Abstract
The growth and aging of the population of Hawai‘i with a high incidence of diabetes mandates a need for more effective strategies to manage the healing of complicated wounds. Maggot debridement therapy (MDT) is one alternative utilized with successful results. Observations have indicated that maggots have the ability to debride wound beds, provide anti-microbial activity and also stimulate wound healing in diabetic patients. None of the patients refused MDT due to aversion of this treatment modality and the majority of patients had minimal discomfort. In 17 of 23 patients with multiple co-morbidities, the treatment of their complex diabetic wounds by MDT resulted in improvement or cure. Maggot debridement therapy is an effective treatment of diabetic wounds.
Introduction
Patients with diabetes have difficulty healing wounds. This is especially true in the elderly whose numbers are increasing, resulting in rising cost for the delivery of health care. The annual cost to manage these wounds exceeds 20 billion dollars,1 with a loss of over two million work days.2 The diabetic foot ulcer in particular is more difficult to treat, costing between $7,000 to $10,000 per ulcer. Many of these ulcers may ultimately require amputation of a limb, where the cost may be as high as $65,000 per person.3
There are numerous dressings to choose from, with costly new products coming to the market on a monthly basis, all claiming to improve outcomes. Maggot Debridement Therapy (MDT) has been infrequently used in the last 60 years due to improved dressings, new surgical techniques, and the surge of new antibiotics to treat non-healing wounds when they become infected.4 Medical-grade maggots became commercially available in 2004,5 and today there is a resurgence of interest in MDT with 12 laboratories in 20 countries dispensing them at low cost.6 They are approved for debridement of wounds with necrotic tissue, including pressure ulcers, venous ulcers, neuropathic foot ulcers, and non-healing traumatic or postsurgical wounds.7 A prospective, randomized study8 of patients with wounds comparing MDT to conventional therapy demonstrated the efficacy of MDT in debriding wounds, but there was no difference in the rate of healing. However, uncontrolled diabetics were excluded from the study. Furthermore, as there are no healthcare facilities using this treatment modality in the State of Hawai‘i, the authors felt that a report of a case series of patients treated locally in Hawai‘i with MDT, might be an impetus for further study and usage in this State.
Methods
Patients with diabetic wounds were evaluated for MDT and written consent for treatment was obtained. The maggots (Lucilia sericata) were obtained from Monarch Labs in Long Beach, California for $98.00 per vial plus shipping. Each vial contained 250–500 maggots that were viable long enough for two MDT treatments. The skin was prepped with Cavilon-No Sting barrier film wipes and then Mastisol was applied to increase the adherence of DuoDERM to the skin. Approximately 40–50 maggots were carefully inserted into the wound bed with a sterile Q-tip and gauze moistened with saline was applied on top of the maggots. The mesh, supplied by Monarch Labs, was then glued to the DuoDERM with rubber cement, forming a mesh “cage,” which confined the maggots to the wound bed (Figure 1). A bulky dressing was applied over the wound.
Figure 1.
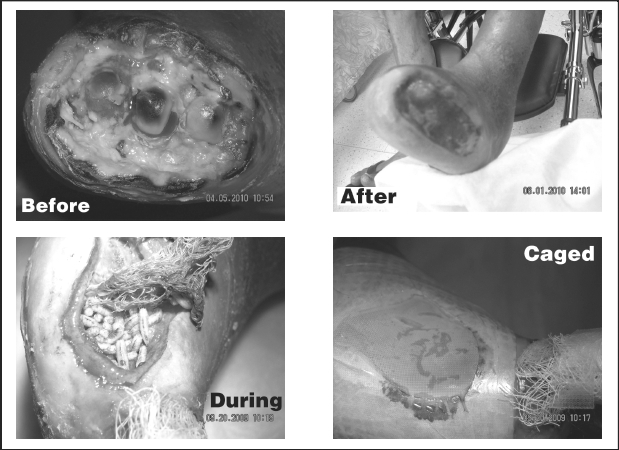
The patients and family members were advised not to disturb the dressing and warned that they would see increased drainage. The patients were then scheduled for follow-up appointments 48 hours later if they were out-patients, or treated in their hospital room 48 hours later if they were in-patients. After 48 hours the wound mesh was removed, and the maggots were then extracted from the wound with wall suction (Figure 1). If the wound was not completely debrided, maggots were reinserted and the wound covered again with mesh. This cycle continued until full debridement was attained.
Twelve of the 23 patients who began MDT as in-patients, were continued on MDT as out-patients. The other eleven patients were all begun on MDT as out-patients. All of the patients had diabetes in addition to multiple other co-morbidities, with 13 of the patients having a hemoglobin A1c > 10 (range 6.1 to 17.3, mean of 10.2). These diabetic patients had the most challenging wounds for debridement often times due to compromised vascularity, not amenable to sharp debridement. Five patients were on chronic hemodialysis with end-stage renal disease (ESRD). Eleven of the 23 patients' wounds had extended to the bone with underlying osteomyelitis
Successful therapy was defined as full-debridement of the wound bed with enhanced granulation tissue formation with or without full closure of the wound. Often, MDT was followed by the application of negative-pressure wound dressings once satisfactory debridement was obtained. This encouraged the rapid formation of granulation tissue. Contraindications to MDT were open wounds in large body cavities and wounds in close proximity to large blood vessels. The team avoided MDT in patients allergic to eggs, soybeans or fly larvae. Coagulopathy was not a contra-indication but such patients were monitored closely since increased bleeding is common during therapy.
Results
The team began MDT in the fall of 2009 in a diabetic patient with a non-healing right hallux amputation. The wound would not close due to multiple co-morbidities (end-stage renal disease, diabetes, and heart disease). A negative- pressure wound dressing led to necrosis of his toes due to insufficient arterial flow. He adamantly refused a below-the-knee amputation. Previous studies9,10 have demonstrated fewer amputations using MDT when compared to conventional therapy. So, MDT was employed to assist in wound debridement and closure of the wound. Subtotal granulation occurred within one month, although devitalized bone still extruded from the wound. After three further months of MDT, complete debridement of exposed bone was obtained (Figure 1).
Over the past nine months the team has treated 23 diabetic patients with MDT. Clinical outcomes are displayed in Table 1. In 17 of 23 patients, successful outcomes were achieved by MDT. These 17 patients exhibited complete debridement with the formation of robust granulation tissue within their wounds. In fact, 6 of these patients formed granulation tissue over exposed tendons, avoiding tendon excision. While MDT may not completely close patients' wounds, partial closure of wounds was obtained in all of the successfully treated patients. For example, one patient with severe lymphedema had been treated since 2006 without any perceptible closure of his venous stasis ulcers. In 10 days, 75% closure of his ulcers was achieved by MDT. Two of the successfully treated patients required a skin graft to achieve full closure, and several others demonstrated further closure of their wounds with negative-pressure dressings. One patient with a large eschar over a below-knee amputation site demonstrated successful debridement of the eschar by MDT but initially did not exhibit granulation in the wound bed. After 30 days, MDT was discontinued and a negative-pressure dressing was applied. After one week further deterioration of the wound bed was observed thought to be due to impaired arterial flow to the extremity. Wet-to-dry dressings were instituted and successful closure of the wound was obtained. Another remarkable feature observed during the treatment of diabetic patients with peripheral neuropathy was the return of normal sensation after several MDT treatments.
Table 1.
Results of MDT
| Age | Sex | Wound | Co-Morbidities | Hgb. A1c | Length of MDT | Outcomes |
| 66 | M | R foot with OM | ESRD, DM, HD, PAD, OB | 6.5 | 60 days | Successful |
| 78 | M | L foot | CABG, CHF, DM , CVA, OB, CRF | 7.7 | 14 days | Unsuccessful - Excessive bleeding |
| 56 | F | R foot with OM | DM, OB | 13.5 | 10 days | Successful |
| 38 | M | R foot with OM | DM, OB, CHF | 11.9 | 2 days | Unsuccessful -Area closed in one treatment |
| 57 | M | R foot with OM | DA, DM, CVA, HD, S, CRF, CHF | 11.9 | 11 days | Successful |
| 61 | M | R foot with OM | DM, ESRD, HD, CVA, CHF | 10.5 | 15 days | Successful |
| 78 | M | BVSU | DM, LE, OB, COPD, CHF, CRF, HD | 6.2 | 10 days | Successful- Three of four ulcers healed |
| 72 | F | R foot with OM | CRF, DM, HD | 12.2 | 2 days | Unsuccessful -Area closed in one treatment |
| 61 | M | R BKA hematoma | DM,HD, CHF, CRF | 8.1 | 30 days | Successful with delayed response |
| 57 | M | R foot | DM, HD, S, ETOH | 12.8 | 10 days | Successful |
| 51 | M | R foot | DM, CRF | 15.6 | 4 days | Successful |
| 60 | F | R foot with OM | DM, HD, OB, PAD | 10.6 | 10 days | Successful |
| 45 | F | L Calf Ulcer | DM, OB, CRF | 8.1 | 1 days | Unsuccessful -Ruptured vein |
| 41 | M | R foot with OM | DM ,CRF, HD | 17.3 | 12 days | Successful |
| 46 | F | L foot | DM, OB, CRF | 11.5 | 4 days | Successful |
| 60 | F | R Calf | DM, OB | 10.6 | 6 days | Unsuccessful due to pyoderma gangrenosum |
| 56 | F | R foot | DM | 12.0 | 14 days | Successful |
| 49 | M | L foot | DM, OB, HD | 9.4 | 10 days | Successful |
| 56 | F | L foot | DM, HD | 6.2 | 8 days | Successful |
| 54 | M | R Hallux with OM | DM | 13.6 | 8 days | Successful |
| 60 | F | L Hallux with OM | DM, ESRD, Stroke, PD | 6.1 | 4 days | Unsuccessful -insufficient opening to dead tissue |
| 58 | M | L foot with OM | DM, HD, ESRD | 6.3 | 4 days | Successful |
| 43 | F | L foot | DM, HD, OB, ESRD | 6.2 | 6 days | Successful |
OM - osteomyelitis; DM - diabetes mellitus; HD - heart disease; OB - obesity; PAD - peripheral arterial disease; ETOH - ethanol abuse; CHF - congestive heart disease; CRF - chronic renal failure; ESRD - end stage renal disease; CVA - cerebral vascular accident; CABG - coronary arterial bypass graft; S - smoker; LE - lymphedema, VSU - venous stasis ulcers, DA - drug addiction, COPD - chronic obstructive pulmonary disease, BKA - below knee amputation.
Six of 23 patients did not significantly benefit from MDT. Three patients who had osteomyelitis of the bones of their feet did not benefit from MDT due to narrow openings of their wounds (i.e., <1cm). Robust granulation tissue formation induced by MDT led to closure of their wounds before the maggots were able to reach the necrotic bone for debridement. In patients that had sufficient exposure of bone, therapy was successful with an apparent halt of bony destruction. A fourth patient had a deep venous stasis ulcer with documented leukocytoplastic vasculitis. The maggots ruptured a vein in her leg causing bleeding which necessitated early discontinuation of the MDT. She was not on anticoagulants. A fifth patient, severely debilitated from a coronary artery bypass graft, congestive heart failure, a cerebral vascular accident, and thrombocytopenia, was anti-coagulated with heparin. The maggots that were placed in his foot ulcer caused excessive bleeding. In a sixth patient who was status-post surgical debridement of a wound, excessive inflammatory reaction at the margins of the wound necessitated discontinuation of MDT. A negative-pressure dressing resulted in enlargement of the wound, which the team later realized was due to pathology, as she was then diagnosed as having pyoderma gangrenosum.
In 60% of diabetic patients treated with MDT, erythema developed in normal skin surrounding the wound. The inflammatory reaction disappeared within 24–48 hours following temporary interruption of MDT, which was thereafter resumed without a resumption of the exhuberant inflammatory reaction. None of the patients discontinued MDT due to an aversion of having maggots placed in their wounds. All of the patients were agreeable to the therapy and most were enthusiastic about this treatment option. Several of the patients complained of discomfort requiring analgesics. This has been previously reported in patients treated with MDT compared to conventional therapy.8 The patients that experienced pain had exposed bone and described the pain as a dull aching sensation that was adequately managed with oral analgesics. One patient temporarily interrupted therapy due to discomfort, but then resumed treatment a short time later without difficulty. Some patients did complain of a “creepy crawling” sensation in their wounds. One patient had maggots escape after getting the dressing wet. A few of the health care professionals were squeamish about assisting in the application and removal of the maggots.
Discussion
Wound debridement, originally thought to be a mechanical effect of the maggots,11 has been shown to be due to three proteolytic enzyme classes that were identified in the maggot excretions.12 Maggot excretions have an inhibitory effect on both Gram-positive and Gram-negative bacteria including methicillin-resistant Staphylococcus aureus, methicillin-sensitive S aureus, Escherichia coli, and Pseudomonas aeruginosa.13 The ammonia excreted by maggots is believed to alter the pH of the wound, which inhibits bacterial growth.14 In 2001 a group of investigators examined the viability of E coli in the gut of the maggot Lucilia sericata and found 67% of the proximal alimentary canal heavily infected. However, in the hindgut there was only 18% viable bacteria, demonstrating the bactericidal effect of maggot gastrointestinal secretions.15
There have been several studies attempting to identify how the maggots increase granulation in the wound bed. A study conducted in 2006 demonstrated an increased migration (but not proliferation) of the fibroblasts which was attributed to the action of serine and metallo-proteinases.16 Another study found high levels of gammainterferon and interleuken-10 in the excretions of maggots that were thought to increase granulation tissue formation.17
The literature recommends not using maggots in a dry wound bed since they require a moist wound to survive.4 The team employed MDT successfully in dry wounds since the maggots create their own moist environment. It is important to advise patients of increased drainage from their wounds while receiving MDT since several of the patients verbalized fear that their condition was worsening when they observed increased drainage. MDT was a preferred method for patients who were not operative candidates due to their underlying vasculopathy. MDT is selective as maggots consume only necrotic tissue, leaving viable tissue intact. Patients needed reassurance that their wounds were not worsening when an exuberant inflammatory response surrounded their wounds. This disappeared in the majority of the patients in 24 to 48 hours, following temporary interruption of MDT. The patient with pyoderma gangrenosum experienced continued inflammation for 96 hours, which was likely due to pathology.
Conclusions
Maggots are able to debride diabetic wounds and stimulate wound healing. This study demonstrates that MDT is an effective strategy for the treatment of complex, diabetic wounds. Furthermore, the authors have shown that MDT works in dry, gangrenous wounds as well. Patient acceptance of, and satisfaction with, MDT was excellent. The majority of the patients tolerated MDT well with only a few experiencing pain that was adequately controlled with oral analgesics.
In 2004, the Food and Drug Administration approved medicalgrade maggots for the treatment of chronic wounds.7 At least one randomized trial8 supports its use compared to conventional therapy. However this trial excluded uncontrolled diabetics. Since many patients with limb ulcers in Hawai‘i have uncontrolled diabetes, the current study focused on this group, where the authors found MDT to be effective treatment.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Harding KG, Morris HL, Patel GK. Science, medicine and future: healing chronic wounds. BMJ. 2002;324(7330):160–163. doi: 10.1136/bmj.324.7330.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherman R. Maggot therapy takes us back to the future of wound care: New and improved maggot therapy for the 21st century. J Diabetes Sci Technol. 2009;3(2):336–344. doi: 10.1177/193229680900300215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation, author. Position statement- The diabetic foot. [December 16, 2010]. Available at: http://www.idf.org/position-statement-diabetic-foot.
- 4.Chan D, Fong D, Leung J, Patil N, Leung Maggot debridement therapy in chronic wound care. Hong Kong Med J. 2007;13(5):382–386. [PubMed] [Google Scholar]
- 5.Sherman R, Shapiro C, Yang R. Maggot therapy for problematic wounds: Uncommon and off-label applications. Advances in Skin & Wound Care. 2007;20(11):602–610. doi: 10.1097/01.ASW.0000284943.70825.a8. [DOI] [PubMed] [Google Scholar]
- 6.Whitaker I, Twine C, Whitaker M, Welck M, Brown C, Ahmed S. Larval therapy from antiquity to the present day: Mechanism of action, clinical applications and future potential. Postgrad Med J. 2007;83(980):409–413. doi: 10.1136/pgmj.2006.055905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration, author. Response letter, medical maggots. [December 16, 2010]. Available at: http://www.accessdata.fda.gov/cdrh_docs/pdf7/K072438.pdf.
- 8.Dunville JC, Worthy G, Bland M, Cullum N, Dowson C, Iglesias C, Mitchell J, Nelson A, Soares M. Larval therapy for leg ulcers (VenUS II): randomized controlled trial. BMJ. 2009;338:b773. doi: 10.1136/bmj.b773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jukema G, Menon A, Bernards A, Steenvoorde P, Rastegar T, Van Dissel J. Amputation-sparing treatment by nature: “surgical maggots revisited. Clinical Infectious Disease. 2002;35:1566–1570. doi: 10.1086/344904. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong D, Salas P, Martin B, Kimbriel H, Nixon B, Boulton A. Maggot therapy in “lower-extremity hospice” wound care: fewer amputations anad more antibiotic free days. J Am Podiart Med Assoc. 2005;95(3):254–257. doi: 10.7547/0950254. [DOI] [PubMed] [Google Scholar]
- 11.Barnard DR. Skeletal-muscular mechanisms of the larva of Lucillia Sericata (Meigen) in relation to feeding habits. Pan-Pac Entomol. 1977;53:223–229. [Google Scholar]
- 12.Chambers L, Woodrow S, Brown AP. Degradation of extracellular matrix components by defined proteinases from the greenbottle larva Lucilia Sericata used for clinical debridement of non-healing wounds. Br J Dermatol. 2003;148:14–23. doi: 10.1046/j.1365-2133.2003.04935.x. [DOI] [PubMed] [Google Scholar]
- 13.Bexfield A, Nigram Y, Thomas S, Ratcliffe NA. Detection and partial characterization of two antibacterial factors for the excretions,/secretions of medical maggot Lucilia Sericata and their activity against methicillin-resistant Staphylococcus aureus (MRSA) Microbes Infect. 2004;6:297–304. doi: 10.1016/j.micinf.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Robinson W. Ammonium bicarbonate secreted by surgical maggots stimulates healing in purulent wounds. Am J Surg. 1940;(47):111–115. [Google Scholar]
- 15.Mumcuoglu KY, Miller J, Friger M, Tarshis M. Destruction of bacteria in the digestive tract of the maggot of Lucilia sericata (Diptera: Calliphoridae) J Med Entomol. 2001;38:161–166. doi: 10.1603/0022-2585-38.2.161. [DOI] [PubMed] [Google Scholar]
- 16.Horobin FJ, Shakesheff KM, Pritchard DI. Promotion of human dermal fibroblast migration, matrix remodeling and modification of fibroblast morphology within a novel 3D model by Lucilia sericata larval secretions. J Invest Dermatol. 2006;126:1690–1696. doi: 10.1038/sj.jid.5700256. [DOI] [PubMed] [Google Scholar]
- 17.Prete PE. Growth effects of Phaenicia sericata larval extract on fibroblasts: mechanism for wound healing by maggot therapy. Life Sci. 1997;60:505–510. doi: 10.1016/s0024-3205(96)00688-1. [DOI] [PubMed] [Google Scholar]


