these areas. Last, this study illustrates that spectral frequency alone does not determine pathogenicity [27], and thus the term pathological HFOs (pHFOs) is now being used more regularly to describe abnormal HFOs associated with epileptogenicity.
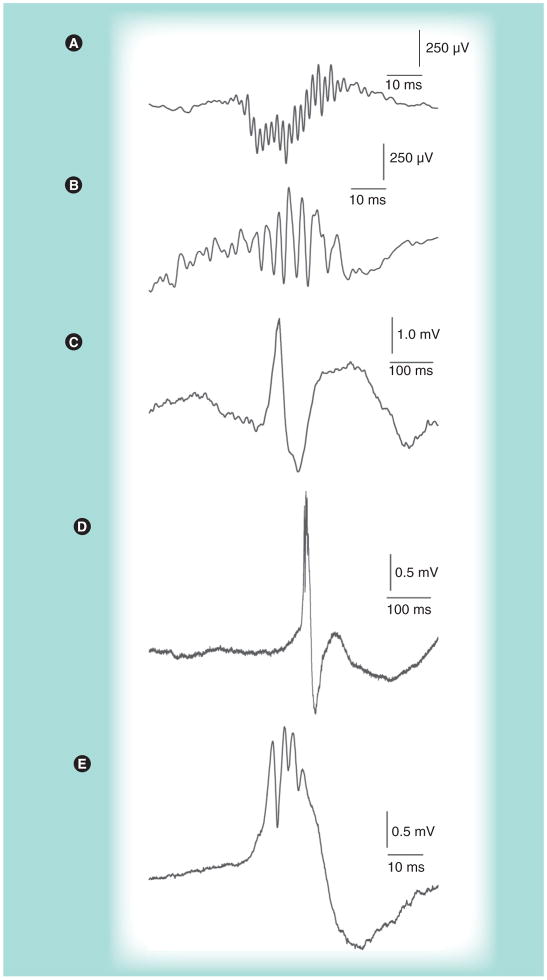
Figure 1. Examples of interictal pathological high-frequency oscillations and EEG spikes.
(A) Pathological high-frequency oscillations (~500 Hz) recorded from the hilar region in the dentate gyrus of pilocarpine-treated epileptic rat. (B) Pathological high-frequency oscillations (~333 Hz) recorded from the microelectrode position in hippocampus ipsilateral to seizure onset of patient with mesial temporal lobe epilepsy and hippocampal sclerosis. (C) Interictal EEG spike followed by longer duration slow wave recorded from entorhinal cortex of a presurgical patient. (D) Interictal EEG spike record in hippocampus of a different patient than in (C). (E) Same as (D), but on an expanded time scale, to more clearly show the pathological high-frequency oscillation (~300 Hz) superimposed on the EEG spike.
In humans, interictal HFOs can be recorded using microelectrodes specially adapted to clinical depth electrodes positioned in hippocampus and entorhinal cortex of patients with medically refractory mesial temporal lobe epilepsy (MTLE) [4,25]. These initial studies showed FR recorded in patients resemble those in epileptic rats (Figure 1B), including their spectral frequency, duration, association with the seizure-onset zone [25,28], and that they exist in cellular layers of entorhinal cortex that correspond with evoked population spike discharge and abnormal synchrony of burst firing [29]. Microelectrode studies in patients also demonstrated that rates of FR are highest during SWS and remain elevated during rapid eye movement sleep [6]. In addition, morphological alterations associated with hippocampal sclerosis could promote the generation of FR [30]. A more recent study found pHFOs in areas of focal cortical dysplasia and nodular heterotopia, and rates of these pHFOs were more strongly linked to areas of seizure onset than anatomical lesion [31]. These latter data suggest that pHFOs are not limited to a specific type of epilepsy, but could be a fundamental property of epileptogenicity common to many types of epilepsy.
It is not yet possible to distinguish normal from pathologic ripple frequency HFOs in humans, but studies have identified several important characteristics of hippocampal ripples that are similar to ripples in the normal nonprimate hippocampus. Human ripples have similar spectral frequencies, they occur bilaterally with the highest rates during SWS and the lowest during rapid eye movement sleep [4,6, 28], and arise from broad areas of tissue supporting generation and synchrony of neuronal discharges [29]. Results from single neuron studies show that in relation to the extracellular ripple frequency HFO, putative interneurons and pyramidal cells discharge in patterns that resemble cell type-specific firing patterns during ripples in the normal hippocampus of rodents [32]. Patient studies have observed temporal coupling between hippocampal ripples and neocortical spindles [33], but there is little evidence that hippocampal ripples are associated with an increase in medial prefrontal single neuron firing as found in nonprimates [34].
Neocortical HFOs also exist during high-voltage spike and wave discharges in rats, which could represent a model of absence epilepsy [16], and are associated with seizure-like events in ketamine-anesthetized cats [35]. It is not clear whether these HFOs are pathological since they are found in animals without epilepsy and bear resemblance to evoked and spontaneous HFOs in normal neocortex. However, unlike HFOs in normal neocortex, fast-spiking neurons do not discharge at fixed latencies in relation to HFOs during the onset of seizure-like events, which suggests that alterations in inhibitory postsynaptic potentials might not be able to regulate neuronal discharges during abnormal neocortical HFOs [35]. More research is needed to better understand the spatiotemporal properties and neuronal mechanisms supporting normal and abnormal neocortical HFOs in the epileptic brain.
Neuronal mechanisms generating interictal pHFOs
It is believed that pHFOs reflect population spikes arising from abnormal synchronously bursting principal neurons within local areas, each could be as small as 1 mm3 [36], surrounded by larger areas of nonbursting tissue [37]. This hypothesis is consistent with the idea that inhibition is altered inside pHFO-generating sites, but strong surrounding these sites, and could explain why application of GABA-A receptor antagonists can spatially extend pHFO-generating sites into surrounding tissue [36]. In addition, studies show that in dentate gyrus, pHFOs have the largest amplitude within the granule cell layer [38], and on average there is a decrease in interneuron firing just prior to an increase in granule cell discharge during pHFOs in dentate gyrus [39]. How abnormal neuronal synchrony is generated during pHFOs is not clear, but in CA3 tissue slices, exposure to high potassium medium produces pHFO field events that can be suppressed with blockade of ionotropic glutamatergic signaling between synchronously firing pyramidal cells [40]. Furthermore, disrupting intrinsic action potential generating mechanisms decreases pHFO amplitude, while increasing the fidelity of pyramidal cell discharges restores pHFO amplitude. These data suggest that increased chemical transmission through recurrent excitatory synapses between pyramidal cells could contribute to the generation of pHFOs.
Recent studies offer possible explanations of how networks could generate pHFOs that could contain spectral frequencies between 100 and 600 Hz. In CA3 of epileptic rats, interfering with neuronal spike timing was associated with an increase in pHFO spectral frequency [41]; the amplitude of these pHFOs was also reduced compared with that typically found in vivo [38]. By contrast, increasing neuronal spike timing reduced pHFO spectral frequency, suggesting that the extent of in-phase and out of phase firing among groups of discharging cells corresponds with lower and higher spectral frequency pHFOs, respectively. Evidence supporting this hypothesis derives from a study that found pHFOs >400 Hz could emerge from local groups of neurons, each firing at a lower frequency than the pHFO, but with firing delays between a half and three quarters of an intra-burst cycle with respect to each other [42].
Results from preceding paragraphs emphasize the functional organization of circuits and synaptic transmission contributing to pHFOs, but nonsynaptic mechanisms, as well as pathology, could strongly effect the generation of pHFOs. Electrical interactions arising from electrical fields created by depolarizing currents, for example ephaptic effects, could increase the probability and synchrony of neuronal firing [43]. Owing to the close proximity and parallel arrangement of neurons in hippocampus and neocortex, ephaptic effects could play a role in generation of pHFOs [44,45]. Electrical interactions mediated through specialized channels, such as gap junctions, offer another mechanism to synchronize neuronal firing. Ripples and pHFOs exist in the absence of synaptic transmission and can be suppressed with agents that block gap junctions [11,46,47]. There is limited evidence for gap junctions between pyramidal cells in hippocampus, but computer simulations indicate that hippocampal networks containing a few (1–3) axon-to-axon gap junctions between pyramidal cells could synchronize neuronal discharges that generate HFOs and possibly pHFOs [48,49]. Finally, studies in epileptic rats and patients have found that hippocampal cell loss correlates with power and rates of FR [30,41], although evidence that pHFOs are present in hippocampal areas without significant cell loss suggest that cell loss and synaptic reorganization are not required for the generation of pHFOs [47].
Pathological HFOs as a biomarker of epilepsy
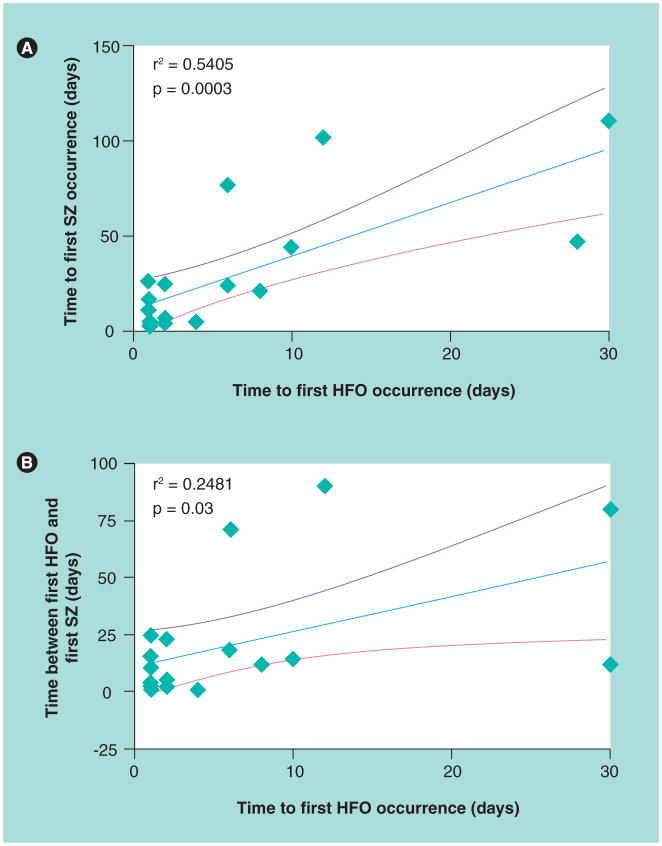
Much of the basic research on pHFOs described in the preceding sections was carried out in chronic models of acquired epilepsy. In this type of model, chemical or electrical stimulation induces an episode of SE that in many animals, several days to weeks later, results in the appearance of spontaneous seizures, which is useful for studies of epileptogenesis. In a chronic model of MTLE, recordings during the development of epilepsy found that shorter latencies to the first appearance of pHFOs correlate with shorter latencies to the first spontaneous seizure (Figure 2A & B) [26]. Furthermore, pHFO-generating sites remain spatially stable over the period of days or months preceding the appearance of spontaneous seizures [50]. These findings suggest that the occurrence and location of pHFOs could predict the development of seizures after an epileptogenic insult and thereby provide a biomarker for epileptogenesis.
Figure 2. Pathological high-frequency oscillations and spontaneous seizures in intrahippocampal kainic acid rat model of mesial temporal lobe epilepsy.
(A) Scatterplot of time in days to first spontaneous seizure in relation to time of first appearance of pathological high-frequency oscillations. (B) Scatterplot illustrating the difference in days between time of first spontaneous seizure and first pathological high-frequency oscillation in relation to time of first appearance of pathological high-frequency oscillation.
HFO: High-frequency oscillation; SZ: Seizure.
Adapted with permission from [26].
Results from chronic models of epilepsy led to the proposal that pHFOs arise from pathologically interconnected neuron clusters [37]. Included in this hypothesis was that synchrony of discharges attributable to abnormal neuronal excitability and synaptic reorganization after SE could contribute to the formation of pathologically interconnected neuron clusters, which might act as endogenous kindling generators that slowly strengthen synaptic connections in target areas. This could eventually lead to the recruitment of additional brain sites into a pathological network that ultimately generates spontaneous seizures.
The body of work from experimental models and retrospective presurgical patient studies provides compelling evidence for the presence of interictal pHFOs inside the seizure onset zone. These results indicate that interictal pHFOs could be used to localize brain areas where seizures begin. Patient studies have found interictal pHFOs, albeit significantly fewer, remote from sites of seizure onset [25,28], which might reflect areas that are part of the epileptogenic zone (i.e., the brain areas necessary and sufficient to generate spontaneous seizures). The epileptogenic zone cannot be measured directly, but is derived from diagnostic tests that indicate the presence of an epileptogenic lesion and electro-graphic localization of sites of seizure onset and early propagation, as well as postsurgical seizure freedom. Based on evidence that showed patients with postsurgical seizure freedom or significantly reduced seizure rates had a greater number of pHFO-generating sites removed than patients who had worse postsurgical seizure outcomes [51,52]; an important goal for prospective studies will be to determine whether inclusion of interictal pHFOs could define the epileptogenic zone more accurately.
Pathological HFOs might also have clinical value in determining treatment and prognosis for patients with medically refractory epilepsy, for example MTLE or neocortical epilepsy. In SE rats with spontaneous hippocampal seizures, a greater number of electrodes recording pHFOs correlate with higher average daily rates of seizures [50], whereas in patients with MTLE and hippocampal sclerosis, reduced hippocampal volumes and lower hippocampal neuron densities correlate with higher rates of FR and lower rates of ripples [30,53]. These data, along with studies cited in the previous paragraph, suggest that rates of pHFOs reflect the severity of epileptogenicity and could help identify patients that would benefit from epilepsy surgery or alternative treatments.
Results from basic research studies in animals and microelectrode recordings with patients have driven the development of clinical EEG monitoring systems to support higher sampling rates and provide greater bandwidth. In addition, it has become more common to adapt standard clinical depth and subdural grid electrodes with smaller diameter and greater numbers of micro-electrodes to study pHFOs [54], and a number of studies using advanced recording systems and hybrid electrodes in a clinical epilepsy setting, described in greater detail in this issue of Biomarkers in Medicine [55], confirm the strong association between pHFOs and human brain areas capable of generating seizures [56–59].
Interictal epileptiform discharges
Interictal EEG spikes
In patients with epilepsy, during the periods between seizure episodes, interictal epileptiform discharges consist of electrographic waves or complexes distinguished from background activity on the EEG. Morphological patterns of interictal epileptiform discharges include spikes (or interictal spikes [IIS]) (Figure 1C & D), which can be described on the basis of duration between 20 and 70 ms, and sharp waves that have duration between 70 and 200 ms [60]. Furthermore, IIS or sharp waves can exist alone or in a complex with a slow wave, and can occur in isolation or brief bursts lasting less than a few seconds that can be difficult to distinguish from some subclinical focal seizures.
Much of the research on IIS derives from studies using acute and chronic experimental animal models of epilepsy (see [61] for review). IIS can be generated under many different conditions; for example, after focal cortical injections of epileptogenic substances, such as penicillin [62,63] or aluminum hydroxide [64]. IIS can also be recorded from tissue slices bathed in low magnesium or calcium [65,66], or high potassium solutions [67], while IIS can occur shortly after kainic acid-induced SE [68], and after episodes of electrical stimulation-induced self-sustaining SE [69]. In spite of the challenges in maintaining spontaneous activity in brain slices, in vitro studies using human resected epileptogenic tissue have also provided insight on abnormal neuronal activity associated with IIS [70–72].
Mechanisms underlying the generation of IIS
Intracellular recordings from penicillin foci show that during IIS, a large percentage of neurons discharge a high-frequency burst of action potentials. Each individual burst is regularly accompanied by a high-amplitude prolonged membrane depolarization termed a ‘paroxysmal depolarization shift’ (PDS) [62], followed by an afterhyperpolarization due in part to increased potassium conductances [73]. Considerable effort has gone into understanding the cellular mechanisms of the PDS in order to identify the causes, and importantly, potential treatment for epilepsy. Results from early studies using the penicillin model suggest the PDS reflects the abnormal or giant excitatory postsynaptic potentials [74–76], and subsequent studies using voltage- and current-clamp techniques provide evidence consistent with a synaptic basis underlying the PDS [77,78].
Factors contributing to the intense synaptic activity underlying IIS likely include the organization of local synaptic connections, densities and locations of ion channels on neurons, and interactions between synaptic and intrinsic currents [79]. In vitro intracellular recordings demonstrate that CA3 and to a lesser extent CA1 hippocampal neurons, and some neocortical neurons, possess endogenous burst firing capabilities that morphologically resemble burst firing that occurs in the presence of convulsant agents [80–82]. These latter studies and others indicate that endogenous bursts are due to intrinsic membrane properties and mediated by voltage-dependent regenerative calcium currents and sodium currents [83–85]. Endogenous bursts might provide the strong synaptic input needed to initiate a PDS and it is likely that, in neurons with burst firing potential, intrinsic membrane currents could also augment the abnormal depolarization that sustains high-frequency discharges. Furthermore, studies have found that changes in intrinsic membrane properties, such as an upregulation in calcium channels [86,87] and alterations in glial function [88], also significantly affect neuronal excitability.
A critical aspect for the generation of IIS is the nearly simultaneous onset of burst firing within the network. Synaptic-mediated impulses can spread more easily within networks containing a high density of recurrent excitatory synapses, such as in hippocampal CA2–CA3. Local excitatory circuits are effective in recruiting and initiating burst firing originating from a few or, under some conditions, a single bursting neuron [89–91]. Because networks generating IIS are larger than those supporting HFOs [36], large extracellular voltage gradients might arise from intense neuronal firing, which could play a prominent role in bringing more neurons closer to the firing threshold [92,93]. Experimental and computer simulations indicate that a low density of gap junctions between pyramidal cells in combination with chemical synapses can have a significant effect on network synchrony during the generation of IIS [49,94,95]. Abnormal excitatory drive promoting synchrony might also arise from axonal sprouting and synaptic reorganization of granule cell mossy fibers often found in experimental SE models and patients with MTLE [96]. In addition to the formation of recurrent excitatory circuits, there is evidence for mossy fiber sprouting onto GABAergic interneurons that is associated with greater paired-pulse suppression in the perforant path of kainic acid-treated epileptic rats [97]. Data from this animal study are consistent with recordings from inside the seizure onset zone in patients with MTLE, which showed evidence of increased perforant path paired-pulse suppression [98] and prolonged suppression of neuronal discharges following evoked neuronal burst firing [99]. These data suggest the periods of neuronal suppression could reflect enhanced inhibition that might be protective by limiting neuronal excitability. Another possibility is that large groups of neurons emerging from a lengthy refractory period might respond simultaneously to excitatory impulses that could increase the synchrony of firing and generation of IIS.
Interictal spikes as a biomarker of epilepsy
Interictal spikes are highly correlated with epilepsy, yet in a small percentage of patients without history of epilepsy, spontaneous or evoked epileptiform discharges can be recorded in the EEG [100,101]. It appears that in many of these cases epileptiform discharges coexist with acute or progressive neurological disorders [102], but the period of follow-up is often too short to determine how many of these individuals develop epilepsy. While appearance of IIS in the EEG can be used diagnostically, studies in presurgical patients with medically intractable focal seizures show that sites generating IIS do not always coincide with the epileptogenic lesion, and rates of IIS often do not predict the onset of seizures and do not correspond with seizure frequency [103,104]. These results suggest IIS might not be a reliable biomarker of focal seizure severity or frequency.
One reason why IIS might not be a dependable biomarker of epileptogenicity is because the functional roles of IIS in epilepsy are not known. Studies using combined hippocampal-entorhinal cortex tissue slices treated with 4-aminopyridine, a convulsant agent that interferes with potassium channels, found that IIS generated in CA3 were associated with a reduction of ictal discharges in the entorhinal cortex [105]. In this same study, severing the Schaffer collateral pathway between CA3 and CA1 was associated with an increase in entorhinal cortex ictal activity, which could subsequently be suppressed using rhythmic, low-frequency electrical stimulation in CA1. These data suggest that hippocampal IIS could suppress the generation of ictal activity in entorhinal cortex, and are consistent with other results that indicate IIS are associated with a reduced incidence of seizures [106,107]. Results such as these have led to the proposal that IIS could have antiseizure effects [108], and emphasize the need for detailed studies to determine whether there are different types of IIS, some that might act to suppress seizures, while others could promote seizures.
An example of a different type of IIS derives from studies that found a strong spatial and temporal coupling between IIS and pHFOs (Figure 1D) [56,57,109]. It was recently proposed that IIS containing pHFOs reflect neurons in the recording area that generate synchronous discharges and actively contribute to the generation and spread of epileptiform activity [110]. By contrast, neurons in areas where IIS do not contain pHFOs may receive abnormal impulses that are below threshold for the generation of synchronous firing that lowers the likelihood that epileptiform input will be transmitted to postsynaptic targets.
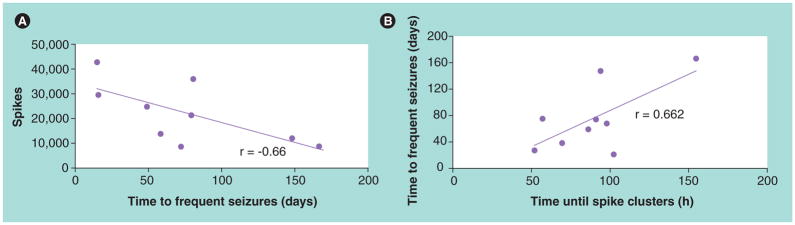
In contrast to the evidence suggesting some IIS reduce seizures, it was proposed that IIS promote seizures and could be a biomarker of epileptogenesis [111,112]. Evidence supporting this hypothesis derives from EEG recordings in kainate-treated epileptic rats, which found most rats had IIS that appeared before the first spontaneous motor seizure [113]. In a separate study, chronic recordings revealed that a greater number of IIS measured 16 days after SE correlated with a shorter time to first spontaneous seizure (Figure 3A). Furthermore, a shorter time to reach half of the eventual steady-state of spontaneous seizures correlated with a shorter time for the appearance of clustered IIS (Figure 3B) [114]. These data indicate that prolonged EEG recordings detecting the presence and patterns of IIS could identify rats that develop epilepsy after convulsive SE. A hypothesis proposed by Staley and colleagues [111] suggests that following brain injury there is a decrease in the number of inter-neurons and reduction in inhibition [115], both of which could produce hyperexcitability and generation of IIS. Injury-related axon sprouting and synaptic reorganization within hyper-excitable networks might be directed back onto itself due to pathological neuronal firing associated with IIS, contributing to a type of abnormal plasticity that increases the strength of recurrent synapses. Thus, IIS might not only guide aberrant sprouting and increase the strength of newly formed synapses, but may maintain these conditions within epileptogenic networks capable of generating spontaneous seizures. If the proposed role of IIS during epileptogenesis is correct, then this would represent a novel target for antiepileptogenic therapy.
Figure 3. Interictal spikes and spontaneous seizures in rats after kainate-induced convulsive status epilepticus.
(A) Scatterplot of number of interictal spikes 16 days after status epilepticus in relation to time to first spontaneous seizure.
(B) Scatterplot illustrating the time in days to frequent seizures with respect to time in hours to appearance of interictal spike clusters. Adapted with permission from [114].
Abnormal Up-spikes
New studies of epileptogenesis are now focusing on patterns and alterations in the Up–Down state (UDS). The UDS initially described by Steriade and coworkers is an electrographic pattern that occurs during SWS [116,117]. It consists of alternating slow wave activity, typically less than 1 Hz, where one phase of the wave is accompanied by an increase in amplitude of electrical activity in the frequency range between 20 and 60 Hz (Up-phase), while the opposite phase corresponds with a reduction or absence of high-frequency electrical activity (Down-phase). The UDS appears in the frontal part of neocortex and propagates through the neocortex, presumably owing to extensive cortico–cortical connections [118]. According to Tononi, UDS activity might be associated with homeostatic downscaling of synaptic processes [119]. The interruption of these processes leads to several neurological dysfunctions such as depression, stress and an increased risk of diabetes and/or obesity [120].
Preliminary studies suggest the pattern of UDS is different in pilocarpine-treated animals with epilepsy compared with animals without epilepsy. In normal mice, there is a gradual increase in the amplitude of γ-activity during the Up-phase, whereas in pilocarpine-treated mice, high-amplitude spikes, termed Up-spikes, interrupt the normal pattern of γ-activity [121]. These alterations in the UDS pattern and appearance of Up-spikes were detected months after pilocarpine-induced SE, but prior to the appearance of spontaneous seizures, suggesting pathological changes in UDS might correspond with epileptogenesis in SE models of acquired epilepsy. Future studies will investigate Up-spikes between pilocarpine-induced SE animals with and without spontaneous seizures to determine whether Up-spikes could be a new electrophysiological biomarker of epileptogenesis.
Future perspective
This review focuses primarily on studies investigating the properties of networks and mechanisms supporting the generation of HFOs in the normal and the epileptic brain. It is anticipated that future basic science and clinical studies will identify aspects of normal ripples and ripple frequency pHFOs that could be used to reliably distinguish between the two electrophysiological events, particularly in areas outside of the dentate gyrus and those in the neocortex. Such distinctions will be important to establish pHFOs as a biomarker of epileptogenesis and epileptogenicity. In addition, research must develop the means to reliably identify pHFOs noninvasively, which up to now has largely depended on intracranial electrode recordings in a surgical epilepsy setting. Magnetoencephalography or functional MRI, possibly in combination with scalp EEG recordings of pHFOs or IIS with pHFOs [122–124], could identify unique electrical or blood flow patterns that are predictive of the epileptogenic region or epileptogenesis. Furthermore, prospective studies should consider intraoperative electrocorticography [52] and extraoperative intracranial recordings of pHFOs to determine whether interictal pHFOs and seizures or pHFOs alone define the epileptogenic region more accurately with better postsurgical seizure freedom. If interictal pHFO recordings prove to be as good as or better than ictal recordings this would also greatly reduce the length and associated risks and costs of invasive studies.
Executive summary.
High-frequency oscillations in normal brain
Spontaneous ripples (100–200 Hz) are present in area CA1 of the hippocampus, as well as CA3, subiculum and the entorhinal cortex; ripple frequency and sensory-evoked high-frequency oscillations (HFOs) occur in neocortex.
Ripples in area CA1 of stratum radiatum, and possibly neocortical HFOs, reflect inhibitory postsynaptic potentials of discharging interneurons that regulate pyramidal cell firing.
Hippocampal ripples and neocortical evoked HFOs are believed to play a role in sensory information processing.
Pathological HFOs in epileptic brain
Interictal fast ripples (250–600 Hz) are strongly associated with brain areas capable of generating spontaneous seizures.
Ripple frequency HFOs in epileptogenic dentate gyrus and the neocortex should be considered pathological HFOs (pHFOs).
Hippocampal pHFOs reflect bursts of population spikes arising chiefly from synchronously firing principal cells.
Interictal pHFOs as a biomarker of epilepsy
The association between pHFOs and epileptogenicity suggest pHFOs could help localize the seizure onset zone and might identify the epileptogenic zone more accurately.
The appearance of pHFOs after epileptogenic injury, for example status epilepticus, and before spontaneous seizures suggests pHFOs could be a biomarker of epileptogenesis in acquired epilepsy.
Interictal spikes as a biomarker of epilepsy
There is little evidence that interictal spikes (IIS) predict seizure frequency or severity of epilepsy.
The functional role of IIS in epilepsy is not known, but some IIS might reduce ictal discharges.
The presence and clustering of IIS after status epilepticus could indicate the subsequent appearance of spontaneous seizures.
Conclusion
Interictal pHFOs reflect basic neuronal disturbances in brain areas capable of generating spontaneous seizures that could identify the epileptogenic region, determine the severity of epileptogenicity and possibly predict the development of epilepsy.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was partially supported by NINDS NS 02808, 33310, 065877 and 071048. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1▪.Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. Focuses on the pyramidal cell and interneuron firing during hippocampal CA1 ripple oscillations. [DOI] [PubMed] [Google Scholar]
- 2.Chrobak JJ, Buzsaki G. High-frequency oscillations in the output of the hippocampal–entorhinal axis of the freely behaving rat. J Neurosci. 1996;16(9):3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci. 1999;19(1):274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪.Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. Describes spontaneous ripples and fast ripples in the hippocampus and entorhinal cortex of patients with epilepsy. [DOI] [PubMed] [Google Scholar]
- 5.Buhl DL, Harris KD, Hormuzdi SG, Monyer H, Buzsaki G. Selective impairment of hippocampal γ oscillations in connexin-36 knock-out mouse in vivo. J Neurosci. 2003;23(3):1013–1018. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J. High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- 7.Ulanovsky N, Moss CF. Hippocampal cellular and network activity in freely moving echolocating bats. Nat Neurosci. 2007;10(2):224–233. doi: 10.1038/nn1829. [DOI] [PubMed] [Google Scholar]
- 8.Buzsaki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- 9.Klausberger T, Marton LF, O’Neill J, et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25(42):9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562(Pt 1):9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11▪.Ylinen A, Bragin A, Nadasdy Z, et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15(1):30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. Provides evidence that ripples in area CA1 of the normal hippocampus reflect inhibitory postsynaptic potentials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buzsaki G. The hippocampo–neortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 13.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 14.Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61(4):587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grenier F, Timofeev I, Steriade M. Focal synchronization of ripples (80–200 Hz) in neocortex and their neuronal correlates. J Neurophysiol. 2001;86(4):1884–1898. doi: 10.1152/jn.2001.86.4.1884. [DOI] [PubMed] [Google Scholar]
- 16.Kandel A, Buzsaki G. Cellular-synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J Neurosci. 1997;17(17):6783–6797. doi: 10.1523/JNEUROSCI.17-17-06783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones MS, Barth DS. Spatiotemporal organization of fast (>200 Hz) electrical oscillations in rat Vibrissa/Barrel cortex. J Neurophysiol. 1999;82(3):1599–1609. doi: 10.1152/jn.1999.82.3.1599. [DOI] [PubMed] [Google Scholar]
- 18.Staba RJ, Brett-Green B, Paulsen M, Barth DS. Effects of ventrobasal lesion and cortical cooling on fast oscillations (>200 Hz) in rat somatosensory cortex. J Neurophysiol. 2003;89:2380–2388. doi: 10.1152/jn.01098.2002. [DOI] [PubMed] [Google Scholar]
- 19.Staba RJ, Bergmann PC, Barth DS. Dissociation of slow waves and fast oscillations above 200 Hz during GABA application in rodent somatosensory cortex. J Physiol. 2004;561(Pt 1):205–214. doi: 10.1113/jphysiol.2004.075325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curio G, Mackert BM, Burghoff M, Koetitz R, Abraham-Fuchs K, Harer W. Localization of evoked neuromagnetic 600 Hz activity in the cerebral somatosensory system. Electroencephalogr Clin Neurophysiol. 1994;91(6):483–487. doi: 10.1016/0013-4694(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 21.Gobbele R, Waberski TD, Simon H, et al. Different origins of low- and high-frequency components (600 Hz) of human somatosensory evoked potentials. Clin Neurophysiol. 2004;115(4):927–937. doi: 10.1016/j.clinph.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Barth DS. Submillisecond synchronization of fast electrical oscillations in neocortex. J Neurosci. 2003;23(6):2502–2510. doi: 10.1523/JNEUROSCI.23-06-02502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staba RJ, Ard TD, Benison AM, Barth DS. Intracortical pathways mediate nonlinear fast oscillation (>200 Hz) interaction in rat barrel cortex. J Neurophysiol. 2004;93(5):2934–2939. doi: 10.1152/jn.01101.2004. [DOI] [PubMed] [Google Scholar]
- 24.Jones MS, MacDonald KD, Choi B, Dudek FE, Barth DS. Intracellular correlates of fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2000;84:1505–1518. doi: 10.1152/jn.2000.84.3.1505. [DOI] [PubMed] [Google Scholar]
- 25▪.Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40(2):127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. Focuses on fast ripples in a chronic experimental model of epilepsy and patients with epilepsy. [DOI] [PubMed] [Google Scholar]
- 26▪.Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45(9):1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. Evidence from this study supports the hypothesis that pathological high-frequency oscillations could be a biomarker of epileptogenesis. [DOI] [PubMed] [Google Scholar]
- 27.Engel J, Jr, Bragin A, Staba RJ, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50(4):598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 28▪.Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. Demonstrates that rates of fast ripples are strongly associated with human mesial temporal seizure onset zone. [DOI] [PubMed] [Google Scholar]
- 29.Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J., Jr Interictal high frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002;52(4):407–415. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- 30.Staba RJ, Frighetto L, Behnke EJ, et al. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48(11):2130–2138. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs J, Levan P, Chatillon CE, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132(Pt 4):1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪.Le Van Quyen M, Bragin A, Staba R, Crepon B, Wilson CL, Engel J., Jr Cell type-specific firing during ripple oscillations in the hippocampal formation of humans. J Neurosci. 2008;28(24):6104–6110. doi: 10.1523/JNEUROSCI.0437-08.2008. Describes the neuronal firing patterns of putative pyramidal cells and interneurons during human ripple oscillations during slow wave sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clemens Z, Molle M, Eross L, Barsi P, Halasz P, Born J. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain. 2007;130(11):2868–2878. doi: 10.1093/brain/awm146. [DOI] [PubMed] [Google Scholar]
- 34.Nir Y, Staba RJ, Andrillon T, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70(1):153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grenier F, Timofeev I, Steriade M. Neocortical very fast oscillations (ripples, 80–200 Hz) during seizures: intracellular correlates. J Neurophysiol. 2003;89(2):841–852. doi: 10.1152/jn.00420.2002. [DOI] [PubMed] [Google Scholar]
- 36.Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22(5):2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37▪.Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41(Suppl 6):S144–S152. doi: 10.1111/j.1528-1157.2000.tb01573.x. Proposes that fast ripples reflect hypersynchronous discharges arising from small clusters of neurons that are involved with epileptogenesis. [DOI] [PubMed] [Google Scholar]
- 38.Bragin A, Wilson CL, Engel J., Jr Voltage depth profiles of high-frequency oscillations after kainic acid-induced status epilepticus. Epilepsia. 2007;48(Suppl 5):35–40. doi: 10.1111/j.1528-1167.2007.01287.x. [DOI] [PubMed] [Google Scholar]
- 39.Bragin A, Benassi SK, Kheiri F, Engel J., Jr Further evidence that pathologic high-frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia. 2011;52(1):45–52. doi: 10.1111/j.1528-1167.2010.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dzhala VI, Staley KJ. Mechanisms of fast ripples in the hippocampus. J Neurosci. 2004;24(40):8896–8906. doi: 10.1523/JNEUROSCI.3112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron. 2007;55(6):930–941. doi: 10.1016/j.neuron.2007.07.040. Found that greater variability in neuronal spike firing was associated with higher spectral frequency high-frequency oscillations, whereas reducing spike-timing jitter was associated with lower frequency high-frequency oscillations. [DOI] [PubMed] [Google Scholar]
- 42▪.Ibarz JM, Foffani G, Cid E, Inostroza M, Menendez de la Prida L. Emergent dynamics of fast ripples in the epileptic hippocampus. J Neurosci. 2010;30(48):16249–16261. doi: 10.1523/JNEUROSCI.3357-10.2010. Using experimental data and computer simulations this study shows that the spectral frequency of fast ripples corresponds with the extent of in- or out-of-phase firing among discharging cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jefferys JG. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995;75(4):689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- 44.Jiruska P, Powell AD, Chang WC, Jefferys JG. Electrographic high-frequency activity and epilepsy. Epilepsy Res. 2010;89(1):60–65. doi: 10.1016/j.eplepsyres.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Köhling R, Staley K. Network mechanisms for fast ripple activity in epileptic tissue. Epilepsy Res. 2011 doi: 10.1016/j.eplepsyres.2011.03.006. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Draguhn A, Traub RD, Schmitz D, Jefferys JGR. Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature. 1998;394:189–192. doi: 10.1038/28184. [DOI] [PubMed] [Google Scholar]
- 47.Jiruska P, Csicsvari J, Powell AD, et al. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci. 2010;30(16):5690–5701. doi: 10.1523/JNEUROSCI.0535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Traub RD, Schmitz D, Jefferys JG, Draguhn A. High-frequency population oscillations are predicted to occur in hippocampal pyramidal neuronal networks interconnected by axoaxonal gap junctions. Neuroscience. 1999;92(2):407–426. doi: 10.1016/s0306-4522(98)00755-6. [DOI] [PubMed] [Google Scholar]
- 49.Traub RD, Whittington MA, Buhl EH, et al. A possible role for gap junctions in generation of very fast EEG oscillations preceding the onset of, and perhaps initiating, seizures. Epilepsia. 2001;42(2):153–170. doi: 10.1046/j.1528-1157.2001.26900.x. [DOI] [PubMed] [Google Scholar]
- 50.Bragin A, Wilson CL, Engel JJ. Spatial stability over time of brain areas generating fast ripples in the epileptic rat. Epilepsia. 2003;44(9):1233–1237. doi: 10.1046/j.1528-1157.2003.18503.x. [DOI] [PubMed] [Google Scholar]
- 51.Jacobs J, Zijlmans M, Zelmann R, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67(2):209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010;75(19):1686–1694. doi: 10.1212/WNL.0b013e3181fc27d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogren JA, Wilson CL, Bragin A, et al. Three dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Ann Neurol. 2009;66(6):783–791. doi: 10.1002/ana.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Gompel JJ, Stead SM, Giannini C, et al. Phase I trial: safety and feasibility of intracranial electroencephalography using hybrid subdural electrodes containing macro- and microelectrode arrays. Neurosurg Focus. 2008;25(3):E23. doi: 10.3171/FOC/2008/25/9/E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Worrell G, Gotman J. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: clinical studies. Biomarkers Med. 2011;5(5):557–566. doi: 10.2217/bmm.11.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130(9):2354–2366. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- 57.Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49(11):1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Worrell GA, Gardner AB, Stead SM, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131(4):928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schevon CA, Trevelyan AJ, Schroeder CE, Goodman RR, McKhann G, Jr, Emerson RG. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain. 2009;132(11):3047–3059. doi: 10.1093/brain/awp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr Clin Neurophysiol. 1974;37(5):538–548. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- 61.de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–567. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 62.Matsumoto H, Marsan CA. Cortical cellular phenomena in experimental epilepsy: interictal manifestations. Exp Neurol. 1964;9:286–304. doi: 10.1016/0014-4886(64)90025-1. [DOI] [PubMed] [Google Scholar]
- 63.Dichter M, Spencer WA. Penicillin-induced interictal discharges from the cat hippocampus. I Characteristics and topographical features. J Neurophysiol. 1969;32:649–662. doi: 10.1152/jn.1969.32.5.649. [DOI] [PubMed] [Google Scholar]
- 64.Prince DA, Futamachi KJ. Intracellular recordings from chronic epileptogenic foci in the monkey. Electroencephalogr Clin Neurophysiol. 1970;29(5):496–510. doi: 10.1016/0013-4694(70)90066-0. [DOI] [PubMed] [Google Scholar]
- 65.Mody I, Lambert JD, Heinemann U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol. 1987;57(3):869–888. doi: 10.1152/jn.1987.57.3.869. [DOI] [PubMed] [Google Scholar]
- 66.Haas HL, Jefferys JG. Low-calcium field burst discharges of CA1 pyramidal neurones in rat hippocampal slices. J Physiol. 1984;354:185–201. doi: 10.1113/jphysiol.1984.sp015371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rutecki PA, Lebeda FJ, Johnston D. Epileptiform activity induced by changes in extracellular potassium in hippocampus. J Neurophysiol. 1985;54(5):1363–1374. doi: 10.1152/jn.1985.54.5.1363. [DOI] [PubMed] [Google Scholar]
- 68.Ben-Ari Y, Tremblay E, Ottersen OP. Injections of kainic acid into the amygdaloid complex of the rat: an electrographic, clinical and histological study in relation to the pathology of epilepsy. Neuroscience. 1980;5(3):515–528. doi: 10.1016/0306-4522(80)90049-4. [DOI] [PubMed] [Google Scholar]
- 69.Lothman EW, Bertram EH, Bekenstein JW, Perlin JB. Self-sustaining limbic status epilepticus induced by ‘continuous’ hippocampal stimulation: electrographic and behavioral characteristics. Epilepsy Res. 1989;3(2):107–119. doi: 10.1016/0920-1211(89)90038-7. [DOI] [PubMed] [Google Scholar]
- 70.Prince DA, Wong RK. Human epileptic neurons studied in vitro. Brain Res. 1981;210(1–2):323–333. doi: 10.1016/0006-8993(81)90905-7. [DOI] [PubMed] [Google Scholar]
- 71.Schwartzkroin PA, Haglund MM. Spontaneous rhythmic synchronous activity in epileptic human and normal monkey temporal lobe. Epilepsia. 1986;27(5):523–533. doi: 10.1111/j.1528-1157.1986.tb03578.x. [DOI] [PubMed] [Google Scholar]
- 72.Hwa GG, Avoli M, Oliver A, Villemure JG. Bicuculline-induced epileptogenesis in the human neocortex maintained in vitro. Exp Brain Res. 1991;83(2):329–339. doi: 10.1007/BF00231156. [DOI] [PubMed] [Google Scholar]
- 73.Schwartzkroin PA, Prince DA. Effects of TEA on hippocampal neurons. Brain Res. 1980;185(1):169–181. doi: 10.1016/0006-8993(80)90680-0. [DOI] [PubMed] [Google Scholar]
- 74.Prince DA. The depolarization shift in ‘epileptic’ neurons. Exp Neurol. 1968;21(4):467–185. doi: 10.1016/0014-4886(68)90066-6. [DOI] [PubMed] [Google Scholar]
- 75.Matsumoto H, Ayala GF, Gumnit RJ. Neuronal behavior and triggering mechanism in cortical epileptic focus. J Neurophysiol. 1969;32(5):688–703. doi: 10.1152/jn.1969.32.5.688. [DOI] [PubMed] [Google Scholar]
- 76.Dichter M, Spencer WA. Penicillin-induced interictal discharges from the cat hippocampus. II Mechanisms underlying origin and restriction. J Neurophysiol. 1969;32(5):663–687. doi: 10.1152/jn.1969.32.5.663. [DOI] [PubMed] [Google Scholar]
- 77.Johnston D, Brown TH. Giant synaptic potential hypothesis for epileptiform activity. Science. 1981;211(4479):294–297. doi: 10.1126/science.7444469. [DOI] [PubMed] [Google Scholar]
- 78.Johnston D, Brown TH. The synaptic nature of the paroxysmal depolarizing shift in hippocampal neurons. Ann Neurol. 1984;16(Suppl):S65–S71. doi: 10.1002/ana.410160711. [DOI] [PubMed] [Google Scholar]
- 79.Dichter MA, Ayala GF. Cellular mechanisms of epilepsy: a status report. Science. 1987;237(4811):157–164. doi: 10.1126/science.3037700. [DOI] [PubMed] [Google Scholar]
- 80.Schwartzkroin PA. Characteristics of CA1 neurons recorded intracellularly in the hippocampal in vitro slice preparation. Brain Res. 1975;85(3):423–436. doi: 10.1016/0006-8993(75)90817-3. [DOI] [PubMed] [Google Scholar]
- 81.Wong RK, Prince DA. Participation of calcium spikes during intrinsic burst firing in hippocampal neurons. Brain Res. 1978;159(2):385–390. doi: 10.1016/0006-8993(78)90544-9. [DOI] [PubMed] [Google Scholar]
- 82.Connors BW, Gutnick MJ, Prince DA. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- 83.Stafstrom CE, Schwindt PC, Chubb MC, Crill WE. Properties of persistent sodium conductance and calcium conductance of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1985;53(1):153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- 84.Azouz R, Jensen MS, Yaari Y. Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol. 1996;492(Pt 1):211–223. doi: 10.1113/jphysiol.1996.sp021302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen S, Su H, Yue C, et al. An increase in persistent sodium current contributes to intrinsic neuronal bursting after status epilepticus. J Neurophysiol. 2011;105(1):117–129. doi: 10.1152/jn.00184.2010. [DOI] [PubMed] [Google Scholar]
- 86.Sanabria ER, Su H, Yaari Y. Initiation of network bursts by Ca2+-dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J Physiol. 2001;532(Pt 1):205–216. doi: 10.1111/j.1469-7793.2001.0205g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87▪.Su H, Sochivko D, Becker A, et al. Upregulation of a T-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J Neurosci. 2002;22(9):3645–3655. doi: 10.1523/JNEUROSCI.22-09-03645.2002. Results from this study suggest that status epilepticus could convert regular firing pyramidal cells to cells with intrinsic burst firing properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88▪.Kang N, Xu J, Xu Q, Nedergaard M, Kang J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;94(6):4121–4130. doi: 10.1152/jn.00448.2005. Provides evidence for astrocytic glutamate release contributing to the generation of epileptiform discharges. [DOI] [PubMed] [Google Scholar]
- 89.MacVicar BA, Dudek FE. Local synaptic circuits in rat hippocampus: interactions between pyramidal cells. Brain Res. 1980;184(1):220–223. doi: 10.1016/0006-8993(80)90602-2. [DOI] [PubMed] [Google Scholar]
- 90.Traub RD, Wong RK. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982;216(4547):745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- 91.Miles R, Wong RKS. Single neurones can initiate synchronized population discharge in the hippocampus. Nature. 1983;306:371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- 92.Jefferys JG, Haas HL. Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature. 1982;300(5891):448–450. doi: 10.1038/300448a0. [DOI] [PubMed] [Google Scholar]
- 93.Taylor CP, Dudek FE. Synchronous neural afterdischarges in rat hippocampal slices without active chemical synapses. Science. 1982;218(4574):810–812. doi: 10.1126/science.7134978. [DOI] [PubMed] [Google Scholar]
- 94.MacVicar BA, Dudek FE. Electrotonic coupling between pyramidal cells: a direct demonstration in rat hippocampal slices. Science. 1981;213(4509):782–785. doi: 10.1126/science.6266013. [DOI] [PubMed] [Google Scholar]
- 95.Schmitz D, Schuchmann S, Fisahn A, et al. Axo-axonal coupling. A novel mechanism for ultrafast neuronal communication. Neuron. 2001;31(5):831–840. doi: 10.1016/s0896-6273(01)00410-x. [DOI] [PubMed] [Google Scholar]
- 96.Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res. 2003;28(11):1649–1658. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- 97.Sloviter RS, Zappone CA, Harvey BD, Frotscher M. Kainic acid-induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: possible anatomical substrate of granule cell hyper-inhibition in chronically epileptic rats. J Comp Neurol. 2006;494(6):944–960. doi: 10.1002/cne.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilson CL, Khan SU, Engel J, Jr, Isokawa M, Babb TL, Behnke EJ. Paired pulse suppression and facilitation in human epileptogenic hippocampal formation. Epilepsy Res. 1998;31:211–230. doi: 10.1016/s0920-1211(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 99.Isokawa-Akesson M, Wilson CL, Babb TL. Inhibition in synchronously firing human hippocampal neurons. Epilepsy Res. 1989;3:236–247. doi: 10.1016/0920-1211(89)90030-2. [DOI] [PubMed] [Google Scholar]
- 100.Gregory RP, Oates T, Merry RT. Electroencephalogram epileptiform abnormalities in candidates for aircrew training. Electroencephalogr Clin Neurophysiol. 1993;86(1):75–77. doi: 10.1016/0013-4694(93)90069-8. [DOI] [PubMed] [Google Scholar]
- 101.Okubo Y, Matsuura M, Asai T, et al. A follow-up study of healthy children with epileptiform EEG discharges. J Epilepsy. 1993;6(4):250–256. [Google Scholar]
- 102.Sam MC, So EL. Significance of epileptiform discharges in patients without epilepsy in the community. Epilepsia. 2001;42(10):1273–1278. doi: 10.1046/j.1528-1157.2001.17101.x. [DOI] [PubMed] [Google Scholar]
- 103.Gotman J, Marciani MG. Electroencephalographic spiking activity, drug levels, and seizure occurrence in epileptic patients. Ann Neurol. 1985;17(6):597–603. doi: 10.1002/ana.410170612. [DOI] [PubMed] [Google Scholar]
- 104.Gotman J. Relationships between interictal spiking and seizures: human and experimental evidence. Can J Neurol Sci. 1991;18(4 Suppl):573–576. doi: 10.1017/s031716710003273x. [DOI] [PubMed] [Google Scholar]
- 105▪.Barbarosie M, Louvel J, Kurcewicz I, Avoli M. CA3-released entorhinal seizures disclose dentate gyrus epileptogenicity and unmask a temporoammonic pathway. J Neurophysiol. 2000;83(3):1115–1124. doi: 10.1152/jn.2000.83.3.1115. Results from this in vitro study suggest that interictal spikes from area CA3 of hippocampus could suppress ictal discharges arising from entorhinal cortex. [DOI] [PubMed] [Google Scholar]
- 106.Engel J, Jr, Ackermann RF. Interictal EEG spikes correlate with decreased, rather than increased, epileptogenicity in amygdaloid kindled rats. Brain Res. 1980;190(2):543–548. doi: 10.1016/0006-8993(80)90296-6. [DOI] [PubMed] [Google Scholar]
- 107.Librizzi L, de Curtis M. Epileptiform ictal discharges are prevented by periodic interictal spiking in the olfactory cortex. Ann Neurol. 2003;53(3):382–389. doi: 10.1002/ana.10471. [DOI] [PubMed] [Google Scholar]
- 108.Avoli M, Biagini G, de Curtis M. Do interictal spikes sustain seizures and epileptogenesis? Epilepsy Curr. 2006;6(6):203–207. doi: 10.1111/j.1535-7511.2006.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Urrestarazu E, Jirsch JD, Le Van P, et al. High-frequency intracerebral EEG activity (100–500 Hz) following interictal spikes. Epilepsia. 2006;47(9):1465–1476. doi: 10.1111/j.1528-1167.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 110.Bragin A, Engel J, Jr, Staba RJ. High-frequency oscillations in epileptic brain. Curr Opin Neurol. 2010;23(2):151–156. doi: 10.1097/WCO.0b013e3283373ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111▪.Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11(4):272–276. doi: 10.1177/1073858405278239. Proposes that interictal spikes could guide abnormal circuit reorganization in networks that initiate spontaneous seizures. [DOI] [PubMed] [Google Scholar]
- 112.Staley KJ, Dudek FE. Interictal spikes and epileptogenesis. Epilepsy Curr. 2006;6(6):199–202. doi: 10.1111/j.1535-7511.2006.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hellier JL, Patrylo PR, Dou P, Nett M, Rose GM, Dudek FE. Assessment of inhibition and epileptiform activity in the septal dentate gyrus of freely behaving rats during the first week after kainate treatment. J Neurosci. 1999;19(22):10053–10064. doi: 10.1523/JNEUROSCI.19-22-10053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114▪.White A, Williams PA, Hellier JL, Clark S, Edward Dudek F, Staley KJ. EEG spike activity precedes epilepsy after kainate-induced status epilepticus. Epilepsia. 2010;51(3):371–383. doi: 10.1111/j.1528-1167.2009.02339.x. Evidence from this study supports the hypothesis that interictal spike could be a biomarker of epileptogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Toth Z, Hollrigel GS, Gorcs T, Soltesz I. Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. J Neurosci. 1997;17(21):8106–8117. doi: 10.1523/JNEUROSCI.17-21-08106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 117.Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (<1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13(8):3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Amzica F, Steriade M. Disconnection of intracortical synaptic linkages disrupts synchronization of a slow oscillation. J Neurosci. 1995;15(6):4658–4677. doi: 10.1523/JNEUROSCI.15-06-04658.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62(2):143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 120.Knutson KL. Impact of sleep and sleep loss on glucose homeostasis and appetite regulation. Sleep Med Clin. 2007;2(2):187–197. doi: 10.1016/j.jsmc.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.He de F, Ma DL, Tang YC, Engel J, Jr, Bragin A, Tang FR. Morpho-physiologic characteristics of dorsal subicular network in mice after pilocarpine-induced status epilepticus. Brain Pathol. 2010;20(1):80–95. doi: 10.1111/j.1750-3639.2009.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kobayashi K, Watanabe Y, Inoue T, Oka M, Yoshinaga H, Ohtsuka Y. Scalp-recorded high-frequency oscillations in childhood sleep-induced electrical status epilepticus. Epilepsia. 2010;51(10):2190–2194. doi: 10.1111/j.1528-1167.2010.02565.x. [DOI] [PubMed] [Google Scholar]
- 123.Andrade-Valenca LP, Dubeau F, Mari F, Zelmann R, Gotman J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology. 2011;77(6):524–531. doi: 10.1212/WNL.0b013e318228bee2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kobayashi K, Yoshinaga H, Toda Y, Inoue T, Oka M, Ohtsuka Y. High-frequency oscillations in idiopathic partial epilepsy of childhood. Epilepsia. 2011 doi: 10.1111/j.1528-1167.2011.03169.x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]