Abstract
The intestine has an unenviable task: to identify and respond to a constant barrage of environmental stimuli that can be both dangerous and beneficial. The proper execution of this task is central to the homeostasis of the host, and as a result the gastrointestinal tract contains more lymphocytes than any other tissue compartment in the body, as well as unique antigen presenting cells with specialized functions. When antigen is initially encountered through the gut, this system generates a robust T-cell mediated hyporesponsiveness called oral tolerance. Although seminal observations of oral tolerance were made a century ago, the relevant mechanisms are only beginning to be unraveled with the use of modern investigational techniques. Food allergy is among the clinical disorders that occur from a failure of this system, and therapies that seek to reestablish tolerance are currently under investigation.
Keywords: oral tolerance, T regulatory cell, Foxp3, dendritic cell, food allergy, microbiota, vitamin A, TGF-β, CD103
INTRODUCTION
Food allergy, which is hypothesized to result from a defect in oral tolerance, is a common, serious, and growing problem in developed countries. Patients with food allergy develop pathological immune responses to ingested food antigens and can rapidly experience harmful adverse symptoms upon re-exposure. Although a recent meta-analysis identified variation in prevalence rates (1), recent survey data from the Centers for Disease Control and Prevention indicate that the current prevalence of food allergy in U.S. children is approximately 4%, an increase of almost 20% in the last decade (2). Increases in food allergy prevalence have also been observed in methodologically rigorous birth cohort studies which use precise sampling techniques and well-defined outcome measures, suggesting that rising prevalence is not simply due to self-diagnosis or increased recognition of the disorder (3). Similar trends in the prevalence of asthma, allergic rhinitis, and atopic dermatitis support the general concept that atopic diseases are increasingly common (4).
Interestingly, the likelihood that spontaneous clinical tolerance will develop in food allergic individuals varies depending upon the allergen. In general, resolution of allergy to egg, milk, wheat, and soy can be expected, although sensitivity may persist into the second decade of life, longer than previously appreciated. In contrast, most patients allergic to peanut, tree nuts, and seafood will not outgrow their disease and must maintain strict elimination diets. The natural history of allergy to other important proteins such as sesame and mustard is largely unknown. In addition, current diagnostic testing for food allergy cannot predict a patient’s risk for anaphylaxis, or determine an individual’s threshold dose to trigger symptoms. Therefore, affected individuals and families must be constantly vigilant to avoid inadvertent exposure; however, even in the most cautious patients accidental ingestions frequently occur (5). The inability to completely eliminate the possibility of anaphylaxis and the associated limitations in everyday activities are great sources of uncertainty and stress on affected families. Over time, health-related quality of life can be adversely affected; in some families, the degree of impairment is similar to other serious chronic diseases of childhood such as type 1 diabetes or ventilator-dependent chronic respiratory failure (6). Although the impact of food allergies is substantial, their prevalence is remarkably low considering the complexities of the mucosal immune system.
The gastrointestinal tract, which is the largest immunologic organ in the body, is constantly exposed to an enormous array of exogenous antigens including commensal bacteria and ingested proteins (7). A single epithelial layer separates this antigenic load from the lymphocytes, antigen presenting cells (APC), stromal cells and other immune cells in the lamina propria that together comprise the mucosal-associated lymphoid tissue (MALT). Within the MALT, unique populations of dendritic cells (DCs) interact with dietary antigens, and determine the fate of the resulting adaptive response, i.e. immunity versus tolerance (8). In this context, immune tolerance is defined as the antigen-specific suppression of cellular or humoral immune responses. When the initial antigen exposure is mediated through the GI tract, a robust T cell-mediated suppression develops called oral tolerance (9).
This review will focus on the relationship between the component parts of the complex gastrointestinal mucosal immune system and IgE-mediated food allergy. Because most of the evidence with regard to immune tolerance to dietary antigens is derived from experimental animals, we will primarily discuss these model systems. Where possible, we will review the evidence for similar phenomena in human biology and the relevant applications for clinical medicine.
PHYSICAL FACTORS OF DIETARY ANTIGENS
Following ingestion, dietary proteins undergo digestion by enzymes in the saliva and stomach as well as by gastric acid. This processing results in reduced protein immunogenicity, likely by the destruction of conformational epitopes. However, specific biochemical properties common amongst different food groups confer resistance to this physical and chemical degradation, which collectively maintain the allergenicity of these proteins upon reaching the small intestine (Table 1). Additional factors that disrupt normal digestion such as co-administration of antacids have been shown in animal models to result in a breakdown in oral tolerance induction (10). Other features of mucosal defense include a hydrophobic layer of mucin oligosaccharides which trap antigen, and both constitutive (i.e., human beta-defensin 1 (hBD-1) and inducible (LL-37, hBD-2 and 3) antimicrobial peptides. Secretory IgA has generally been considered to provide important tolerogenic function by binding to luminal antigens and preventing absorption (i.e., “immune exclusion”), although its specific importance has been controversial (11). A recent study showed that mice deficient in the receptor which secretes IgA and IgM into the intestinal lumen are hypersensitive to IgG-mediated anaphylaxis; nonetheless they can be tolerized by an oral feed prior to systemic priming (12). In this model, tolerance was transferrable by CD4+CD25+ splenocytes, suggesting that cellular mechanisms can compensate for an impaired immune exclusion mechanism. However, a recent case-control study from a larger placebo-controlled trial examining probiotics for allergy prevention in high risk infants showed that the risk of atopy was inversely correlated with fecal IgA levels (13). These data serve as one example of the complex and complementary forces which act to suppress immunity in the gut.
Table 1.
Biochemical Factors Which Promote Allergenicity
| Molecular Weight < 70 kD |
| Glycosylation |
| Resistance to thermal or chemical denaturation |
| Abundance in food source |
| Linear epitopes |
| Solubility in water |
Gastrointestinal epithelial dysfunction has been thought to contribute to food hypersensitivity through both increased sensitization due to a leaky barrier and the heightened T-helper-2 (Th2) effector response that ensues. Epithelial junction complexes, also called adherens junctions, and tight junctions provide structural integrity to the gut barrier (14). However, this barrier is not completely formed at birth, and although not well understood, structural integrity may take time to fully develop. (7). In mice, the permeability of this barrier is further influenced by exposures to microbial pathogens such as viruses, alcohol, NSAIDs, and other toxins, as well as cytokines such as IL-9, immune cells and apoptotic pathways. These environmental exposures ultimately result in changes in gene expression and phosphorylation of tight junction proteins such as occludins, claudins, and JAM-ZO1 proteins, which in turn are associated with changes in intestinal mast cells and allergic sensitization (15,16).
Interestingly, intestinal permeability was assessed in food-allergic infants by calculating the urinary ratio after ingestion of freely diffusible lactulose and normally unabsorbed mannitol (17).. Food-allergic infants were noted to have a lower ratio, indicating increased intestinal permeability, when compared with normal healthy young children. Investigators examined this ratio in children who had been on an allergen-free diet for at least 6 months and determined that intestinal permeability remained increased in food-allergic children, despite the absence of food allergen stimulation. Further evidence linking GI epithelial barrier dysfunction and food allergy comes from studies in immunosuppressed patients after solid organ transplant who developed food allergy while on systemic calcineurin inhibitors; initially, investigators assumed this allergy was the result of transfer of sensitized donor lymphocytes. However, it is now theorized that medication-induced decreases in cellular ATP levels altered the integrity of junctional complexes, resulting in increased intestinal permeability (18). Mutations in the gene encoding filaggrin also lead to profound epidermal barrier dysfunction and are highly prevalent in atopic dermatitis patients, which is in turn associated with an increased prevalence of food allergy. Acquired barrier defects associated with decreased filaggrin expression have been observed in the esophagus of patients with eosinophilic esophagitis, and are thought to be secondary to IL-13 (19). However, no studies to date have examined the mechanistic relationship of filaggrin mutations to IgE priming in the gut or clinical food allergy (20).
Increasing evidence suggests that the mucosal epithelium is likely to be more involved in tolerance than simply by acting as a physical barrier. Epithelial cells are known to express MHC class II molecules on their basolateral membranes and thus may act as nonprofessional APCs which do not express conventional costimulatory molecules, favoring anergy (21). In addition, factors derived from the gut epithelium are generally believed to condition the DCs in the stroma, dampening immune responses and promoting gut homeostasis (22). One such factor, constitutively expressed by the gut epithelium, is thymic stromal lymphopoetin (TSLP). TSLP is an IL-7 like cytokine that has been shown to activate expression of OX40L on dendritic cells and drive Th2 differentiation. Thus, TSLP is a critical mediator of allergic inflammation in the lung and skin. By contrast, in the gut TSLP appears to play a regulatory role, limiting deleterious Th1 and Th17 inflammation in models of helminth infection and colitis (23). Although incompletely understood, this regulation may occur at the level of the DC, which expresses the TSLP receptor and has been shown to develop tolerogenic properties after TSLP exposure. Interestingly, regulatory responses to dietary allergens are evidently normal in TSLP receptor-deficient animals (24). These findings suggest that though allergic sensitization in the gut may be mediated or regulated via TSLP, it is not required for oral tolerance. Little is known about the role of TSLP in food sensitization in humans. However, a recent study identified TSLP gene expression in the esophagus of patients with eosinophilic esophagitis and showed that genetic variants in TSLP and the TSLP receptor are associated with the disease (25).
THE IMPORTANCE OF ANTIGEN DOSE & TIMING
Mucosal responses to soluble protein antigens early in life tend to be Th2 biased, which has led to the general idea that this response occurs by default in both animals and humans (26). Genetics plays a clear role in mouse models, in which certain strains have exaggerated Th2 bias whereas others tend to be resistant to sensitization (27). More recent evidence supports that impairment in regulatory T cell (T reg) induction and innate immunity may also contribute to Th2 polarization in early life (28). Although these findings might be expected in atopic infants, prospective birth cohort studies have shown that IgE production to egg, milk, and peanut commonly occurs, even in healthy infants (29). In non-allergic individuals, this Th2 bias appears to be transient, and IgE levels fall, possibly through a counterbalancing induction of antigen-specific Th1 responses (i.e. IFN-γ); in contrast, these Th2 responses consolidate and strengthen in allergic children, perhaps through induction of IL-4 signaling (30).
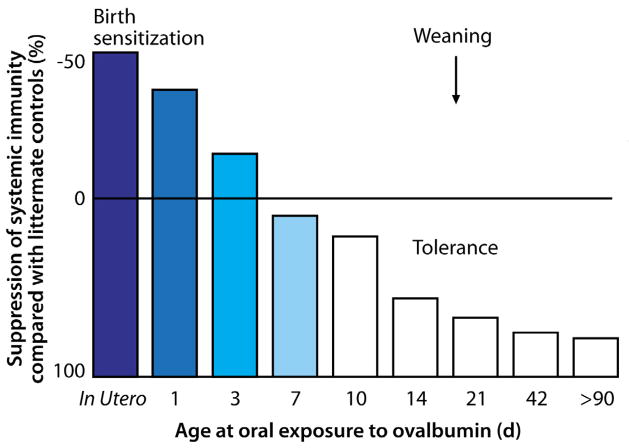
In mouse models, high-dose exposure to antigen in early life, even a single isolated dose, can produce lymphocyte anergy, whereas low-dose exposure, especially when repeated, induces T reg development (31). Interestingly though, the differences in the actual dosing in these studies is quite small. Emerging evidence in human disease suggests that exposure to the proper dose of antigen during this critical period in early life is important for the shaping of the appropriate immune response to foods. Several epidemiologic studies have implicated delayed introduction in the increased prevalence of peanut allergy (32,33). Similarly there is evidence that delayed introduction of cereals is associated with a higher risk of wheat allergy (34), although methodologic limitations in retrospective studies make definitive conclusions difficult. Recently, European and American guidelines for the introduction of potentially allergenic solid foods were revised to reflect the position that insufficient high-quality evidence exists to support delayed weaning as a preventative (i.e., tolerogenic) strategy (35,36). However, early introduction is not automatically better, since mature immune regulation may require time. In a set of classic experiments, Strobel showed that immunologic priming is enhanced in neonatal mouse pups fed antigen in the first few days of life, whereas tolerance develops only after waiting 7–10 days to introduce antigen (37) (Figure 1); if and how this “window” of priming versus tolerance translates to humans is unknown. Cow’s milk is typically the first potentially allergenic exposure, often occurring early in life, and yet cow’s milk is by far the most common food to which children are allergic (31). Although oral tolerance has been shown to occur across a range of doses, frequent or continuous exposure to relatively low doses typically results in robust oral tolerance induction. Defining the most appropriate time and dose for tolerance induction in humans is a great research need. Interventional studies are underway to investigate the importance of early life oral exposure in tolerance development.
Figure 1. Immunity, not tolerance, occurs after allergen exposure in early life.
Neonatal mouse pups were exposed to ovalbumin (OVA) via intra-amniotic injection 24–36 hours prior to birth or fed OVA (1 mg/gram body weight) or saline at day 1, 3, 7, 14, or 42. When rechallenged, animals exposed to OVA prior to the seventh day of life did not develop tolerance but instead robust humoral and cell-mediated immune responses, which persisted up to 14 weeks. Although it had long been known that tolerance was the default response to oral antigen administration in adult mice, these experiments demonstrated that oral exposure in early life could result in active immunologic priming rather than suppression.
THE MICROBIOME AND ORAL TOLERANCE
Another critical influence on the gastrointestinal mucosal immune response is the microbial stimulation provided by the enteric flora, which by adulthood number approximately 100 trillion in the large intestine, providing essential nutritional and immunologic benefits (38). Previous studies of germ-free mice demonstrated that colonization of the GI tract is required for proper organization and development of both mucosal and secondary lymphoid tissues. These animals have impaired antibody responses and do not develop oral tolerance (39). Thus, the interaction beginning at birth between the intestinal microbiome and the MALT probably represents the primary stimulus for proper postnatal immune development. There is increasing evidence that signals from innate immune receptors such as Toll-like receptors (TLRs) play an important role in intestinal homeostasis (40), in the genesis of T regs (41), and on the outcome of allergic disease (42). Investigators have identified specific microbial products (i.e. polysaccharide A from Bacteroides fragilis) that interact with TLRs and promote downstream induction of T regs, which subsequently modulate intestinal inflammation in mouse models of experimental colitis (41). However, determining the specific microbial signal(s) that are most critical in development of tolerogenic versus allergic outcomes has proven challenging, as evidenced by the overall disappointing results of probiotic trials on prevention and treatment of allergic disease (43). This difficulty may be largely technical, because historically most studies have relied on culture-based methods to attempt to identify an incredibly diverse ecosystem, of which many members are fastidious or simply unculturable. New deep-sequencing technologies which focus on the unique 16s ribosomal subunit of bacterial RNA allow investigators to identify previously unknown organisms and promise to revolutionize our understanding of these critical microbes (44). Coupling 16s rRNA sequencing techniques with high-throughput approaches allows profiling of entire bacterial communities, forming the basis of the Human Microbiome Project to characterize the entire flora (http://nihroadmap.nih.gov/hmp/). Using traditional methods, it has already been shown that allergic children are less frequently colonized with enterococci and bifidobacteria during the first year of life than are healthy children. (45). The HMP will very likely shed important new light on this critical influence of mucosal immune responses.
THE FUNDAMENTAL IMPORTANCE OF ANTIGEN-PRESENTING CELLS
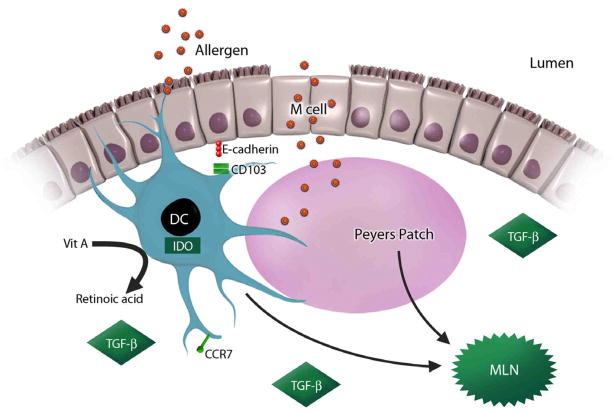
A complex interplay of the aforementioned physical factors, antigen character, dose, and timing, and the adjuvant effects of innate immune stimulation (i.e. microbiota, microbial toxins, etc.) ultimately shape the development of oral tolerance through the same final common pathway: by directly or indirectly influencing the APC. Much recent research has begun to demonstrate that mucosal DCs are probably the most critical determinant of allergic sensitization versus tolerance in naïve individuals. These DCs can encounter ingested antigen in one of three ways: by extending dendrites through the paracellular space between epithelial cells to sample luminal contents, by directly interacting with the epithelial cells, and by taking up antigen in the Peyer’s patch (PP), specialized lymphoid tissue which is immediately adjacent to microfold (M) cells (46). The properties of the antigen itself - particulate versus soluble - to a certain extent determine the route of exposure, and it is likely that each route will involve distinct DC populations. The relative contributions of each of these DC populations to oral tolerance is incompletely understood but beginning to be unraveled, and the CCR7-dependent migration of CD103+ CD11c+ DCs to the mesenteric lymph node (MLN) appears to be a critical step (47).
Although multiple mechanisms of tolerance are likely, and may include anergy or deletion of T cells, substantial evidence links oral tolerance to the ability of mucosal DCs to induce Foxp3+ regulatory T cells in the MLN (48). The integrin CD103, as well as retinoic acid (RA), indoleamine-2,3-dioxygenase (IDO), B7 family costimulatory molecules, and transforming growth factor-beta (TGF-β) all appear to act on the DC to positively influence this conversion (49–53). By contrast, lamina propria DCs that do not express CD103 are strongly pro-inflammatory. These data suggest that tolerogenic DCs may receive site-specific signals from the intestinal epithelium through interactions with E-cadherin, a ligand of CD103; it is likely that spatial conditioning by the mucosal epithelium provides a unique microenvironment which orchestrates antigen presentation towards appropriate inflammatory versus tolerogenic outcomes in the MLN(54)(Figure 2). Exposure of mucosal DCs to RA also has been shown to induce expression of the α4β7 integrin and CCR9 on lymphocytes, which provide them signals to recirculate back to the MALT (55–57). Importantly, studies of humans have observed similar RA-associated phenotypic and functional features of CD103+ MLN DCs, implicating them as potential therapeutic targets for the regulation of intestinal immune responses (58). In addition to its role in peripheral conversion of Foxp3+ T regs, RA can also condition DCs to induce T-cell- independent IgA production (57,59) in the gut of mice and humans, which may be a complementary mechanism in hyporesponsiveness to dietary proteins. Taken together, these data confirm that retinoic acid, acting in concert with TGF-β, powerfully modulates tolerogenic intestinal immune responses by participating in the peripheral conversion of inducible Foxp3+ regulatory T cells in the MLN, imprinting lymphocytes with gut-homing signatures, and stimulating IgA production (60–62). Interestingly, dietary vitamin A is the sole source of RA, since it cannot be synthesized by humans or animals; thus the effect of RA on the intestinal immune response represents a key adaptation to local environmental factors.
Figure 2. Microenvironmental signals initiate tolerogenic dendritic cell responses.
Oral tolerance depends on CD103+ mucosal dendritic cells encountering luminal antigens and transporting them to the mesenteric lymph nodes. This process requires CCR7 and is positively influenced by local factors such as retinoic acid, TGF-β, and IDO. CD103+ dendritic cells conditioned by these stimuli then interact with cognate T cells to actively suppress immune reactivity.
APCs other than conventional DCs may also participate in oral tolerance induction. For example, intestinal macrophages can also efficiently induce Foxp3+ T regs in an IL-10, RA, and TGF-β-dependent fashion (63). Plasmacytoid DCs (pDC), a specialized DC subset known for the ability to produce vast quantities of type I interferons, can also activate inducible Foxp3+ as well as IL-10+ Tr1 regulatory T cells in both mice and humans under various conditions (64–67). pDCs in the murine liver, which account for almost 30% of the CD11c+ DC population there, can induce oral tolerance to fed protein and hapten by inducing anergy or deletion of 70–80% of antigen-specific CD4+ and CD8+ T cells in addition to generating CD4+CD25+ T regs (68,69). This suggests that oral tolerance may be operative via multiple mechanisms in multiple tissue compartments. Hepatic tolerance was studied recently in humans by isolating liver DCs from surgical specimens. Compared to peripheral blood-derived DCs, liver DCs produced vastly greater amounts of IL-10, greater suppression of T-cell responses in mixed lymphocyte culture, and the exclusive IL-10 dependent generation of CD4+CD25+FoxP3+ regulatory T cells from naïve precursors (70).
T CELLS: PRIMARY EFFECTORS OF ORAL TOLERANCE
Integration of environmental information by DCs results in specific activation and differentiation of T cell subsets, including the Foxp3+ T reg, which we will consider first, as the primary effector of oral tolerance. However, other mechanisms that affect T cell activity also influence tolerance, such as anergy and deletion. In addition, although most work has focused on T cells expressing the alpha/beta T cell receptor and CD4, there appears to be at least some role for other types of T cells in tolerance models, such as CD8+ T cells, gamma/delta T cells, and perhaps natural killer T (NKT) cells (9,71).
Repeated exposures to low doses of antigen are thought to be the optimal stimulus for the development of T regs, which suppress immune responses through soluble or cell-bound regulatory cytokines, such as IL-10 and TGF-β (7). These antigen-specific regulatory cells migrate to lymphoid organs, where they inhibit the generation of effector cells, and to target organs, where they release cytokines that are capable of nonspecific suppression (9). Natural CD4+CD25+ regulatory T cells (nTreg) develop in the thymus and express the specific transcription factor Foxp3, which confers regulatory function to these cells to block both TH1 and TH2 responses. nTreg mediate suppression through cell surface–bound TGF-β, although suppression can possibly occur independently of TGF-β (72).
Inducible regulatory T cells (iTreg) are CD4+ cells which can differentiate from naïve precursors, acquiring regulatory properties in the periphery after exposure to antigen. In many cases these cells acquire the expression of Foxp3 and they exist in at least two forms, distinguished by the anti-inflammatory cytokines produced: IL-10 (Tr1 cells) and TGF-β (Th3 cells). Whereas nTregs are thought to primarily govern peripheral tolerance to self antigens, iTregs are more likely responsible for tolerance to exogenous substances like allergens. A series of elegant experiments confirmed that Foxp3+ iTregs are required to establish mucosal tolerance to inhaled allergen in mice (73). Induction of iTregs was also recently shown to convey tolerance in a food allergy model. In this system, the lectin SIGNR1 expressed by lamina propria DCs recognized glycosylated residues on fed antigen, leading to IL-10 production and the generation of functional IL-10+ Tr1 cells and oral tolerance (74). In humans, Tr1 cells are known to develop after frequent exposure to allergen and are associated with the development of clinical tolerance (75).
It is likely that defects in regulatory T cell activity contribute to the development of food allergy. Whereas in mice, substantial data link T regs to mucosal allergen tolerance (72), in humans the evidence is limited and largely indirect. The importance of T regs in human intestinal function is primarily demonstrated in IPEX syndrome patients, in whom severe enteropathy and food allergy are part of a fatal immunoregulatory disorder caused by FOXP3 mutations (76). A deletion in the noncoding region of FOXP3 that impairs mRNA splicing has been associated with a milder IPEX variant that consists of severe enteropathy, atopic dermatitis, and food allergies (77). Probably the most widely cited study of more typical food allergy patients examined children with non-IgE-mediated cow’s milk intolerance, in which clinical tolerance usually develops within a year or two of diagnosis (78). Compared with children who maintain clinically active intolerance, the development of tolerance is associated with higher numbers of circulating CD4+CD25+ regulatory T cells and decreased in vitro proliferative responses to bovine β-lactoglobin in peripheral blood mononuclear cells (PBMCs); depletion of CD25+ cells from the PBMCs of tolerant children led to a 5-fold increase in proliferation against β-lactoglobin in vitro. However, the relevance of these findings to IgE-mediated cow’s milk allergy is not known. In another in vitro study of milk-allergic children, lymphocytes were cultured from the duodenal mucosa in the presence of milk. These cells displayed a typical Th2 cytokine profile when restimulated, but only very low amounts of TGF-β and IL-10, suggesting a defect in iTreg function (79). In a seminal study, Shreffler et al provided substantial evidence that T regs are involved in clinical tolerance to food allergens by examining the cells from milk-allergic, heated-milk tolerant (i.e., intermediate phenotype), formerly milk-allergic, and healthy control children, all of whom had normal total percentages of polyclonal T regs. However, whereas control subjects had low numbers of milk-specific T regs, there was a dose-dependent increase in the numbers of milk-specific T regs from allergic to heated milk-tolerant to fully tolerant children. Furthermore, these cells, which had a phenotype of CD4+ CD25hi CD45RO+ CD27+ CTLA-4+ CD127−, were indirectly shown to have functional suppressive capacity (80).
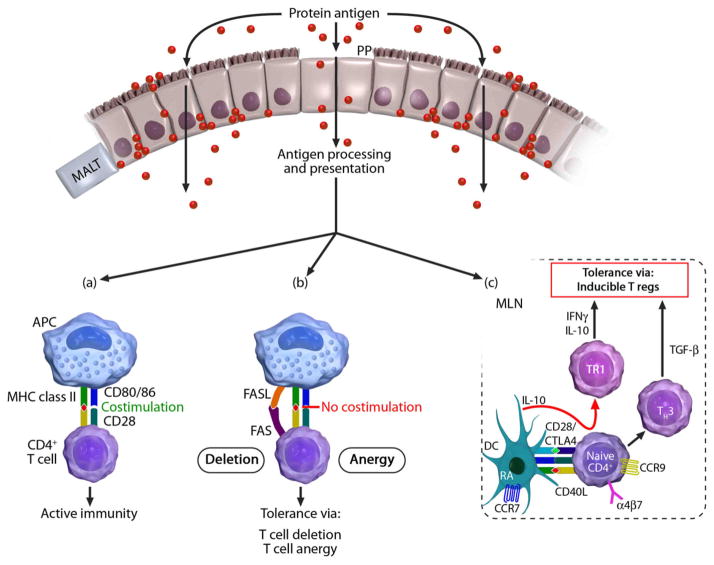
In contrast to the low-dose exposures that induce T regs, high-dose tolerance is mediated by lymphocyte anergy or clonal deletion (31) (Figure 3). Anergy can occur through T cell receptor ligation in the absence of costimulatory signals provided by soluble cytokines, such as IL-2, or by interactions between receptors on T cells (CD28) and counter-receptors on APCs (CD80, CD86, and CTLA-4) (81). Clonal deletion in oral tolerance occurs by means of FAS-mediated apoptosis (82). Low- and high-dose tolerance are likely not mutually exclusive and may have overlapping functionality (9). TGF-β is secreted during clonal anergy and deletion, and in the absence of the cofactors such as IL-6 that with TGF-β drive Th17 responses, likely contributes to an immunosuppressive environment in the gut. Although there is evidence that it is liberated during apoptosis, the primary source of TGF-β remains one of the most significant unresolved questions in tolerance; other contributors of TGF-β in this environment include the gut epithelium and immature mucosal DCs. Ultimately, no matter the source, TGF-β is required for the differentiation of inducible Foxp3+ T regs in mouse models of oral tolerance (48).
Figure 3. T lymphocytes are the ultimate effectors of oral tolerance.
Once antigens pass through the gut epithelium and are taken up, processed, and presented by antigen-presenting cells on MHC II, one of several fates is possible, depending on the conditions present: (a), An active immune response ensues when the T cell receives input from its co-stimulatory molecule CD28, which binds CD80/86 on the antigen-presenting cell; (b), Reactive clones are rendered anergic or deleted if the TCR-peptide-MHC interaction occurs without costimulation, or in the presence of FAS-FASL interaction, respectively; (c), Inducible or adaptive T regulatory cells, which express the gut-homing receptors CCR9 and α4β7, are generated in the mesenteric lymph node by interactions with tolerogenic dendritic cells. Tr1 cells are formed in the presence of the suppressive cytokine IL-10 and go on to themselves produce IL-10, whereas TH3 cells produce TGF-β.
Interestingly, maternal breast milk may be another important source of TGF-β relevant to mucosal tolerance. Elegant animal models of neonatal weaning have begun to dissect the respective contributions of breast milk TGF-β (83) to allergic asthma and breast milk allergen (84) to food allergy. All mammalian milks contain TGF-β, and receptors for TGF-β have been identified in the rodent small intestine (85), where they mediate uptake of exogenous TGF-β, which acts as a negative regulator of inflammation (86); oral administration of exogenous TGF-β has been shown to enhance oral tolerance to coadministered ovalbumin and beta-lactoglobulin (87,88). Further studies of neonatal mice indicate that allergen-IgG immune complexes are formed in breast milk, which may act on an intestinal Fc gamma receptor in a TGF-β independent way to induce mucosal tolerance and protection from allergic asthma through T reg induction (89).
Although breastfeeding has been repeatedly demonstrated to have a positive effect on the prevention or delay of atopic dermatitis in humans, it is admittedly a weak effect and does not translate to the prevention of food allergy or asthma. However, clinical studies of allergy prevention are beset by methodologic flaws that weaken their associations, and few have performed in depth mechanistic analysis. Certainly food allergens are known to pass into human milk, which also contains TGF-β, of primarily the TGF-β2 isotype (87); however, mothers of allergic children may themselves be on an elimination diet. Therefore exposure during breastfeeding is complex, and most studies of breastfeeding on clinical outcomes did not analyze the content of the milk. Breast milk concentrations of TGF-β have been shown to fluctuate between women and throughout the day (90), and mothers of allergic children have been shown to have lower breast milk TGF-β levels (85). Likewise, food-allergic children have fewer TGF-β+ cells in the small intestine when compared to healthy controls and children with celiac disease (91). With roles in epithelial growth, IgA production, DC maturation, and T reg differentiation, TGF-β clearly provides key signals for oral tolerance induction. Further study is needed to unravel the complex and crucial activities of this pleiotropic molecule.
BRINGING ORAL TOLERANCE INDUCTION TO THE CLINIC
There is a great clinical need for a disease-modifying therapy for food-allergic patients. Both allergen-specific and non-specific approaches to tolerance induction are currently being studied, and promising pilot data in humans exist (92–95). Although clinical desensitization and immune modulation has been demonstrated, the strength of the current evidence from early clinical trial designs is insufficient to change practice. Findings from several current trials utilizing randomized, double-blinded, placebo-controlled designs are eagerly anticipated. Although the induction of bona fide immune tolerance in food-allergic patients is a long-term goal, it is not clear if this is possible in any of the approaches under study. If so, the mechanisms of an interventional therapy may not resemble the kind of mucosal tolerance that occurs naturally.
CONCLUSIONS
The gastrointestinal mucosal immune system must constantly analyze antigenic information and respond appropriately to pathogens, commensals, and food antigens. These responses require a complex immunoregulatory network, of which the hallmark is the induction of oral tolerance. Both host- and antigen-specific properties, as well as dietary and other environmental factors are important in determining the proper adaptive immune response. Multiple immunologic determinants, including APCs/DCs, T cells, cytokines, and antibody, play essential roles in shaping the tolerogenic response, and specific characteristics of each of these key players are being identified. Advances in our understanding of the complexities of the gastrointestinal mucosal immune response and the mechanisms of oral tolerance induction will inform the development of future therapies and improve the care and quality of life of patients with food allergy.
Acknowledgments
Funding sources:
Food Allergy & Anaphylaxis Network; Food Allergy Project; Gerber Foundation; NIH Grant 1 R01-AI06874-01A1, NIH T32 Training Grant, and NIH Grant 1 UL1 RR024128-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research (contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH); Dorothy & Frank Robins Family; and the National Peanut Board.
Abbreviations
- APC
antigen presenting cell
- CD
cluster of differentiation
- DC
dendritic cell
- Foxp3
forkhead box p3
- MALT
mucosa-associated lymphoid tissue
- MHC
major histocompatibility complex
- MLN
mesenteric lymph node
- PBMC
peripheral blood mononuclear cell
- PP
Peyer’s patch
- RA
retinoic acid
- TGF-β
transforming growth factor-beta
- TSLP
thymic stromal lymphopoetin
- TLR
Toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chafen JJS, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, et al. Diagnosing and Managing Common Food Allergies: A Systematic Review. JAMA. 2010 May 12;303(18):1848–1856. doi: 10.1001/jama.2010.582. [DOI] [PubMed] [Google Scholar]
- 2.Branum AM, Lukacs SL. Food Allergy Among Children in the United States. Pediatrics. 2009 Dec 1;124(6):1549–1555. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- 3.Venter C, Arshad SH, Grundy J, Pereira B, Clayton CB, Voigt K, et al. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010;65(1):103–108. doi: 10.1111/j.1398-9995.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Sheikh A, Strachan DP, Anderson HR. Time trends in allergic disorders in the UK. Thorax. 2007 Jan;62(1):91–96. doi: 10.1136/thx.2004.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010 Feb;125(2, Supplement 2):S116–S125. doi: 10.1016/j.jaci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 6.Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy. 2010;65(8):933–945. doi: 10.1111/j.1398-9995.2010.02342.x. [DOI] [PubMed] [Google Scholar]
- 7.Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005;115(1):3–12. doi: 10.1016/j.jaci.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008 Jun;8(6):435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faria AMC, Weiner HL. Oral tolerance. Immunol Rev. 2005;206(1):232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Untersmayr E, Jensen-Jarolim E. The role of protein digestibility and antacids on food allergy outcomes. J Allergy Clin Immunol. 2008 Jun;121(6):1301–1308. doi: 10.1016/j.jaci.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandtzaeg P. Update on mucosal immunoglobulin A in gastrointestinal disease. Curr Opin Gastro 9000. doi: 10.1097/MOG.0b013e32833dccf8. Publish Ahead of Print. Available from: http://journals.lww.com/co-gastroenterology/Fulltext/publishahead/Update_on_mucosal_immunoglobulin_A_in.99878.aspx. [DOI] [PubMed]
- 12.Karlsson MR, Johansen F, Kahu H, Macpherson A, Brandtzaeg P. Hypersensitivity and oral tolerance in the absence of a secretory immune system. Allergy. 2010;65(5):561–570. doi: 10.1111/j.1398-9995.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 13.Kukkonen K, Kuitunen M, Haahtela T, Korpela R, Poussa T, Savilahti E. High intestinal IgA associates with reduced risk of IgE-associated allergic diseases. Pediatr Allergy Immunol. 2010;21(1-Part-I):67–73. doi: 10.1111/j.1399-3038.2009.00907.x. [DOI] [PubMed] [Google Scholar]
- 14.Dahan S, Roth-Walter F, Arnaboldi P, Agarwal S, Mayer L. Epithelia: lymphocyte interactions in the gut. Immunol Rev. 2007;215(1):243–253. doi: 10.1111/j.1600-065X.2006.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, et al. IL-9– and mast cell–mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008 Apr 14;205(4):897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groschwitz KR, Hogan SP. Intestinal barrier function: Molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009 Jul;124(1):3–20. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ventura M, Polimeno L, Amoruso A, Gatti F, Annoscia E, Marinaro M, et al. Intestinal permeability in patients with adverse reactions to food. Dig Liver Dis. 2006 Oct;38(10):732–736. doi: 10.1016/j.dld.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Levy Y, Davidovits M, Cleper R, Shapiro R. New-onset post-transplantation food allergy in children – Is it attributable only to the immunosuppressive protocol? Pediatr Transplant. 2009 Feb;13(1):63–69. doi: 10.1111/j.1399-3046.2007.00883.x. [DOI] [PubMed] [Google Scholar]
- 19.Blanchard C, Stucke EM, Burwinkel K, Caldwell JM, Collins MH, Ahrens A, et al. Coordinate Interaction between IL-13 and Epithelial Differentiation Cluster Genes in Eosinophilic Esophagitis. J Immunol. 2010 Apr 1;184(7):4033–4041. doi: 10.4049/jimmunol.0903069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Oord R, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ. 339:b2243. doi: 10.1136/bmj.b2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hershberg RM, Cho DH, Youakim A, Bradley MB, Lee JS, Framson PE, et al. Highly polarized HLA class II antigen processing and presentation by human intestinal epithelial cells. J Clin Invest. 1998;102(4):792–803. doi: 10.1172/JCI3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iliev ID, Matteoli G, Rescigno M. The yin and yang of intestinal epithelial cells in controlling dendritic cell function. J Exp Med. 2007;204(10):2253–7. doi: 10.1084/jem.20062535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010 Apr;11(4):289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blazquez AB, Berin MC. Gastrointestinal Dendritic Cells Promote Th2 Skewing via OX40L. J Immunol. 2008;180(7):4441–50. doi: 10.4049/jimmunol.180.7.4441. [DOI] [PubMed] [Google Scholar]
- 25.Sherrill JD, Gao P, Stucke EM, Blanchard C, Collins MH, Putnam PE, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol. 2010 Jul;126(1):160–165.e3. doi: 10.1016/j.jaci.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiner HL, Friedman A, Miller A, Khoury SJ, Al-Sabbagh A, Santos L, et al. Oral Tolerance: Immunologic Mechanisms and Treatment of Animal and Human Organ-Specific Autoimmune Diseases by Oral Administration of Autoantigens. Ann Rev Immunol. 1994 Apr 1;12(1):809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 27.Berin MC, Zheng Y, Domaradzki M, Li XM, Sampson HA. Role of TLR4 in allergic sensitization to food proteins in mice. Allergy. 2006;61(1):64–71. doi: 10.1111/j.1398-9995.2006.01012.x. [DOI] [PubMed] [Google Scholar]
- 28.Prescott SL. Allergic disease: understanding how in utero events set the scene. Proc Nutr Soc. 2010;69(03):366–372. doi: 10.1017/S0029665110001874. [DOI] [PubMed] [Google Scholar]
- 29.Sigurs N, Hattevig G, Kjellman B, Kjellman NM, Nilsson L, Bjorksten B. Appearance of atopic disease in relation to serum IgE antibodies in children followed up from birth for 4 to 15 years. J Allergy Clin Immunol. 1994 Oct;94(4):757–763. doi: 10.1016/0091-6749(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 30.Holt PG. Prenatal versus postnatal priming of allergen specific immunologic memory: The debate continues. J Allergy Clin Immunol. 2008 Oct;122(4):717–718. doi: 10.1016/j.jaci.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Burks AW, Laubach S, Jones SM. Oral tolerance, food allergy, and immunotherapy: Implications for future treatment. J Allergy Clin Immunol. 2008 Jun;121(6):1344–1350. doi: 10.1016/j.jaci.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 32.Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008 Nov;122(5):984–991. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 33.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009 Feb;123(2):417–423. doi: 10.1016/j.jaci.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Poole JA, Barriga K, Leung DY, Hoffman M, Eisenbarth GS, Rewers M, et al. Timing of Initial Exposure to Cereal Grains and the Risk of Wheat Allergy. Pediatrics. 2006 Jun 1;117(6):2175–2182. doi: 10.1542/peds.2005-1803. [DOI] [PubMed] [Google Scholar]
- 35.Greer FR, Sicherer SH, Burks A. Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Timing of Introduction of Complementary Foods, and Hydrolyzed Formulas. Pediatrics. 2008;121(1):183–91. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 36.Høst A, Halken S, Muraro A, Dreborg S, Niggemann B, Aalberse R, et al. Dietary prevention of allergic diseases in infants and small children. Pediatr Allergy Immunol. 2008;19(1):1–4. doi: 10.1111/j.1399-3038.2007.00680.x. [DOI] [PubMed] [Google Scholar]
- 37.Strobel S, Ferguson A. Immune Responses to Fed Protein Antigens in Mice. 3. Systemic Tolerance or Priming Is Related to Age at Which Antigen Is First Encountered. Pediatr Res. 1984;18(7) doi: 10.1203/00006450-198407000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009 May;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mowat A, Parker L, Beacock-Sharp H, Millington OR, Chirdo F. Oral tolerance: overview and historical perspectives. Ann NY Acad Sci. 2004;1029(1):1–8. doi: 10.1196/annals.1309.001. Oral Tolerance: New Insights and Prospects for Clinical Application. [DOI] [PubMed] [Google Scholar]
- 40.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Nat Acad Sci. 2010 Jul 6;107(27):12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holt PG, Strickland DH. Soothing signals: transplacental transmission of resistance to asthma and allergy. J Exp Med. 2009 Dec 21;206(13):2861–2864. doi: 10.1084/jem.20092469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao T, Chang C, Hsu Y, Huang J. Review Article: Probiotics for allergic diseases: Realities and myths. Pediatr Allergy Immunol. 2010;21(6):900–919. doi: 10.1111/j.1399-3038.2009.00955.x. [DOI] [PubMed] [Google Scholar]
- 44.Medini D, Serruto D, Parkhill J, Relman DA, Donati C, Moxon R, et al. Microbiology in the post-genomic era. Nat Rev Micro. 2008 Jun;6(6):419–430. doi: 10.1038/nrmicro1901. [DOI] [PubMed] [Google Scholar]
- 45.Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001 Oct;108(4):516–520. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- 46.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3(4):331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 47.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006 Mar 20;203(3):519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curotto de Lafaille MA, Lafaille JJ. Natural and Adaptive Foxp3+ Regulatory T Cells: More of the Same or a Division of Labor? Immunity. 2009 May 22;30(5):626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and Regulatory T Cell Differentiation Mediated by Retinoic Acid. Science. 2007 Jul 13;317(5835):256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 50.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun C, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-{beta} and retinoic acid dependent mechanism. J Exp Med. 2007 Aug 6;204(8):1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun C, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007 Aug 6;204(8):1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010 May 1;59(5):595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 53.Fukaya T, Takagi H, Sato Y, Sato K, Eizumi K, Taya H, et al. Crucial roles of B7-H1 and B7-DC expressed on mesenteric lymph node dendritic cells in the generation of antigen-specific CD4+Foxp3+ regulatory T cells in the establishment of oral tolerance. Blood. 2010 Jun 23; doi: 10.1182/blood-2009-10-250472. blood-2009-10-250472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belkaid Y, Oldenhove G. Tuning Microenvironments: Induction of Regulatory T Cells by Dendritic Cells. Immunity. 2008 Sep 19;29(3):362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007 Aug 6;204(8):1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A Metabolites Induce Gut-Homing FoxP3+ Regulatory T Cells. J Immunol. 2007 Sep 15;179(6):3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 57.Mora JR, Iwata M, Eksteen B, Song S, Junt T, Senman B, et al. Generation of Gut-Homing IgA-Secreting B Cells by Intestinal Dendritic Cells. Science. 2006 Nov 17;314(5802):1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 58.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008 Sep 1;205(9):2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uematsu S, Fujimoto K, Jang MH, Yang B, Jung Y, Nishiyama M, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008 Jul;9(7):769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 60.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin Immunol. 2009 Feb;21(1):8–13. doi: 10.1016/j.smim.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Mucida D, Park Y, Cheroutre H. Seminars in Immunology. Elsevier; 2009. From the diet to the nucleus: Vitamin A and TGF-β join efforts at the mucosal interface of the intestine; pp. 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strober W. Vitamin A rewrites the ABCs of oral tolerance. Mucosal Immunol. 2008;1(2):92–5. doi: 10.1038/mi.2007.22. [DOI] [PubMed] [Google Scholar]
- 63.Denning TL, Wang Y, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007 Oct;8(10):1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 64.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The Indoleamine 2, 3-Dioxygenase Pathway Is Essential for Human Plasmacytoid Dendritic Cell-Induced Adaptive T Regulatory Cell Generation. J Immunol. 2008;181(8):5396–404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma MD, Baban B, Chandler P, Hou D, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2, 3-dioxygenase. J Clin Invest. 2007;117(9):2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204(1):105–15. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. Human Plasmacytoid Dendritic Cells Activated by CpG Oligodeoxynucleotides Induce the Generation of CD4+ CD25+ Regulatory T Cells. J Immunol. 2004;173(7):4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 68.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, et al. Plasmacytoid Dendritic Cells Mediate Oral Tolerance. Immunity. 2008 Sep 19;29(3):464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dubois B, Joubert G, Gomez de Aguero M, Gouanvic M, Goubier A, Kaiserlian D. Sequential Role of Plasmacytoid Dendritic Cells and Regulatory T Cells in Oral Tolerance. Gastroenterology. 2009 Sep;137(3):1019–1028. doi: 10.1053/j.gastro.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 70.Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, et al. Human Liver Dendritic Cells Promote T Cell Hyporesponsiveness. J Immunol. 2009 Feb 15;182(4):1901–1911. doi: 10.4049/jimmunol.0803404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Middendorp S, Nieuwenhuis EES. NKT cells in mucosal immunity. Mucosal Immunol. 2009 Jul 8;2(5):393–402. doi: 10.1038/mi.2009.99. [DOI] [PubMed] [Google Scholar]
- 72.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008 Mar;9(3):239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ Regulatory T Cell-Dependent and -Independent Control of Allergic Inflammation. Immunity. 2008 Jul 18;29(1):114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 74.Zhou Y, Kawasaki H, Hsu S, Lee RT, Yao X, Plunkett B, et al. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat Med. 2010 doi: 10.1038/nm.2201. online; advance online publication. Available from: http://dx.doi.org/10.1038/nm.2201. [DOI] [PMC free article] [PubMed]
- 75.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, et al. Immune Responses in Healthy and Allergic Individuals Are Characterized by a Fine Balance between Allergen-specific T Regulatory 1 and T Helper 2 Cells. J Exp Med. 2004;199(11):1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Gen. 2001 Jan;27(1):20. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 77.Torgerson TR, Linane A, Moes N, Anover S, Mateo V, Rieux-Laucat F, et al. Severe Food Allergy as a Variant of IPEX Syndrome Caused by a Deletion in a Noncoding Region of the FOXP3 Gene. Gastroenterology. 2007 May;132(5):1705–1717. doi: 10.1053/j.gastro.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 78.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ Regulatory T Cells in Children who Have Outgrown Cow’s Milk Allergy. J Exp Med. 2004 Jun 21;199(12):1679–1688. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beyer K, Castro R, Birnbaum A, Benkov K, Pittman N, Sampson HA. Human milk-specific mucosal lymphocytes of the gastrointestinal tract display a TH2 cytokine profile. J Allergy Clin Immunol. 2002 Apr;109(4):707–713. doi: 10.1067/mai.2002.122503. [DOI] [PubMed] [Google Scholar]
- 80.Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009 Jan;123(1):43–52.e7. doi: 10.1016/j.jaci.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 81.Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunol Rev. 2003;192(1):161–180. doi: 10.1034/j.1600-065x.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995 Jul 13;376(6536):177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 83.Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, et al. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med. 2008 Feb;14(2):170–175. doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- 84.Lopez-Exposito I, Song Y, Jarvinen KM, Srivastava K, Li X. Maternal peanut exposure during pregnancy and lactation reduces peanut allergy risk in offspring. J Allergy Clin Immunol. 2009 Nov;124(5):1039–1046. doi: 10.1016/j.jaci.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Penttila IA. Milk-Derived Transforming Growth Factor-[beta] and the Infant Immune Response. J Pediatrics. 2010 Feb;156(2, Supplement 1):S21–S25. doi: 10.1016/j.jpeds.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 86.Li MO, Flavell RA. Contextual Regulation of Inflammation: A Duet by Transforming Growth Factor-[beta] and Interleukin-10. Immunity. 2008 Apr 11;28(4):468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 87.Ando T, Hatsushika K, Wako M, Ohba T, Koyama K, Ohnuma Y, et al. Orally administered TGF-[beta] is biologically active in the intestinal mucosa and enhances oral tolerance. J Allergy Clin Immunol. 2007 Oct;120(4):916–923. doi: 10.1016/j.jaci.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 88.Penttila I. Effects of Transforming Growth Factor-Beta and Formula Feeding on Systemic Immune Responses to Dietary [beta]-Lactoglobulin in Allergy-Prone Rats. Pediatr Res. 2006;59(5):650–5. doi: 10.1203/01.pdr.0000203149.75465.74. [DOI] [PubMed] [Google Scholar]
- 89.Mosconi E, Rekima A, Seitz-Polski B, Kanda A, Fleury S, Tissandie E, et al. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol. 2010;3(5):461–474. doi: 10.1038/mi.2010.23. print. [DOI] [PubMed] [Google Scholar]
- 90.Hawkes JS, Bryan D, James MJ, Gibson RA. Cytokines (IL-1[beta], IL-6, TNF-[alpha], TGF-[beta]1, and TGF-[beta]2) and Prostaglandin E2 in Human Milk during the First Three Months Postpartum. Pediatr Res. 1999;46(2):194–9. doi: 10.1203/00006450-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 91.Pérez-Machado M, Ashwood P, Thomson M, Latcham F, Sim R, Walker-Smith J, et al. Reduced transforming growth factor-β1-producing T cells in the duodenal mucosa of children with food allergy. Eur J Immunol. 2003;33(8):2307–2315. doi: 10.1002/eji.200323308. [DOI] [PubMed] [Google Scholar]
- 92.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LCL, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010 Jul;126(1):83–91.e1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 94.Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2008 Dec;122(6):1154–1160. doi: 10.1016/j.jaci.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clark AT, Islam S, King Y, Deighton J, Anagnostou K, Ewan PW. Successful oral tolerance induction in severe peanut allergy. Allergy. 2009;64(8):1218–1220. doi: 10.1111/j.1398-9995.2009.01982.x. [DOI] [PubMed] [Google Scholar]