Abstract
Ranolazine is a clinically approved drug for treating cardiac ventricular dysrhythmias and angina. Its mechanism(s) of protection is not clearly understood but evidence points to blocking the late Na+ current that arises during ischemia, blocking mitochondrial complex I activity, or modulating mitochondrial metabolism. Here we tested the effect of ranolazine treatment before ischemia at the mitochondrial level in intact isolated hearts and in mitochondria isolated from hearts at different times of reperfusion. Left ventricular (LV) pressure (LVP), coronary flow (CF), and O2 metabolism were measured in guinea pig isolated hearts perfused with Krebs-Ringer’s solution; mitochondrial (m) O2•−, Ca2+, NADH/FAD (redox state), and cytosolic (c) Ca2+ were assessed on-line in the LV free wall by fluorescence spectrophotometry. Ranolazine (5 µM), infused for 1 min just before 30 min of global ischemia, itself did not change O2•−, cCa2+, mCa2+ or redox state. During late ischemia and reperfusion (IR) O2•− emission and m[Ca2+] increased less in the ranolazine group vs. the control group. Ranolazine decreased c[Ca2+] only during ischemia while NADH and FAD were not different during IR in the ranolazine vs. control groups. Throughout reperfusion LVP and CF were higher, and ventricular fibrillation was less frequent. Infarct size was smaller in the ranolazine group than the control group. Mitochondria isolated from ranolazine-treated hearts had mild resistance to permeability transition pore (mPTP) opening and less cytochrome c release than control hearts. Ranolazine may provide functional protection of the heart during IR injury by reducing cCa2+ and mCa2+ loading secondary to its effect to block the late Na+ current. Subsequently it indirectly reduces O2•− emission, preserves bioenergetics, delays mPTP opening, and restricts loss of cytochrome c, thereby reducing necrosis and apoptosis.
Keywords: mitochondrial Ca2+, oxidative stress, permeability transition pore, ranolazine
1. Introduction
Coronary heart disease remains the leading cause of death worldwide [1] despite improvement in its diagnosis and treatment. The most effective strategy for treating cardiac injury and limiting infarct size is early restoration of coronary blood flow to the ischemic myocardium. However, this approach, while essential, is often associated with functional and structural damage during reperfusion [2], which is a consequence of the abnormalities that occur during the ischemic period [3]. Although the complex mechanisms of ischemia and reperfusion (IR) injury are not well understood, many studies indicate that both excess reactive oxygen species (ROS) production and cytosolic (c) Ca2+ overload play key roles. More recently, mitochondrial dysfunction has been proposed to lie at the center of the etiology of IR injury because mitochondria act as both sources and targets of the injury process. Indeed, mitochondria sustain progressive damage during a lengthening of cardiac ischemia [4], and in the process become a major source of excess ROS generation [5–7] and the initiator of necrosis and apoptosis. Mitochondrial (m) Ca2+ overload occurs concurrently with excess ROS production and this leads to a subsequent increase in permeability of the inner mitochondrial membrane to large solutes, commonly referred to as the mitochondrial permeability transition pore (mPTP). An important consequence of mPTP opening is expansion of the matrix which, when sufficient, leads to rupture of the outer mitochondrial membrane and subsequently necrosis [8] and probably apoptosis [9].Thus protecting mitochondria against this vicious cycle of perpetual damage by excess mitochondrial ROS production and mCa2+ overload associated with IR may preserve the structural and functional integrity of mitochondria and other cell structures to reduce cardiac injury.
Ranolazine (RS-43285) [10] is a clinically used anti-anginal drug [11–13] that has also been shown to reduce ischemic damage in animal models [14–16]. The specific cardioprotective mechanism(s) of ranolazine is not well understood especially since, and contrary to other antianginal agents, it exerts its effects without significantly changing hemodynamic parameters [17]. Several mechanisms have been proposed for ranolazine’s cardioprotective effects. One is that ranolazine acts by inhibiting fatty acid oxidation during ischemia and shifting the metabolism towards glucose oxidation, which is more efficient during ischemia due to the limited availability of O2 [18]. Another proposal is that ranolazine selectively blocks a ROS-induced late sarcolemmal Na+ channel current [19], which may reduce the rise in c[Na+] and c[Ca2+] during IR. It has also been suggested that ranolazine inhibits complex I of the electron transport chain (ETC) to afford protection [20]. Together, these studies clearly show that ranolazine protects against cardiac IR injury and suggest that mitochondrial mechanisms may underlie this protection. However, it is unknown if ranolazine has a direct or indirect role on mitochondria to effect cardiac protection.
For this study we tested if ranolazine given just before ischemia protected mitochondrial function in situ during IR in the intact beating heart as well as in isolated mitochondria sequestered at 10 min reperfusion. In the intact hearts we assessed mitochondrial redox state by noting changes in nicotinamide adenine dinucleotide hydrogen (NADH) and flavin adenine dinucleotide (FAD), two indicators of the mitochondrial bioenergetic state, as well as increases in mitochondrial O2•− emission, c[Ca2+] and m[Ca2+] overload, all of which are markers of myocardial injury during IR. We postulated that a decrease in mCa2+ loading secondary to a decrease in cCa2+ loading during IR reflects inhibition of the late Na+ channel current, whereas an increase in mO2•− emission and NADH during ranolazine perfusion indicates a possible role for complex I blockade as a mechanism for cardioprotection by ranolazine.
2. Materials and Methods
2.1. Isolated Heart Preparation
This investigation conformed to the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health Publication No. 85-23, Revised 1996). The Medical College of Wisconsin Biomedical Resources Studies Committee approved this study. Guinea pig hearts (n=64) were isolated and prepared as described in detail in our previous studies [21–26]. Briefly, animals were anesthetized with ketamine (50 mg/kg) and heparin was administered to prevent clotting. Following decapitation and thoracotomy, hearts were removed and perfused at 55 mmHg via the aortic root with a Krebs Ringer’s solution (KR: in mM 138 Na+, 4.5 K+, 1.2 Mg2+, 2.5 Ca2+, 134 Cl−, 15 HCO3−, 1.2 H2PO4−, 11.5 glucose, 2 pyruvate, 16 mannitol, 0.1 probenecid, 0.05 EDTA, and 5 U/L insulin) gassed with 3% CO2, 97% O2 (pH 7.4) at 37°C. Pyruvate improves performance in isolated perfused guinea pig hearts [27,28], and mannitol, a non-metabolized sugar, supports the oncotic pressure of the KR solution. Probenecid was added to accentuate loading and intracellular retention of fluorescent dyes [29]. A saline-filled balloon catheter attached to a transducer was used to measure left ventricular pressure (LVP). Coronary flow (CF) was measured using an ultrasonic flowmeter (model T106X; Transonic Systems, Ithaca, NY) placed directly into the aortic inflow line as previously described [30]. Spontaneous heart rate was monitored with bipolar electrodes placed in the right atrial and ventricular walls [30]. If ventricular fibrillations, which usually occur upon reperfusion, did not convert spontaneously to sinus rhythm after 30 s, 100 µg lidocaine was injected into the aortic cannula [21]. Cardiac O2 consumption (MVO2) was calculated as coronary flow • heart weight−1 • (PaO2 − PvO2) • 0.028 µl O2 / ml (37°C) at 760 mmHg.
2.2. Measurements of Redox State, O2•− and [Ca2+]
All fluorescence signals were detected at 103 to 105 photons/s. FAD fluorescence is derived only from mitochondria; the majority of the NADH signal also arises from mitochondria [31–33]. The majority of superoxide (O2•−) likely originates from cardiac mitochondria with only a small amount derived from non-mitochondrial sources [22]. It is known that vascular smooth muscle and endothelial cells generate O2•− but their contribution compared to cardiac myocytes is very small. After the quenching of cCa2+ signals by MnCl2 the majority of the Ca2+ signal is derived from the mitochondrial compartment [34] because of its large volume relative to cell volume [35,36].
NADH and FAD, c[Ca2+], m[Ca2+], and ROS were measured near continuously through the left ventricular (LV) free wall using one of four excitation and emission fluorescence spectra [21–26] in different subsets of hearts. A trifurcated fiberoptic probe (3.8 mm2/bundle) was placed against the LV to excite and record light signals at specific wavelengths using spectrophotofluorometers (SLM Instruments Inc, Urbana, IL; or Photon Technology International, Birmingham, NJ). Light penetration and its fluorescence sensitivity are dependent on the light source intensity and wavelength. For Ca2+ the signal is transmural but attenuated at the endocardial surface to 20 to 30% of that at the epicardial surface [37].
In a subset of hearts, as described previously [23,24,38], 10 µM dihydroethidium (DHE), a fluorescent probe used to detect the O2•− radical [7,39,40], was loaded for 20 min followed by wash out of residual dye; the LV free wall was excited at 540 nm, and light emission was recorded at 590 nm. O2•− non-enzymatically converts DHE to 2-hydroxyethidium (2-OH-E+) a precursor that appears to be rapidly made, is labile, and fluoresces at a slightly shorter wavelength than the heme-peroxidase oxidation product ethidium that can intercalate with DNA [39,40]. In other hearts NADH autofluorescence was assessed at 350 nm excitation and 450 and 390 nm emissions, and FAD autofluorescence at 480 nm excitation and 540 nm emission [25,33,38,41].
Alternatively, other hearts were loaded with 6 µM indo 1 AM for 30 min to measure total Ca2+ transients using an excitation of 350 nm and emissions of 390 and 450 nm. In order to measure m[Ca2+], hearts were perfused (before IR) for 15 min with 100 µM MnCl2 to quench the cytosolic indo 1 signal [42,43] and then washed out for 15 min. MnCl2 slightly increased LVP which returned to its original value upon washout. The remaining fluorescent signal after quenching represented the m[Ca2+] signal [34]. For the c[Ca2+] measurement, 100 µM MnCl2 was perfused for 15 min by the end of the reperfusion to quench the cytosolic indo 1 signal. c[Ca2+] was then calculated from the total [Ca2+] after subtracting the mitochondrial component [44]. To estimate actual [Ca2+] during the entire protocol, NADH autofluorescence was subtracted from the underlying changes in Ca2+ fluorescent signals for each group [30,38,45]. Each signal was digitized and recorded at 200 Hz on computers for later analyses using specifically designed computational programs and commercial software (Matlab). pH alone in the range of 6.2–8.0 does not alter the DHE fluorescence in our model [23]. Also in preliminary experiments, we found that a decrease in pH from 7.4 to 6.5, which may occur during ischemia, did not significantly change the indo 1 AM fluorescence signal. This excludes the possibility of lactic acidosis buildup during ischemia from having significant direct effects on Ca2+, O2•−, and NADH signals during ischemia.
2.3. Protocol
The study had three groups: IR alone (control, n=24), 5 µM ranolazine + IR (ranolazine, n=25), and perfusion without IR or ranolazine (time control, n=15). Each group contained subsets of hearts to independently measure and record either NADH plus FAD, c[Ca2+], m[Ca2+], or O2•−. Each heart initially underwent a stabilization period followed by dye loading and washout of unbound dye to specifically measure either c[Ca2+], m[Ca2+] or O2•−. NADH and FAD autofluorescence were assessed simultaneously [30,38,45,46] using a protocol similar to that of the indo 1 fluorescence dye-loaded hearts.
All hearts undergoing IR were perfused only briefly (1 min) with ranolazine, or no treatment (vehicle), immediately before the onset of 30 min of no flow global ischemia. This protocol assured that hearts were treated with the drug only briefly before ischemia and that the drug was present in the heart during the ischemic period but not on reperfusion. This treatment effect is not a classical preconditioning protocol in which the drug is washed out completely before ischemia. Ranolazine perfusion alone caused a small but significant depression of LVP and a significant increase in CF. We have shown previously that after a one min drug perfusion before ischemia a given drug is no longer present at the initiation of reperfusion [38]; thus, we expect ranolazine is not present on reperfusion.
Ranolazine itself did not alter the fluorescence characteristics or spectra of any dye. Therefore, any observed changes in fluorescent spectra were most likely attributed only to the effects induced by IR. After ischemia each heart was reperfused with KR solution without ranolazine for 120 min. At the end of each experiment hearts were removed and atria were discarded; ventricles were cut into thin transverse sections (4–5) of approximately 3 mm each and incubated in buffered 0.1% 2,3,5-triphenyltetrazolium chloride (TTC) to stain viable tissue from necrotic tissue for estimating infarct size using a cumulative planimetry technique [47].
2.4. Assessment of Mitochondrial Membrane Integrity
In another set of experiments, isolated hearts (n=6) were treated as described above ± ranolazine treatment followed by 30 min ischemia, but subjected to only 10 min reperfusion. Hearts were then immediately removed from the perfusion apparatus and mitochondria were isolated as described by us previously [48–50]. This time point (10 min reperfusion) was chosen because mitochondria may be more susceptible to mPTP opening due to the disturbance in Ca2+ homeostasis, the increase in ROS production, and increase in pH, without loss of non-viable mitochondria.
In brief, ventricles were excised, placed in an isolation buffer (buffer A) (200 mM mannitol, 50 mM sucrose, 5 mM KH2PO4, 5 mM MOPS, 1 mM EGTA, and 0.1% BSA, with pH adjusted to 7.15 with KOH), and minced into 1-mm3 pieces. The suspension was homogenized for 15 s in 2.5 ml isolation buffer containing 5 U/ml protease (Bacillus licheniformis) and for another 15 s after adding 17 ml isolation buffer. The suspension was centrifuged at 8,000 g for 10 min, the pellet was re-suspended in 25 ml of isolation buffer and centrifuged at 750 g for 10 min, the supernatant was centrifuged again at 8,000 g for 10 min, and the final pellet was suspended in 0.5 ml of isolation buffer and kept on ice. Protein content was determined by the Bradford method [51]. All isolation procedures were conducted at 4°C and isolated mitochondrial experiments were conducted at room temperature (25°C). Before initiating the mitochondrial experiments, respiratory control index (state 3/state 4 respiration, RCI) was determined to be 11.6±0.3 for the complex I substrate pyruvate; this demonstrated a strong coupling between mitochondrial respiration and phosphorylation in guinea pig heart isolated mitochondria.
2.4.1. Mitochondrial Membrane Potential and mPTP Opening
Mitochondria were suspended in respiration buffer (buffer B) (130 mM KCl, 5 mM K2HPO4, 20 mM MOPS, 2.5 mM EGTA, 1 µM Na4P2O7, 10 mM pyruvate, and 0.1% BSA, with pH adjusted to 7.15 with KOH) inside a cuvette-based spectrophotometer (model QM-8, Photon Technology International, Birmingham, NJ). Membrane potential (ΔΨm) was monitored in the presence of the fluorescent dye rhodamine 123 (50 nM) using excitation and emission wavelengths of 503 and 527 nm, respectively [49,50]. After a 2 min stabilization period, pulses of CaCl2 (25 µM) were given every min until ΔΨm completely depolarized indicating mPTP opening. In some experiments, cyclosporine A (0.5 µM), which inhibits mPTP opening, was added to the mitochondrial suspension at the beginning of the protocol to confirm mPTP opening by excess added CaCl2. In addition to the pulse method we used the slow infusion approach of CaCl2 (25 µM/min) into the mitochondrial suspension. The responses of mitochondria were not different between the two approaches.
2.4.2 Mitochondrial Ca2+ Retention and mPTP Opening
In addition to ΔΨm for assessing mitochondrial membrane integrity, m[Ca2+] was measured as described recently [52], while mitochondria were challenged with extra-matrix pulses of CaCl2 (25 µM). In brief, isolated guinea pig heart mitochondria were incubated with 1 µM indo 1 AM in the same isolation buffer (buffer A) described above for 20 min at room temperature with continuous stirring. The suspension was then diluted with isolation buffer and centrifuged at 8,000 g for 10 min to remove excess dye. Mitochondria were then re-suspended in isolation buffer and stored on ice. The protein content was determined by the Bradford method. Assays were carried out with mitochondria suspended in respiration buffer (buffer B) inside the cuvette-based spectrophotometer at room temperature (25°C) with the light beam filtered at an excitation wavelength of 350 nm and emission wavelengths of 390 nm and 450 nm.
2.5. Cytochrome c Levels in Cytosolic and Mitochondrial Fractions
Mitochondria were isolated as described above with minor modifications to separate the cytosolic from the mitochondrial fractions by differential centrifugation [53]. All isolation procedures were conducted at 4°C. Briefly, heart tissues were minced and homogenized as described above. However, after the first spin the supernatant was further centrifuged at 14,000 g for 30 min to obtain pure cytosolic fractions. The pellet from the first spin was resuspended in isolation buffer and centrifuged at 750 g for 10 min. The supernatant was collected and centrifuged again at 8,000 g for 10 min, and the final pellet was resuspended in isolation buffer and represented the mitochondrial fraction. Cytosolic and mitochondrial fractions were stored at −80°C until use. Protein content was determined by the Bradford method [51] followed by standard Western blotting procedures.
Cytosolic and mitochondrial samples were boiled in Laemmli buffer followed by resolving on SDS-PAGE as described by Laemmli [54] and transferred onto a polyvinylidene difluoride (PVDF) membrane using the Transblot system (Bio-Rad, Richmond, CA). Membranes were blocked in Tween-20 Tris-buffered saline (TTBS) buffer (25 mM TrisCl pH 7.5, 150 mM NaCl and 0.1% Tween 20) containing 5% milk for 1 h, and then incubated with cytochrome c primary antibody (1:1000 dilution, Invitrogen, CA) overnight at 4°C. After washing 3 times with TTBS the next day, the membranes were incubated in an appropriate secondary antibody conjugated to horseradish peroxidase and then immersed in an enhanced chemiluminescence detection solution (ECL plus from GE Healthcare, Buckinghamshire, UK) and exposed to X-ray film for autoradiography. After stripping cytochrome c antibody, protein loading of cytosolic fractions was confirmed using an antibody against β–actin (1:200, Santa Cruz, CA) and purity was confirmed by checking for mitochondrial contamination using an antibody against voltage dependent anion channel (VDAC) (1:1000, Cell Signaling, Danvers, MA).
2.6. Statistical Analysis
For the intact heart studies, measurements for each group were compared at baseline, during the brief treatment with or without ranolazine before ischemia, at 15 and the last 5 (25–30) min of ischemia, and at the first 5 (1–5), 30, 60, and 120 min reperfusion. All data are expressed as mean ± SEM. Values for NADH, FAD and O2•− are expressed in arbitrary fluorescence units (afu), and c[Ca2+] and m[Ca2+] are given in nM after subtracting background autofluorescence [55]. Between groups and within group comparisons were performed by two-way analysis of variance to determine significance (SigmaPlot 11.0, Systat Software Inc, San Jose, CA). If F values were significant (P < 0.05), post hoc comparisons of means tests (Student-Newman-Keuls) were used to compare the groups within each subset. In mitochondrial studies, statistical analysis was performed using one-way analysis of variance. All data are expressed as mean ± SEM. Differences between means were considered significant when P < 0.05 (two-tailed).
3. Results
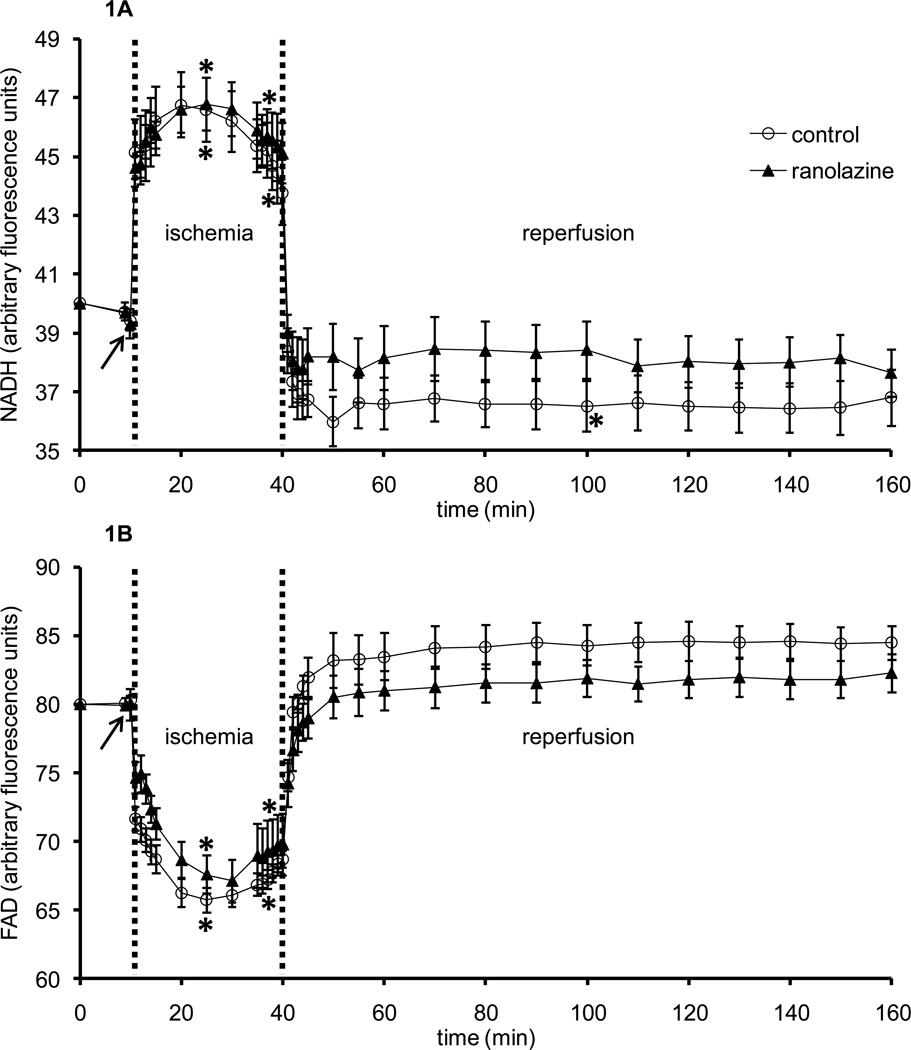
Baseline values were not different among groups for all measurements in intact heart studies. To assess the role of mitochondria in overall cardiac functional and metabolic recovery by ranolazine, mitochondrial bioenergetics, i.e., NADH and FAD, DHE (indicative of mitochondrial O2•− emission), and indo 1 fluorescence (indicative of [Ca2+]) were monitored in different subsets of hearts. Figures 1A and 1B show, respectively, timeline changes in NADH (baseline value 40±0.2 afu) and FAD (baseline value 80±0.3 afu), indicators of mitochondrial redox state, during the 1 min period of ranolazine or no treatment (arrow), during ischemia, and throughout reperfusion. An increase in NADH and a decrease in FAD indicate a more reduced mitochondrial redox state [38,45]. Brief perfusion of ranolazine did not change NADH or FAD. Ischemia caused an abrupt, significant increase in NADH autofluorescence and a reciprocal decrease in FAD autofluorescence in both groups. On reperfusion, NADH and FAD autofluorescence signals returned to baseline values in the control and ranolazine treated hearts without a significant difference between the two groups.
Figure 1.
Changes in NADH autofluorescence (A) and FAD autofluorescence (B) during 1 min treatment with or without ranolazine, and during and after 30 min of no flow global ischemia. Arrow indicates 1 min ranolazine (n=6) or control (n=6) perfusion immediately before ischemia. *P < 0.05, 1 min perfusion before ischemia, during ischemia and reperfusion vs. baseline values; #P < 0.05 ranolazine vs. control.
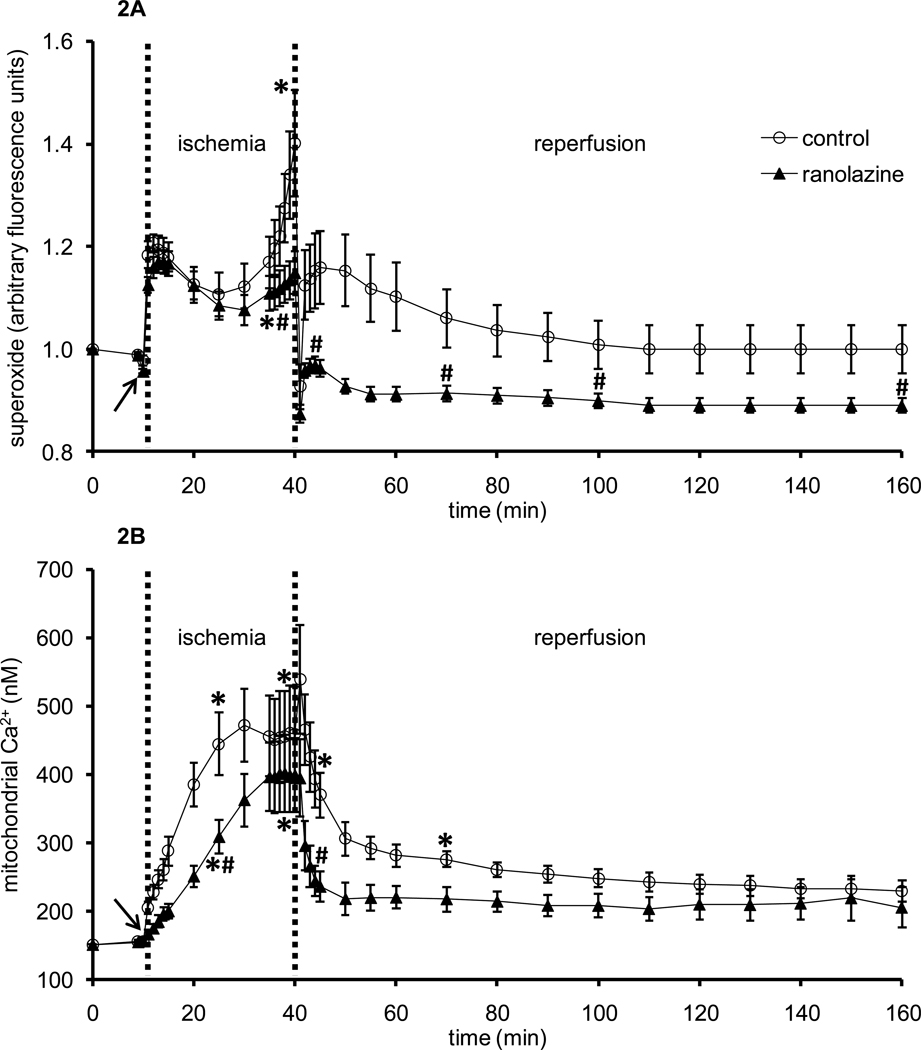
Whereas NADH and FAD are markers of the mitochondrial redox state during IR injury, O2•− emission, c[Ca2+], and m[Ca2+] overload are key effectors in the mechanism of cellular injury following IR. Figure 2A shows timeline changes in O2•− during the 1 min period of ranolazine or no treatment (arrow), during ischemia, and throughout reperfusion. Ranolazine perfusion, per se, did not alter O2•− emission. Ischemia initially caused a marked increase in O2•− in both groups; by 25 min ischemia O2•− increased more in the control IR group and remained higher during reperfusion than in the ranolazine treated group. Figure 2B shows timeline changes in average m[Ca2+] (baseline value 151±2 nM) during the brief perfusion of ranolazine or no treatment (arrow), during ischemia, and throughout reperfusion. Ranolazine perfusion, per se, did not change m[Ca2+]. Ischemia caused m[Ca2+] to increase in both groups but more so in the control group (445±45 nM) than in the ranolazine group (308±25 nM) at 15 min ischemia. At 1 min reperfusion, m[Ca2+] increased in the control group while it did not change in the ranolazine group. During the remainder of reperfusion, m[Ca2+] continued to decrease towards baseline in both groups, but it was significantly less in the ranolazine treated group than in the untreated control group.
Figure 2.
Changes in DHE fluorescence (indicative of superoxide, O2•−) (A) and indo 1 fluorescence (indicative of m[Ca2+] (B) during 1 min treatment with or without ranolazine, and during and after 30 min of no flow global ischemia. Arrow indicates 1 min ranolazine (n=8 for superoxide; n=6 for m[Ca2+]) or control (n=5 for superoxide; n=6 for m[Ca2+]) perfusion immediately before ischemia. *P < 0.05, 1 min perfusion before ischemia, during ischemia and reperfusion vs. baseline values; #P < 0.05 ranolazine vs. control.
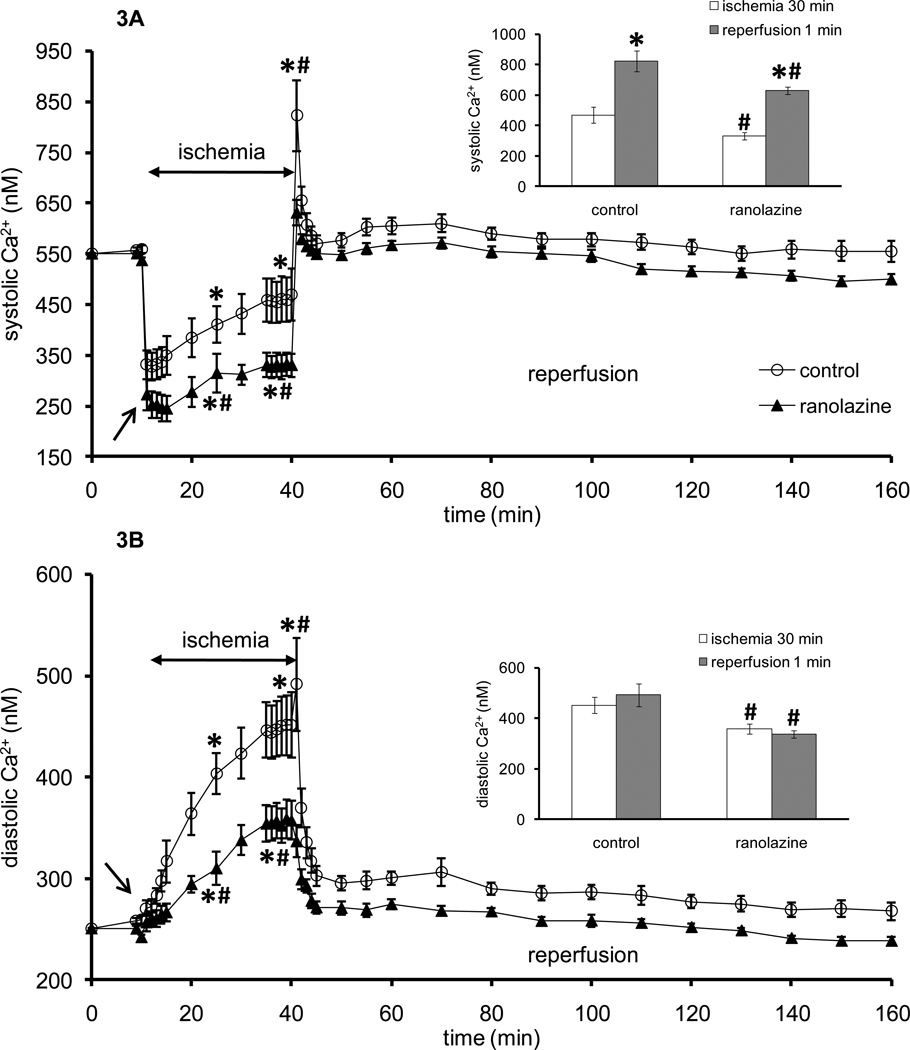
Increases in m[Ca2+] during IR insult follow increases in c[Ca2+]. Figures 3A and 3B show timeline changes in systolic (baseline value 550±4 nM) and diastolic (baseline value 250±3 nM) [Ca2+] before, during, and after ischemia. Note that after 5–8 min ischemia hearts cease to beat and so there is no difference between systolic and diastolic [Ca2+]. Systolic [Ca2+] decreased once ischemia was initiated but then c[Ca2+] increased gradually during ischemia in both groups, but more so in the control group (410±36 nM) than in the ranolazine group (315±38 nM) at 15 min ischemia. At the beginning of reperfusion (1 min), systolic and diastolic [Ca2+] increased significantly in the control group (823±70 nM, 492±46 nM, respectively) compared to the ranolazine group (631±25 nM, 337±16 nM, respectively) and then decreased and remained similar in both groups throughout reperfusion.
Figure 3.
Changes in systolic (A) and diastolic (B) indo 1 fluorescence (indicative of systolic and diastolic [Ca2+], respectively) during 1 min treatment with or without ranolazine, and during and after 30 min of no flow global ischemia. During early ischemia Ca2+ transients continue to occur; once these transients cease, systolic and diastolic Ca2+ are the same. Arrow indicates 1 min ranolazine (n=5) or control (n=7) perfusion immediately before ischemia. *P < 0.05, 1 min perfusion before ischemia, during ischemia and reperfusion vs. baseline values; #P < 0.05 ranolazine vs. control. Insets show systolic (A) and diastolic (B) [Ca2+] at 30 min ischemia (white columns) and at 1 min reperfusion (dark columns). *P < 0.05 [Ca2+] at 1 min reperfusion vs. 30 min ischemia. #P < 0.05 ranolazine vs. control.
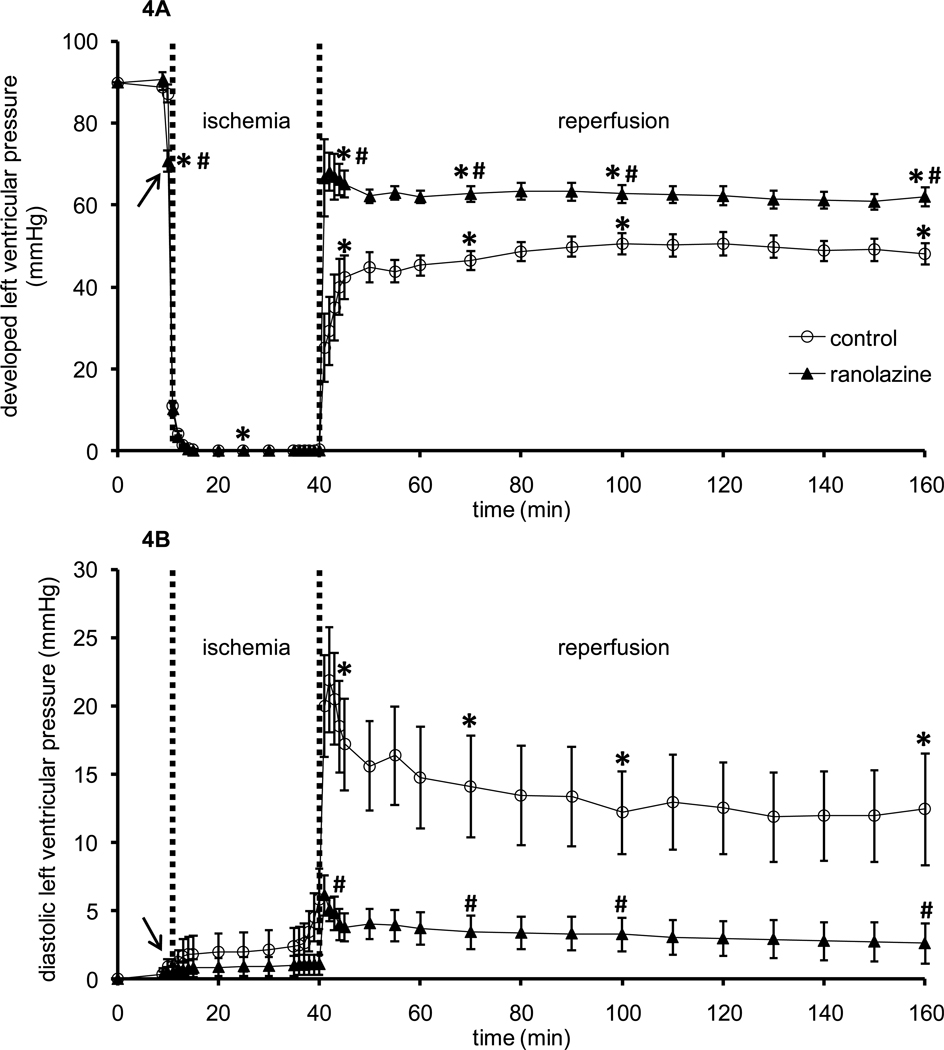
Figures 4A and 4B show timeline changes in systolic minus diastolic LVP (developed LVP, baseline value 90±2 mmHg) and diastolic LVP (diaLVP, baseline value set to 0 mmHg), respectively. Ranolazine by itself caused a small decrease in developed LVP. Upon reperfusion, ranolazine-treated hearts exhibited higher developed LVP (67±9 mmHg) and lower diastolic LVP (6±2 mmHg) vs. control hearts (developed LVP 25±8 mmHg, diastolic LVP 20±4 mmHg). Throughout reperfusion, developed LVP remained significantly lower in control hearts than in ranolazine-treated hearts.
Figure 4.
Changes in developed left ventricular pressure (A) and diastolic left ventricular pressure (B) during 1 min treatment with or without ranolazine, and during and after 30 min of no flow global ischemia. Arrow indicates 1 min ranolazine (n=14) or control (n=11) perfusion immediately before ischemia. *P < 0.05, 1 min perfusion before ischemia, during ischemia and reperfusion vs. baseline values; #P < 0.05 ranolazine vs. control.
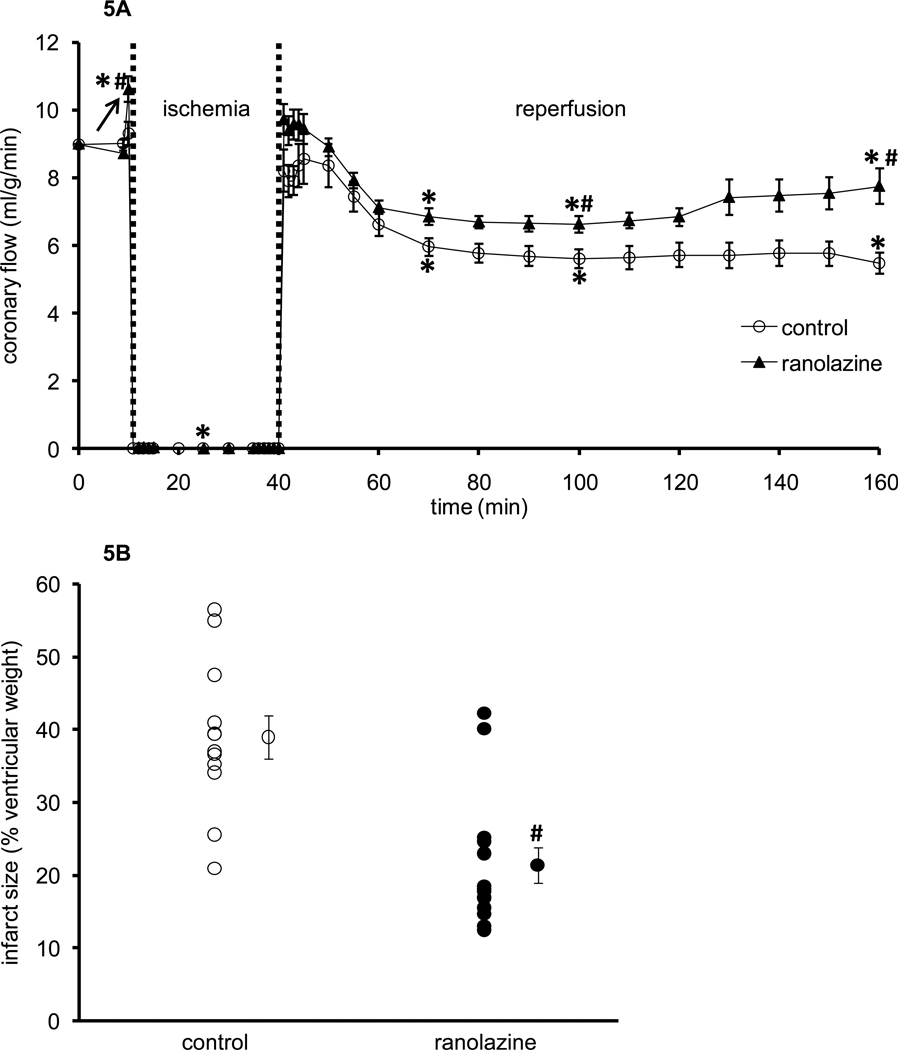
Figure 5A shows timeline changes in coronary flow (baseline value 9±0.1 ml/g/min) before, during, and after ischemia in both groups. Ranolazine perfusion (arrow) caused a small increase in coronary flow. During reperfusion coronary flow decreased in both groups but remained significantly higher in ranolazine-treated hearts throughout reperfusion, i.e. 7.8±0.5 ml/g/min vs. 5.5±0.3 ml/g/min in control hearts at 120 min reperfusion. In the time control experiments (no ischemia), all measured variables at 120 min were not significantly different from baseline values (data not shown) [38]. This suggests stability of the heart in this perfusion model.
Figure 5.
Changes in coronary flow (A) during 1 min treatment with or without ranolazine, and during and after 30 min of no flow global ischemia. Arrow indicates 1 min ranolazine (n=14) or control (n=11) perfusion immediately before ischemia. Panel B shows infarct size as a percentage of total ventricular weight measured after 120 min reperfusion for ranolazine (n=14) and control groups (n=11). *P < 0.05, 1 min perfusion before ischemia, during ischemia and reperfusion vs. baseline values; #P < 0.05 ranolazine vs. control.
Table 1 shows improved cardiac contractility, relaxation, and O2 consumption in ranolazine-treated hearts vs. control hearts during the reperfusion period. Heart rate was not different between the two groups throughout the reperfusion. Ranolazine treatment reduced the incidence of ventricular fibrillation which occurred during the first 10 min of reperfusion compared to IR only control hearts (i.e. 17% vs. 100%).
Table 1.
Changes in Heart rate, O2 Consumption and cardiac contractility and relaxation at baseline, during 1 min of treatment with ranolazine or KR, and at selected intervals during reperfusion.
| Baseline | Treatment 1 min |
Reperfusion 5 min |
Reperfusion 30 min |
Reperfusion 60 min |
Reperfusion 120 min |
|
|---|---|---|---|---|---|---|
| Heart Rate | ||||||
| control | 246±9 | 252±14 | 302±43 | 254±11 | 251±11 | 244±8 |
| ranolazine | 256±3 | 209±6*# | 257±6# | 261±5 | 262±5 | 263±4 |
| O2 Consumption | ||||||
| control | 84±5 | 86±6 | 77±6 | 51±3* | 52±5* | 52±6* |
| ranolazine | 82±4 | 84±6 | 83±5 | 68±4# | 69±4# | 72±5# |
| dLVP/dtmax | ||||||
| control | 2135±238 | 2125±217 | 1096±208* | 1118±116* | 1225±127* | 1216±133* |
| ranolazine | 2038±150 | 1563±115# | 1684±101# | 1793±104# | 1818±102# | 1768±107# |
| dLVP/dtmin | ||||||
| control | −1517±163 | −1518±168 | −631±121* | −868±100* | −898±109* | −888±112* |
| ranolazine | −1401±106 | −1095±90# | −938±69*# | −1173±80# | −1181±78 | −1136±80 |
Values are mean±sem for control (n=11) and ranolazine (n=14)
Heart rate (beats); O2 Consumption (O2•g−1•min−1); Cardiac contractility and relaxation (mmHg/s)
P < 0.05 vs. baseline values;
P < 0.05, ranolazine vs. control.
Figure 5B shows ventricular infarct size as a percentage of total ventricular weight (area at risk) for both groups after 120 min reperfusion. Infarct size was larger in control hearts (39±3%) than in ranolazine-treated hearts (21±2%).
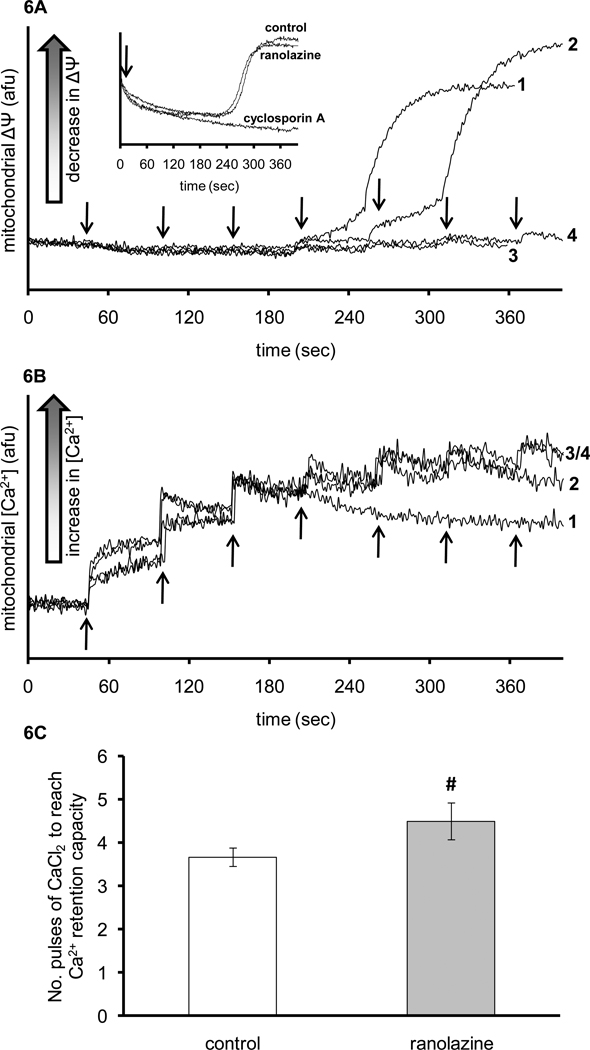
Figure 6 shows representative traces for ΔΨm (Panel A) and m[Ca2+] (Panel B) measured during Ca2+ pulse challenges in mitochondria isolated from control (line 1, 3) and ranolazine-treated (line 2, 4) hearts. Mitochondria were exposed to pulses of 25 µM CaCl2 injected every min into the mitochondrial suspension to instigate pathologic Ca2+ overload. Mitochondria isolated from control IR hearts exhibited earlier mitochondrial depolarization and lower Ca2+ retention capacity than mitochondria isolated from ranolazine-treated hearts. Lines 3 and 4 are similar to lines 1 and 2 except that cyclosporine A (CSA, 0.5 µM) was added to the suspension to prevent mPTP opening. ΔΨm did not change with added CaCl2 pulses in the presence of CSA, which indicates that the mitochondrial depolarization observed in lines 1 and 2 is due to opening of mPTP. Figure 6C summarizes the number of pulses of 25 µM CaCl2 needed to reach maximal Ca2+ retention capacity and to initiate mitochondrial depolarization. IR only hearts required fewer pulses (3.8±0.2) to induce ΔΨm collapse compared to ranolazine-treated hearts (4.8±0.7) suggesting a relative delay in mPTP opening. Inset in Figure 6A shows membrane potential measurement in freshly isolated mitochondria from hearts that were neither subjected to IR nor perfused with KR as time control. The data were obtained from mitochondria that were challenged with constant infusion of 25 µM/min CaCl2. Ranolazine, cyclosporine A, or vehicle (control) was added to the mitochondrial suspension at the beginning of the run. Ranolazine did not directly delay mPTP opening as did cyclosporine A and it was not different from vehicle (determined by the depolarization in ΔΨm).
Figure 6.
Representative traces of rhodamine 123 fluorescence (indicative of membrane potential, ΔΨm) (A) and indo 1 fluorescence (indicative of m[Ca2+]) (B) in mitochondria isolated from IR only hearts (traces 1, 3; n=3) and ranolazine + IR hearts (traces 2, 4; n=3). Mitochondria (0.5 mg/ml) were suspended in KCl-based experimental buffer containing 10 mM pyruvate. In traces 3 and 4, the mitochondrial suspension also contained 0.5 µM cyclosporine A to prevent mPTP opening. The arrows indicate when pulses of 25 µM of CaCl2 were added to the mitochondrial suspension. Panel C shows a summary of the number of CaCl2 pulses needed to reach maximal Ca2+ retention capacity and to initiate ΔΨm depolarization. #P < 0.05 ranolazine vs. control. Inset in panel A represents traces of rhodamine 123 fluorescence (membrane potential, ΔΨm) with 25 µM/min of CaCl2 constantly infused in the mitochondrial suspension with the addition of either ranolazine, cyclosporine A or vehicle as control. Arrow in the inset indicates when ranolazine, cyclosporine A, or vehicle was added.
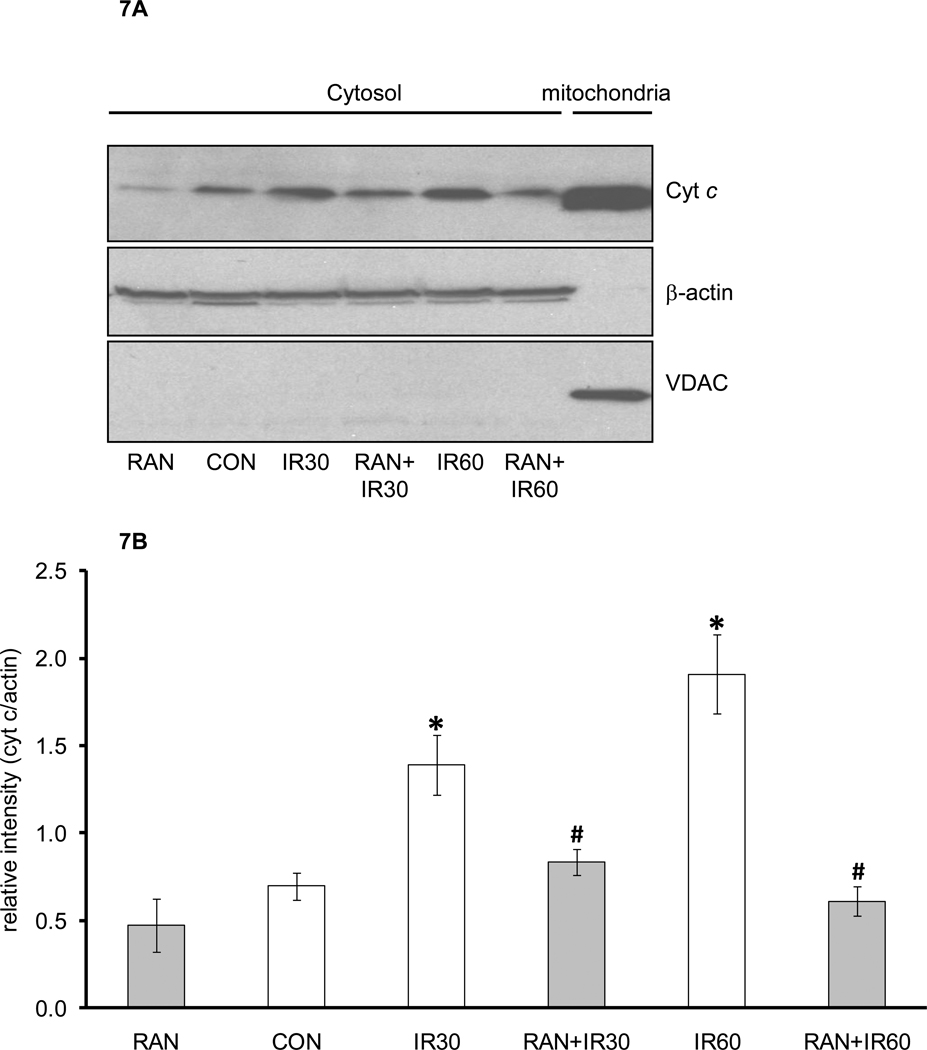
Figure 7 shows mitochondrial cytochrome c release assayed in cytosolic fractions obtained from the time control, IR only, and ranolazine + IR groups (Panel A). Compared to the time-matched controls, the control treated hearts at 30 min of reperfusion (IR30) and 60 min of reperfusion (IR60) displayed a higher amount of cytochrome c in the cytosol. When hearts were treated briefly with ranolazine before ischemia, the amount of cytochrome c released into the cytosol was markedly abated. Protein levels of β–actin were similar among groups, indicating equal protein loading of the cytosolic fraction. The lack of expression of VDAC in the cytosol demonstrated that the cytosolic fractions were not contaminated by the mitochondrial fractions. Panel B summarizes the levels of cytochrome c band densities in the cytosol following IR. Cytochrome c release was higher after 60 min reperfusion than at 30 min reperfusion. Ranolazine treatment before ischemia attenuated cytochrome c release at 30 min and 60 min reperfusion, but there was no significant difference in cytochrome c release between these two time points.
Figure 7.
Representative cytochrome c measurements (A) using Western blotting in cytosolic and mitochondrial fractions. Data are summarized in panel B. Abbreviations are: RAN, hearts treated with ranolazine only without ischemia; CON, hearts not treated with ranolazine and not exposed to ischemia; IR30, hearts exposed to 30 min ischemia and 30 min reperfusion without treatment with ranolazine; RAN+IR30, hearts treated with ranolazine followed by 30 min ischemia and 30 min reperfusion; IR60, hearts exposed to 30 min ischemia followed by 60 min reperfusion without treatment with ranolazine; RAN+IR60, hearts treated with ranolazine followed by 30 min ischemia and 60 min reperfusion. *P < 0.05 ischemia groups vs. no ischemia groups. #P < 0.05 RAN+IR30 or RAN+IR60 vs. IR30 or IR60.
4. Discussion
Our results indicate clearly that ranolazine, when present during ischemia, protects isolated hearts against IR injury as shown by the higher developed LVP and lower diastolic LVP on reperfusion, by the smaller infarct size, and by the decreased incidence of dysrhythmias. This improvement in cardiac function was associated with less O2•− production and reduced cCa2+ and mCa2+ overload without an effect on redox state (NADH, FAD). Ranolazine treatment improved coronary flow on reperfusion suggesting less injury to vascular and endothelial cells than to heart muscle cells. During 1 min perfusion before IR, ranolazine did not change O2•−, cCa2+, mCa2+, NADH, or FAD. In addition, mitochondria isolated from ranolazine-treated hearts showed a small resistance to collapse of ΔΨm due to mCa2+ overload as evidenced by a delay in mPTP opening and less cytochrome c release. Although ranolazine treatment improved LVP and coronary flow on reperfusion, it is unlikely that the higher flow was responsible for the higher LVP because artificially increasing flow after ischemia does not improve contractility (unpublished data) suggesting there is more injury to contractile cells than to vascular or endothelial cells.
Mitochondria play a crucial role in IR injury and hence are promising targets for novel antiischemic therapies. The importance of mitochondria as both targets and mediators of IR is becoming increasingly recognized [4]. It is often demonstrated that excess mitochondrial ROS and mCa2+ overload are the two major factors that are intertwined in the pathology of IR injury. But how they are correlated, or how they influence each other, is a subject of intense debate. As noted in a review article by Brookes et al. [56], excess m[Ca2+] can cause an increase in ROS by different mechanisms such as by stimulation of citric acid cycle and oxidative phosphorylation to increase electron leak, by enhancing NO• generation which blocks complex IV and causes electron leak from complex III, and by enhancing cytochrome c dislocation. On the other hand, ROS was shown to enhance Ca2+ release from the sarcoplasmic reticulum [57]. These events could underlie our findings for Ca2+-induced ROS production in IR injury and the reduction of both after treatment with ranolazine. An increase in m[Ca2+] [38,58] is caused by the increase in c[Ca2+] as shown in our study (Figure 3) and reported by others [21,59,60]. The increase in c[Ca2+] and m[Ca2+] may result from an increase in c[Na+] and activation of the Na+/Ca2+ exchange. The increase in c[Na+] results from failed Na+/K+ ATPase pump activity during ischemia and early reperfusion, coupled with an increased pH gradient between the intracellular and extracellular spaces and the activation of the relatively quiescent Na+/H+ exchanger [61].
However, increased c[Na+] may also arise in part from activation of the late Na+ current by some toxic ischemic metabolites [62,63] and by ROS that are generated during reperfusion [64]. Thus ranolazine, as an inhibitor of the late Na+ current, could reduce c[Na+] during ischemia and subsequently reduce c[Ca2+]. Song et al. [65] reported that ranolazine attenuated H2O2-induced intracellular Na+ and Ca2+ overload in rabbit isolated ventricular myocytes. Our current study supports and extends the study by Song et al. by demonstrating that ranolazine not only causes a decrease in c[Ca2+] (Figure 3), but also more importantly, resulted in a decrease in m[Ca2+] during ischemia in intact beating hearts (Figure 2B). Although m[Ca2+] also increased during late ischemia in ranolazine-treated hearts, mitochondria from these hearts were better able to rapidly expel the excess Ca2+ and lower their Ca2+ content immediately on reperfusion than the control IR only hearts. This is highly relevant since the most damaging Ca2+ influx appears to occur during early reperfusion [66].
Mitochondria maintain a delicate balance between ROS generation and ROS scavenging; this difference is called ROS emission. However, under pathological conditions such as in cardiac IR, the balance that keeps ROS to a minimum is altered due to the increase in ROS generation coupled with a decrease in ROS scavenging capability. The decrease in ROS scavenging capacity during ischemia is likely a result of multiple factors such as the loss of reducing equivalents (NADH, FADH2) which are necessary to recharge the ROS scavenging capacity of glutathione, thioredoxin, peroxiredoxins and other scavenging systems [67], or due to release of cytochrome c (also a ROS scavenger) in the cytosol as a result of mPTP opening, or transient partial permeabilization of the outer mitochondrial membrane [67]. It is unlikely that ranolazine reduces ROS during ischemia by providing more NADH for the glutathione system since our results show that ranolazine did not further increase mitochondrial NADH during IR (Figure 1A) compared to IR controls.
On the other hand, ranolazine treatment reduced the release of cytochrome c into the cytosol (Figure 7). Cytochrome c can accept or donate an electron depending on the redox state of its heme (Fe). Thus it can scavenge O2•− through its capacity to be reduced alternatively by the transfer of electrons from O2•−, thereby reducing ROS emission [67,68]. It is therefore possible that by preserving the scavenging capacity of cytochrome c, and/or by increasing the efficiency of electron transfer through the ETC, ranolazine treatment reduced ROS especially during reperfusion.
The increase in mitochondrial O2•− generation arises primarily from an impaired electron flux via the ETC due to the low mitochondrial O2 concentration, and O2•− production primarily occurs from mitochondrial complexes I and III [6,56] with complex III playing a central role in this process [69]. In a previous study [38] using a similar experimental protocol, we showed that blocking complex I of the ETC with amobarbital, a reversible complex I blocker, decreased electron flow to complex III and subsequently reduced ROS generation and protected mitochondria against ischemic injury. Like amobarbital, ranolazine is reported to block complex I [20], albeit as a weaker inhibitor compared to amobarbital or rotenone, especially in energetically coupled mitochondria. However, in uncoupled mitochondria, ranolazine had a greater effect to inhibit complex I [20]. It was suggested that this occurs because the lower membrane potential in uncoupled mitochondria, with a more acidic environment, favors greater protonation and uptake of ranolazine, or that it is only the charged form of ranolazine that is inhibitory [20]. It is important to note that our experiments showed no evidence of a direct effect of ranolazine to block complex I in the isolated heart or in isolated mitochondrial studies.
We observed similar levels of NADH during IR in ranolazine-treated and control hearts which may seem to be in contradiction with our previous studies [38,45,70] that show higher NADH levels are associated with better cardiac recovery. This could result from the fact that ranolazine has been reported to stimulate glucose oxidation in ischemic, and reperfused ischemic hearts [18] by indirectly increasing the amount of the active dephosphorylated form of the pyruvate dehydrogenase complex [71], which may lead to more usage of the available NADH.
In conclusion, ranolazine protected mitochondria against IR as shown by the lesser O2•− production and reduced cCa2+ and mCa2+ overload. This was associated with a small delay in mPTP opening but there was no strong evidence for a direct effect of ranolazine treatment on a component of the pore. We observed in freshly isolated mitochondria (inset in Figure 6A) that ranolazine did not directly delay mPTP opening-induced membrane potential depolarization as does cyclosporine A. Moreover, in companion studies, we found that ranolazine preserved the mitochondrial supercomplexes in part by restoring cardiolipin and so preserved electron flux in mitochondrial Fe-S clusters during IR [72–74].Yet, it is still undetermined if these results are due to direct or indirect effect of ranolazine on mitochondria. These observations along with our other preliminary data (not shown), and importantly, the results reported in this current study all imply an indirect mechanism for ranolazine to protect cardiomyocyte mitochondria. Although ranolazine-treated hearts showed a slightly higher resistance to mPTP opening and reduced cytochrome c release, we attribute these findings to indirect preservation of mitochondria. Overall, our results indicate that ranolazine may confer protection against IR in our model by reducing cCa2+ and mCa2+ loading and ROS, possibly secondary to blocking the late Na+ channel current. Even though we did not observe any direct evidence of a mitochondrial effect of ranolazine, it clearly contributes to mitochondrial protection via its overall cardioprotective effect.
ACKNOWLEGMENTS
The authors thank Anita Tredeau and Steven Contney for their valuable assistance.
This work was published in part in abstract form: Aldakkak et al. FASEB J, 2009, 2010 and was supported by the American Heart Association (0855940G, D.F. Stowe), the National Institutes of Health (R01 HL095122, A.K.S. Camara, and R01 HL089514, D.F. Stowe), and the Veterans Administration (VA Merit 8204-05P, D.F. Stowe).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None.
Contributor Information
Mohammed Aldakkak, Email: maldakka@mcw.edu.
Amadou KS Camara, Email: aksc@mcw.edu.
James S Heisner, Email: jheisner@mcw.edu.
Meiying Yang, Email: myang@mcw.edu.
David F Stowe, Email: dfstowe@mcw.edu.
REFERENCES
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960;70:68–78. [PubMed] [Google Scholar]
- 3.Mewton N, Ivanes F, Cour M, Ovize M. Postconditioning: from experimental proof to clinical concept. Dis Model Mech. 2010;3:39–44. doi: 10.1242/dmm.004309. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–C147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- 5.Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 6.Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- 8.Baines CP. The mitochondrial permeability transition pore and the cardiac necrotic program. Pediatr Cardiol. 2011;32:258–262. doi: 10.1007/s00246-010-9880-9. [DOI] [PubMed] [Google Scholar]
- 9.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain D, Dasgupta P, Hughes LO, Lahiri A, Raftery EB. Ranolazine (RS-43285): a preliminary study of a new anti-anginal agent with selective effect on ischaemic myocardium. Eur J Clin Pharmacol. 1990;38:111–114. doi: 10.1007/BF00265967. [DOI] [PubMed] [Google Scholar]
- 11.Mega JL, Hochman JS, Scirica BM, Murphy SA, Sloan S, McCabe CH, Merlini P, Morrow DA. Clinical features and outcomes of women with unstable ischemic heart disease: observations from metabolic efficiency with ranolazine for less ischemia in non-ST-elevation acute coronary syndromes-thrombolysis in myocardial infarction 36 (MERLIN-TIMI 36) Circulation. 2010;121:1809–1817. doi: 10.1161/CIRCULATIONAHA.109.897231. [DOI] [PubMed] [Google Scholar]
- 12.Scirica BM, Braunwald E, Belardinelli L, Hedgepeth CM, Spinar J, Wang W, Qin J, Karwatowska-Prokopczuk E, Verheugt FW, Morrow DA. Relationship between nonsustained ventricular tachycardia after non-ST-elevation acute coronary syndrome and sudden cardiac death: observations from the metabolic efficiency with ranolazine for less ischemia in non-ST-elevation acute coronary syndrome-thrombolysis in myocardial infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2010;122:455–462. doi: 10.1161/CIRCULATIONAHA.110.937136. [DOI] [PubMed] [Google Scholar]
- 13.Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, Molhoek P, Verheugt FW, Gersh BJ, McCabe CH, Braunwald E. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 14.Allely MC, Alps BJ. The effects of the novel anti-anginal compound RS 43285 on myocardial conduction in the anaesthetized dog. Br J Pharmacol. 1988;93:375–382. doi: 10.1111/j.1476-5381.1988.tb11444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allely MC, Alps BJ. Prevention of myocardial enzyme release by ranolazine in a primate model of ischaemia with reperfusion. Br J Pharmacol. 1990;99:5–6. doi: 10.1111/j.1476-5381.1990.tb14641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gralinski MR, Black SC, Kilgore KS, Chou AY, McCormack JG, Lucchesi BR. Cardioprotective effects of ranolazine (RS-43285) in the isolated perfused rabbit heart. Cardiovasc Res. 1994;28:1231–1237. doi: 10.1093/cvr/28.8.1231. [DOI] [PubMed] [Google Scholar]
- 17.Stanley WC. Ranolazine: new approach for the treatment of stable angina pectoris. Expert Rev Cardiovasc Ther. 2005;3:821–829. doi: 10.1586/14779072.3.5.821. [DOI] [PubMed] [Google Scholar]
- 18.McCormack JG, Barr RL, Wolff AA, Lopaschuk GD. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation. 1996;93:135–142. doi: 10.1161/01.cir.93.1.135. [DOI] [PubMed] [Google Scholar]
- 19.Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004;44:192–199. doi: 10.1097/00005344-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Wyatt KM, Skene C, Veitch K, Hue L, McCormack JG. The antianginal agent ranolazine is a weak inhibitor of the respiratory complex I, but with greater potency in broken or uncoupled than in coupled mitochondria. Biochem Pharmacol. 1995;50:1599–1606. doi: 10.1016/0006-2952(95)02042-x. [DOI] [PubMed] [Google Scholar]
- 21.An J, Varadarajan SG, Camara A, Chen Q, Novalija E, Gross GJ, Stowe DF. Blocking Na+/H+ exchange reduces [Na+]i and [Ca2+]i load after ischemia and improves function in intact hearts. Am J Physiol Heart Circ Physiol. 2001;281:H2398–H2409. doi: 10.1152/ajpheart.2001.281.6.H2398. [DOI] [PubMed] [Google Scholar]
- 22.Camara AK, Riess ML, Kevin LG, Novalija E, Stowe DF. Hypothermia augments reactive oxygen species detected in the guinea pig isolated perfused heart. Am J Physiol Heart Circ Physiol. 2004;286:H1289–H1299. doi: 10.1152/ajpheart.00811.2003. [DOI] [PubMed] [Google Scholar]
- 23.Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H566–H574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- 24.Kevin LG, Novalija E, Riess ML, Camara AK, Rhodes SS, Stowe DF. Sevoflurane exposure generates superoxide but leads to decreased superoxide during ischemia and reperfusion in isolated hearts. Anesth Analg. 2003;96:949–955. doi: 10.1213/01.ANE.0000052515.25465.35. table of contents. [DOI] [PubMed] [Google Scholar]
- 25.Riess ML, Camara AK, Chen Q, Novalija E, Rhodes SS, Stowe DF. Altered NADH and improved function by anesthetic and ischemic preconditioning in guinea pig intact hearts. Am J Physiol Heart Circ Physiol. 2002;283:H53–H60. doi: 10.1152/ajpheart.01057.2001. [DOI] [PubMed] [Google Scholar]
- 26.Riess ML, Camara AK, Novalija E, Chen Q, Rhodes SS, Stowe DF. Anesthetic preconditioning attenuates mitochondrial Ca2+ overload during ischemia in Guinea pig intact hearts: reversal by 5-hydroxydecanoic acid. Anesth Analg. 2002;95:1540–1546. doi: 10.1097/00000539-200212000-00013. table of contents. [DOI] [PubMed] [Google Scholar]
- 27.Bunger R. The stabilizing effect of pyruvate on the function and metabolism of isolated perfused guinea pig hearts. Fortschr Med. 1978;96:1246. [PubMed] [Google Scholar]
- 28.Bunger R, Haddy FJ, Querengasser A, Gerlach E. An isolated guinea pig heart preparation with in vivo like features. Pflugers Arch. 1975;353:317–326. doi: 10.1007/BF00587028. [DOI] [PubMed] [Google Scholar]
- 29.Scaduto RC, Jr., Grotyohann LW. Hydrolysis of Ca2+-sensitive fluorescent probes by perfused rat heart. Am J Physiol Heart Circ Physiol. 2003;285:H2118–H2124. doi: 10.1152/ajpheart.00881.2001. [DOI] [PubMed] [Google Scholar]
- 30.Camara AK, Aldakkak M, Heisner JS, Rhodes SS, Riess ML, An J, Heinen A, Stowe DF. ROS scavenging before 27°C ischemia protects hearts and reduces mitochondrial ROS, Ca2+ overload, and changes in redox state. Am J Physiol Cell Physiol. 2007;292:C2021–C2031. doi: 10.1152/ajpcell.00231.2006. [DOI] [PubMed] [Google Scholar]
- 31.Brandes R, Bers DM. Increased work in cardiac trabeculae causes decreased mitochondrial NADH fluorescence followed by slow recovery. Biophys J. 1996;71:1024–1035. doi: 10.1016/S0006-3495(96)79303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chance B, Williamson JR, Jamieson D, Schoenner B. Properties and kinetics of reduced pyridine nucleotide fluorescence of the isolated and in vivo rat heart. Biochem Z. 1965;341:357–377. [Google Scholar]
- 33.Nuutinen EM. Subcellular origin of the surface fluorescence of reduced nicotinamide nucleotides in the isolated perfused rat heart. Basic Res Cardiol. 1984;79:49–58. doi: 10.1007/BF01935806. [DOI] [PubMed] [Google Scholar]
- 34.Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991;261:H1123–H1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- 35.Barth E, Stammler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992;24:669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 36.Vinnakota KC, Bassingthwaighte JB. Myocardial density and composition: a basis for calculating intracellular metabolite concentrations. Am J Physiol Heart Circ Physiol. 2004;286:H1742–H1749. doi: 10.1152/ajpheart.00478.2003. [DOI] [PubMed] [Google Scholar]
- 37.Rhodes SS, Ropella KM, Camara AK, Chen Q, Riess ML, Stowe DF. How inotropic drugs alter dynamic and static indices of cyclic myoplasmic [Ca2+] to contractility relationships in intact hearts. J Cardiovasc Pharmacol. 2003;42:539–553. doi: 10.1097/00005344-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008;77:406–415. doi: 10.1016/j.cardiores.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 41.Riess ML, Camara AK, Kevin LG, An J, Stowe DF. Reduced reactive O2 species formation and preserved mitochondrial NADH and [Ca2+] levels during short-term 17°C ischemia in intact hearts. Cardiovasc Res. 2004;61:580–590. doi: 10.1016/j.cardiores.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 42.Brandes R, Figueredo VM, Camacho SA, Baker AJ, Weiner MW. Investigation of factors affecting fluorometric quantitation of cytosolic [Ca2+] in perfused hearts. Biophys J. 1993;65:1983–1993. doi: 10.1016/S0006-3495(93)81275-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stowe DF, Fujita S, An J, Paulsen RA, Varadarajan SG, Smart SC. Modulation of myocardial function and [Ca2+] sensitivity by moderate hypothermia in guinea pig isolated hearts. Am J Physiol. 1999;277:H2321–H2332. doi: 10.1152/ajpheart.1999.277.6.H2321. [DOI] [PubMed] [Google Scholar]
- 44.Rhodes SS, Ropella KM, Camara AK, Chen Q, Riess ML, Pagel PS, Stowe DF. Ischemia-reperfusion injury changes the dynamics of Ca2+-contraction coupling due to inotropic drugs in isolated hearts. J Appl Physiol. 2006;100:940–950. doi: 10.1152/japplphysiol.00285.2005. [DOI] [PubMed] [Google Scholar]
- 45.Aldakkak M, Stowe DF, Heisner JS, Spence M, Camara AK. Enhanced Na+/H+ exchange during ischemia and reperfusion impairs mitochondrial bioenergetics and myocardial function. J Cardiovasc Pharmacol. 2008;52:236–244. doi: 10.1097/FJC.0b013e3181831337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.An J, Camara AK, Rhodes SS, Riess ML, Stowe DF. Warm ischemic preconditioning improves mitochondrial redox balance during and after mild hypothermic ischemia in guinea pig isolated hearts. Am J Physiol Heart Circ Physiol. 2005;288:H2620–H2627. doi: 10.1152/ajpheart.01124.2004. [DOI] [PubMed] [Google Scholar]
- 47.Riess ML, Rhodes SS, Stowe DF, Aldakkak M, Camara AK. Comparison of cumulative planimetry versus manual dissection to assess experimental infarct size in isolated hearts. J Pharmacol Toxicol Methods. 2009;60:275–280. doi: 10.1016/j.vascn.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aldakkak M, Stowe DF, Cheng Q, Kwok WM, Camara AK. Mitochondrial matrix K+ flux independent of large-conductance Ca2+-activated K+ channel opening. Am J Physiol Cell Physiol. 2010;298:C530–C541. doi: 10.1152/ajpcell.00468.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinen A, Aldakkak M, Stowe DF, Rhodes SS, Riess ML, Varadarajan SG, Camara AK. Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2007;293:H1400–H1407. doi: 10.1152/ajpheart.00198.2007. [DOI] [PubMed] [Google Scholar]
- 50.Heinen A, Camara AK, Aldakkak M, Rhodes SS, Riess ML, Stowe DF. Mitochondrial Ca2+-induced K+ influx increases respiration and enhances ROS production while maintaining membrane potential. Am J Physiol Cell Physiol. 2007;292:C148–C156. doi: 10.1152/ajpcell.00215.2006. [DOI] [PubMed] [Google Scholar]
- 51.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 52.Haumann J, Dash RK, Stowe DF, Boelens AD, Beard DA, Camara AK. Mitochondrial free [Ca2+] increases during ATP/ADP antiport and ADP phosphorylation: exploration of mechanisms. Biophys J. 2010;99:997–1006. doi: 10.1016/j.bpj.2010.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng J, Zhu M, Schaub MC, Gehrig P, Roschitzki B, Lucchinetti E, Zaugg M. Phosphoproteome analysis of isoflurane-protected heart mitochondria: phosphorylation of adenine nucleotide translocator-1 on Tyr194 regulates mitochondrial function. Cardiovasc Res. 2008;80:20–29. doi: 10.1093/cvr/cvn161. [DOI] [PubMed] [Google Scholar]
- 54.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 55.Stowe DF, Varadarajan SG, An J, Smart SC. Reduced cytosolic Ca2+ loading and improved cardiac function after cardioplegic cold storage of guinea pig isolated hearts. Circulation. 2000;102:1172–1177. doi: 10.1161/01.cir.102.10.1172. [DOI] [PubMed] [Google Scholar]
- 56.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 57.Favero TG, Zable AC, Abramson JJ. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1995;270:25557–25563. doi: 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- 58.Miyamae M, Camacho SA, Weiner MW, Figueredo VM. Attenuation of postischemic reperfusion injury is related to prevention of [Ca2+]m overload in rat hearts. Am J Physiol. 1996;271:H2145–H2153. doi: 10.1152/ajpheart.1996.271.5.H2145. [DOI] [PubMed] [Google Scholar]
- 59.Camara AK, An J, Chen Q, Novalija E, Varadarajan SG, Schelling P, Stowe DF. Na+/H+ exchange inhibition with cardioplegia reduces cytosolic [Ca2+] and myocardial damage after cold ischemia. J Cardiovasc Pharmacol. 2003;41:686–698. doi: 10.1097/00005344-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Javadov S, Choi A, Rajapurohitam V, Zeidan A, Basnakian AG, Karmazyn M. NHE-1 inhibition-induced cardioprotection against ischaemia/reperfusion is associated with attenuation of the mitochondrial permeability transition. Cardiovasc Res. 2008;77:416–424. doi: 10.1093/cvr/cvm039. [DOI] [PubMed] [Google Scholar]
- 61.Teshima Y, Akao M, Jones SP, Marban E. Cariporide (HOE642), a selective Na+-H+ exchange inhibitor, inhibits the mitochondrial death pathway. Circulation. 2003;108:2275–2281. doi: 10.1161/01.CIR.0000093277.20968.C7. [DOI] [PubMed] [Google Scholar]
- 62.Undrovinas AI, Fleidervish IA, Makielski JC. Inward sodium current at resting potentials in single cardiac myocytes induced by the ischemic metabolite lysophosphatidylcholine. Circ Res. 1992;71:1231–1241. doi: 10.1161/01.res.71.5.1231. [DOI] [PubMed] [Google Scholar]
- 63.Wu J, Corr PB. Palmitoyl carnitine modifies sodium currents and induces transient inward current in ventricular myocytes. Am J Physiol. 1994;266:H1034–H1046. doi: 10.1152/ajpheart.1994.266.3.H1034. [DOI] [PubMed] [Google Scholar]
- 64.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol. 1997;500(Pt 3):631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 66.Allen DG, Xiao XH. Role of the cardiac Na+/H+ exchanger during ischemia and reperfusion. Cardiovasc Res. 2003;57:934–941. doi: 10.1016/s0008-6363(02)00836-2. [DOI] [PubMed] [Google Scholar]
- 67.Camara AK, Lesnefsky EJ, Stowe DF. Potential therapeutic benefits of strategies directed to mitochondria. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2009.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 69.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 70.Aldakkak M, Stowe DF, Lesnefsky EJ, Heisner JS, Chen Q, Camara AK. Modulation of mitochondrial bioenergetics in the isolated Guinea pig beating heart by potassium and lidocaine cardioplegia: implications for cardioprotection. J Cardiovasc Pharmacol. 2009;54:298–309. doi: 10.1097/FJC.0b013e3181b2b842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clarke B, Spedding M, Patmore L, McCormack JG. Protective effects of ranolazine in guinea-pig hearts during low-flow ischaemia and their association with increases in active pyruvate dehydrogenase. Br J Pharmacol. 1993;109:748–750. doi: 10.1111/j.1476-5381.1993.tb13637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gadicherla A, Antholine W, Camara AK, Heisner J, Aldakkak M, Boelens A, Stowe DF. Free radical generation and electron flux in mitochondrial Fe-S centers during cardiac injury; changes with mitochondrial protective drug ranolazine. Biophysics J. 2010 (Abstract) [Google Scholar]
- 73.Gadicherla A, Antholine W, Heisner J, Camara AK, Aldakkak M, Boelens A, Yang M, Stowe DF. Protection of NADH-linked Fe-S clusters in cardiac mitochondria by ranolazine. Experimental Biology. 2010 (Abstract) [Google Scholar]
- 74.Gadicherla A, Yang M, Camara AK, Aldakkak M, Boelens A, Wakim B, Stowe DF. Ranolazine preserves the integrity of mitochondrial supercomplexes. Biophysics J. 2010 (Abstract) [Google Scholar]