Abstract
Community-acquired extended-spectrum beta-lactamase E coli (ESBLEC) have not been previously described in Honolulu. Its emergence as a community-acquired pathogen is concerning. This case series describes three patients who were diagnosed with community-acquired ESBLEC bacteriuria in 2010.
Introduction
First detected in Germany in 1983, extended-spectrum beta-lactamase producing Enterobacteriaceae were considered to be a microbiological anomaly. 1 As years progressed, this anomaly became more and more present throughout Europe and into the rest of the world, which was found especially in K pneumoniae and E coli. Prior to the turn of the century, extended-spectrum beta-lactamase E coli (ESBLEC) had always been known to be nosocomial in origin secondary to overuse and misuse of cephalosporins.1 The emergence of community-acquired ESBLEC in the 21st century is concerning. The unique microbiological properties of community-acquired ESBLEC limit the antibiotic options available for proper management. In this case series, we describe and discuss three cases of community-acquired ESBLEC infections occurring in Honolulu in 2010.
Patient 1
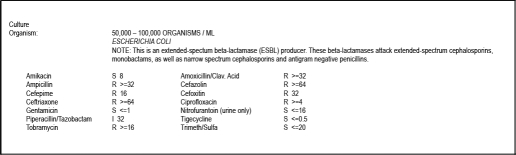
Patient 1 is an 82-year-old, widowed, Japanese, retired woman who presented to her primary care physician's office with a chief complaint of foul-smelling urine for seven days, not associated with hesitancy, dysuria, fever, or chills. She had no pertinent past medical history. She does not reside in a nursing home, nor has she been exposed to a health care facility within the last 6 months. Physical exam was benign. Urinalysis and reflex culture revealed Citrobacter koserii urinary tract infection (UTI) with appropriate sensitivity to ciprofloxacin. She was instructed to complete her three day course of ciprofloxacin, and to follow up if urinary symptoms persist. Seven days later, she reported back to her physician with continued symptoms. Repeat urinalysis revealed the following: Appearance, Clear; Color, Yellow; white blood cells, 15–20/hpf; Specific Gravity, 1.105; pH, 6.5; Leukocytes, 3+. Her urine was again sent off for culture and sensitivity. The patient was switched from ciprofloxacin to amoxicillin-clavulanate and was instructed to follow up in three days if urinary symptoms persist. Two days later, cultures returned positive for ESBL E coli. Sensitivities are listed in Figure 1. A telephone conversation with the patient revealed that her symptoms resolved after two days on amoxicillin-clavulanate, despite displaying resistance. No new therapy was started. She has not presented with new UTI symptoms to this date.
Figure 1.
Urine Culture and Sensitivity Report for Patient 1.
Patient 2
Patient 2 is a 60-year-old, divorced, Japanese, retired woman with a past medical history significant for rheumatoid arthritis treated with methotrexate. She presented to her primary care physician with symptoms of fever, nausea, malaise, and chills following the consumption of raw oysters. After her physician prescribed a 10-day course of azithromycin, her symptoms resolved. Forty-five days later, she visited Las Vegas where she had developed similar flu-like symptoms. She visited an emergency room in Las Vegas where she was assessed and given azithromycin and ibuprofen. No diagnosis was made at discharge. Seven days after returning home to Hawai‘i, she presented to her primary care provider with fever, chills, malaise, and nausea. A complete blood count (CBC) and basic metabolic panel (BMP) were significant for white blood cell (WBC) of 13,600 per µL (13% bands), a blood urea nitrogen (BUN) of 41 mg/dL, and a creatinine of 1.5 mg/dL. She was treated with empiric azithromycin without improvement, and was admitted to the hospital 2 days later due to failed outpatient management. On admission, she revealed that she had increased urinary frequency for a “really long time,” of which she attributed it to her long-term use of diuretics for bilateral leg edema. She did not have hesitancy or pain. Several lab tests including CBC, blood culture, BMP, urinalysis, and urine culture were ordered on admission. Her WBC count was 18,000 per µL. Urinalysis showed: Appearance, Cloudy; Color, Yellow; Specific Gravity, 1.020; pH, 5.5; Nitrites, Positive; Leukocyte Esterase, Negative; WBC 0–5/hpf; Bacteria, Moderate. BMP was unremarkable. Urine and blood cultures were pending. She was initially treated with pipercillin-tazobactam for her suspected urosepsis, but was changed to ciprofloxacin due to an anaphylactic reaction. She was then switched from ciprofloxacin to meropenem once cultures returned positive for ESBL E coli with sensitivities listed in Figure 2. Blood cultures also revealed ESBL E coli with the same sensitivities. Two hours after meropenem infusion, the patient developed wheezing, tachycardia, and hypertension. Meropenem was discontinued. Treatment strategies including amikacin and tigecycline were discussed with the patient, but were refused by the patient due to the possible adverse reactions associated with amikacin. A re-challenge with meropenem was deemed as the best course of antibiotic action. The patient was pre-medicated with famotidine, diphenhydramine, and methylprednisolone, and then re-challenged with meropenem. No adverse events occurred. Meropenem was continued for two weeks without any adverse events. The patient made a full recovery and was discharged eight days after admission.
Figure 2.
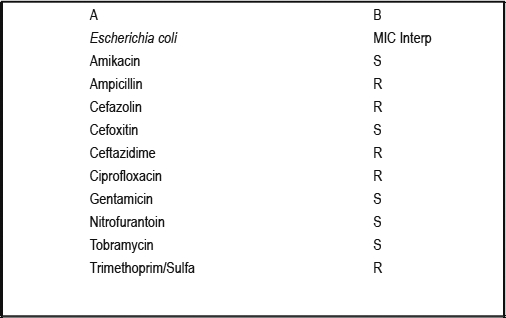
Urine Culture and Sensitivity Report for Patient 2.
Patient 3
Patient 3 is a 76-year-old, married, Japanese, retired man with a past medical history significant for benign prostatic hyperplasia (BPH) and asthma treated with oral prednisone. He does not reside in a nursing home, nor has he been exposed to a health care facility within 6 months. He presented to his primary care physician for a regular 3-month check up for ongoing asthma. Routine urinalysis was performed. The patient reported being in good health and had no complaints of asthma exacerbations or urinary symptoms. Physical exam was benign. Routine urinalysis showed: Appearance, Clear; Color, Light Orange; WBC, 30–40/hpf; Specific Gravity, 1.025; Blood, 1+; pH, 5.0; Leukocytes, 3+. In the face of the abnormal UA, the specimen was sent off for culture and sensitivity. The patient was not given antibiotics due to the lack of presenting symptoms. Two days later, culture and sensitivities revealed ESBL E coli with the sensitivities listed in Figure 3. An infectious diseases specialist was consulted and a 10-day course of nitrofurantoin was initiated in light of his underlying BPH. The patient never developed any complaints of UTI symptoms.
Figure 3.
Urine Culture and Sensitivity Report for Patient 3.
Discussion
This case series is the first published report of community-acquired ESBLEC in Honolulu. The three above cases describe ESBLEC infections lacking nosocomial origins.
Previous studies have described different gene-types of ESBLs in E coli: TEM, SHV, and CTX-M. 1–5 In terms of community acquired ESBL E coli, CTX-M beta-lactamases appear to be the main subtype.1,5,6 CTX-M subtype ESBLEC is capable of being acquired in different ways. One way would be fecal-oral spread either through human-human, animal-human, or fomite contact.7 Second, de novo acquisition of the CTX-M gene into E coli can occur through plasmid conjugation from Kluyvera species, a non-pathogenic Enterobacteriaceae present in our normal gut flora.8–11 These plasmids may also harbor genes for resistance to aminoglycosides, chloramphenicol, sulfonamides, trimethoprim, and tetracyclines.12–14
Risk factors for developing community-acquired ESBLEC urinary tract infections include diabetes mellitus, recent use of fluoroquinolones or cephalosporins, recurrent urinary tract infections, and age greater than 60 years old.14–16
According to the Clinical Laboratory Standards Institute (CLSI), identification of ESBLEC requires one of two criteria: double disk diffusion or minimum inhibitory concentration (MIC) analysis.16 Double-disk diffusion has been the laboratory standard, and was the method used to confirm ESBLEC in our samples. Our cases of community-acquired ESBLEC were confirmed using double-disk diffusion containing clavulanic acid with cefotaxime and ceftazidime. Enhancement of the zone ≥5 mm of the cefotaxime or ceftazidime disk on the side facing the clavulanic acid indicated the presence of an ESBL. ESBLEC can also be detected by examining MICs of a broth dilution containing one of the five extended-spectrum beta-lactam antibiotics (cefpodoxime, ceftazidime, aztreonam, cefotaxime, or ceftriaxone) alone and in addition to 4 µg/mL clavulanic acid. A decrease in the MIC ≥3 twofold dilutions in the presence of clavulanic acid indicates the presence of ESBL.16–17
Despite the results of the susceptibility report, if an organism has been identified as ESBLEC, all cephalosporins and aztreonam should be deemed as inappropriate antibiotic therapy.16 An inoculum effect may be an explanation behind varying susceptibilities.18,19 An increased inoculum may be associated with a marked increase in MIC. Regardless of the sensitivities, with enough inoculum, the ESBLEC is assumed to overcome all extended spectrum beta-lactams. Additionally, if the CTX-M gene is present, there is a concern that the ESBLEC organism acquired a plasmid which may also give resistance to aminoglycosides, chloramphenicol, sulfonamides, trimethoprim, and tetracyclines.12–14
Carbapenems have been described as mainstays of treatment for ESBLEC infections.5,20–22 Meropenem may be the best choice in the event of ESBLEC meningitis due to having fewer chances of neurologic side effects compared to imipenem.23 The use of a cephalosporin with a beta-lactamase inhibitor has been controversial and prone to treatment failure, and thus should not be used as first-line treatment.21,24
In the outpatient setting, oral antibiotics such as fosfomycin or nitrofurantoin have been proven to be effective antibiotics against ESBLEC UTIs (>94% in-vitro susceptibility).24 Fosfomycin-resistant strains of ESBLEC are starting to emerge with increased use.26
The clinical significance of ESBLEC is two-fold. First, the mortality rate of susceptibility/treatment mismatched patients has ranged from 42–100%.27–29 Second is the previously-mentioned inoculum effect of ESBLEC. Susceptibility reports may be deceiving by showing susceptibility to various antibiotics. As mentioned before, all cephalosporins, aztreonam, aminoglycosides, chloramphenicol, sulfonamides, trimethoprim, and tetracyclines should be avoided due to in-vivo and in-vitro differences in susceptibility via an inoculum effect.
In retrospect, gene studies and pulsed-field gel electrophoresis would have been worthwhile techniques to employ on the urine samples of our three patients. Such tests would allow us to determine if the ESBLEC cases were from the same origin through genetic similarities. To our knowledge, these three cases have at least 1 degree of separation; none of which would be from a hospital setting. If gene studies revealed CTX-M genes, it would provide stronger evidence that our patients had community-acquired ESBLEC.
Conclusions
The emergence of community acquired ESBLEC is concerning and is a serious threat to our community. Antibiotic susceptibility of these new superbugs is limited so the evolution of antibiotic resistance may be outpacing the development of new, active antibiotics. These new superbugs are challenging our approach to effective antimicrobial therapy.
Footnotes
None of the authors identify a conflict of interest.
References
- 1.Bradford PA. Extended-spectrum B-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rupp ME, Fey PD. Extended spectrum B-lactamase (ESBL)-producing Enterobacteriaceae. Drugs. 2003;63:353–365. doi: 10.2165/00003495-200363040-00002. [DOI] [PubMed] [Google Scholar]
- 3.Livermore DM. B-lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauernfeind AI, Stemplinger R, Jungwirth P, et al. Sequences of B-lactamase genes encoding CTX-M1 (MEN-1) and CTX-M2 and relationship of their amino acid sequences with those of other B-lactamases. Antimicrob Agents Chemother. 1996;40:509–513. doi: 10.1128/aac.40.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis JS, Herrera M, Wickes B, Patterson JE, Jorgensen JH. First report of the emergence of CTX-M-type extended-spectrum β-lactamases (ESBLs) as the predominant ESBL isolated in a U.S. health care system. Antimicrob Agents Chemother. 2007;51(11):4015–4021. doi: 10.1128/AAC.00576-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernard H, Tancrede C, Livrelli V, et al. A novel plasmid-mediated extended-spectrum B-lactamase not derived from TEM- or SHV-type enzymes. J Antimicrob Chemother. 1992;29:590–592. doi: 10.1093/jac/29.5.590. [DOI] [PubMed] [Google Scholar]
- 7.Meunier D, Jouy E, Lazizzera C, Kobisch M, Madec JY. CTX-M1 and CTX-M15 type beta-lactamases in clinical Escherichia coli isolates recovered from food-producing animals in France. Int J Antimicrob Agents. 2006;28:402–407. doi: 10.1016/j.ijantimicag.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Poirel L, Kampfer P, Nordmann P. Chromosome-encoded Ambler class A B-lactamase of Kluyvera georgiana, a probable progenitor of a subgroup of CTX-M extended-spectrum B-lactamases. Antimicrob Agents Chemother. 2002;46:4038–4040. doi: 10.1128/AAC.46.12.4038-4040.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humeniuk C, Arlet G, Gautier V, et al. B-lactamases of Kluyvera ascorbata, probable progenitors of some plasmid-encoded CTX-M types. Antimicrob Agents Chemother. 2002;46:3045–3049. doi: 10.1128/AAC.46.9.3045-3049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decousser JW, Poirel L, Nordmann P. Characterization of a chromosomally encoded extendedspectrum class A B-lactamase from Kluyvera cryocrescens. Antimicrob Agents Chemother. 2001;45:3595–3598. doi: 10.1128/AAC.45.12.3595-3598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woerther PL, Angebault C, Lescat M, Ruppe E, Skurnik D, et al. Emergence and dissemination of extended-spectrum β-lactamase-producing Escherichia coli in the community: lessons from the study of a remote and controlled population. J Infect Dis. 2010;202(4):515–523. doi: 10.1086/654883. [DOI] [PubMed] [Google Scholar]
- 12.Morosini MI, Garcia-Castillo M, Coque TM, et al. Antibiotic co-resistance in extended-spectrum B-lactamase-producing Enterobacteriaceae and the in vitro activity of tigecycline. Antimicrob Agents Chemother. 2006;50:2695–2699. doi: 10.1128/AAC.00155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordmann P, Poirel L. Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J Antimicrob Chemother. 2005;5:463–469. doi: 10.1093/jac/dki245. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Bano J, Navarro MD, Romero L, et al. Epidemiology and clinical features of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in nonhospitalized patients. J Clin Microbiol. 2004;42:1089–1094. doi: 10.1128/JCM.42.3.1089-1094.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colodner R, Rock W, Chazan N, et al. Risk factors for development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. Eur J Clin Micriobiol Infect Dis. 2004;23:163–167. doi: 10.1007/s10096-003-1084-2. [DOI] [PubMed] [Google Scholar]
- 16.Clinical Laboratory Standards Institute, author. Performance standards for antimicrobial susceptibility testing; twentieth informational supplement (aerobic dilution) M100-S20. Wayne, Pa: CLSI; 2010. [Google Scholar]
- 17.Steward CD, Rasheed JK, Hubert SK, et al. Characterization of clinical isolates of Klebsiella pneumoniae from 19 laboratories using the National Committee for Clinical Laboratory Standards extended-spectrum b-lactamase detection methods. J Clin Microbiol. 2001;39:2684–2872. doi: 10.1128/JCM.39.8.2864-2872.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson KS, Moland ES. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum b-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2001;45:3548–3554. doi: 10.1128/AAC.45.12.3548-3554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Queenan AM, Foleno B, Gownley C, Wira E, Bush K. Effects of inoculum and beta-lactamase activity in AmpC- and extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae clinical isolates tested by using NCCLS ESBL methodology. J Clin Microbiol. 2004;1:269–275. doi: 10.1128/JCM.42.1.269-275.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paterson DL, Von Ko W, Gottberg A, et al. In vitro susceptibility and clinical outcome of bacteremia due to extended-spectrum beta-lactamase producing Klebsiella pneumoniae. Clin Infect Dis. 1998;27:956. [Google Scholar]
- 21.Paterson DL. Recommendation for treatment of severe infections caused by Enterobacteriaceae producing extended-spectrum b-lactamases (ESBLs) Clin Microbiol Infect. 2000;6:460–463. doi: 10.1046/j.1469-0691.2000.00107.x. [DOI] [PubMed] [Google Scholar]
- 22.Jacoby G, Han P, Tran J. Comparative in vitro activities of carbapenem L-749,345 and other antimicrobials against multiresistant Gram-negative clinical pathogens. Antimicrob Agents Chemother. 1997;41:1830–1831. doi: 10.1128/aac.41.8.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segal-Maurer S, Mariano N, Qavi A, Urban C, Rahal JJ. Successful treatment of ceftazidime-resistant Klebsiella pneumoniae ventriculitis with intravenous meropenem and intraventricular polymyxin B: case report and review. Clin Infect Dis. 1999;28:1134–1138. doi: 10.1086/514754. [DOI] [PubMed] [Google Scholar]
- 24.Paterson DL, Singh N, Gayowski T, Marino IR. Fatal infection due to extended-spectrum beta-lactamase producing Escherichia coli: implications for antibiotic choice for spontaneous bacterial peritonitis. Clin Infect Dis. 1999;28:683–684. doi: 10.1086/517217. [DOI] [PubMed] [Google Scholar]
- 25.Auer S, Wojna A, Hell M. Oral treatment options for ambulatory patients with urinary tract infections caused by extended-spectrum-B-lactamase-producing Escherichia coli. Antimicrob Agents Chemother. 2010;54:4006–4008. doi: 10.1128/AAC.01760-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oteo J, Bautista V, Lara N, Cuevas O, Arroyo M, et al. Parallel increase in community use of fosfomycin and resistance to fosfomycin in extended-spectrum-β-lactamase (ESBL)-producing Escherichia coli. J Antimicrob Chemother. 2010;65(11):2459–2463. doi: 10.1093/jac/dkq346. [DOI] [PubMed] [Google Scholar]
- 27.Meyer KS, Urban C, Eagan JA, et al. Nosocomial outbreak of Klebsiella infection resistant to late-generation cephalosporins. Ann Intern Med. 1993;119:353–358. doi: 10.7326/0003-4819-119-5-199309010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Naumovski L, Quinn JP, Miyashiro D, et al. Outbreak of ceftazidime resistance due to a novel extended-spectrum β-lactamase in isolates from cancer patients. Antimicrob Agents Chemother. 1992;36:1991–1996. doi: 10.1128/aac.36.9.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiappa DA, Hayden MK, Matushek MG, et al. Ceftazidime resistant Klebsiella pneumoniae and Escherichia coli bloodstream infection: a case-control and molecular epidemiologic investigation. J Infect Dis. 1996;174:529–536. doi: 10.1093/infdis/174.3.529. [DOI] [PubMed] [Google Scholar]