Abstract
Plastid trnL-trnF and nuclear ribosomal ITS sequences were obtained from selected wild-type individuals of Polygonum minus Huds. in Peninsular Malaysia. The 380 bp trnL-trnF sequences of the Polygonum minus accessions were identical. Therefore, the trnL-trnF failed to distinguish between the Polygonum minus accessions. However, the divergence of ITS sequences (650 bp) among the Polygonum minus accessions was 1%, indicating that these accessions could be distinguished by the ITS sequences. A phylogenetic relationship based on the ITS sequences was inferred using neighbor-joining, maximum parsimony and Bayesian inference. All of the tree topologies indicated that Polygonum minus from Peninsular Malaysia is unique and different from the synonymous Persicaria minor (Huds.) Opiz and Polygonum kawagoeanum Makino.
Keywords: ITS, phylogenetics, plastid DNA, Polygonum minus, trnL-trnF
1. Introduction
Current advances in plant molecular biology techniques and approaches provide a convenient and rapid evaluation of the differences in informational content, derived from DNA sequence data, of related individuals [1,2]. Polymerase chain reaction (PCR)-based techniques have been used comprehensively as plant molecular markers for significant phylogenetic presumption among relatively closely related species [3]. The impact of genetic data from chloroplast DNA (cpDNA) and nuclear ribosomal DNA (nrDNA) can be clearly seen in the fields of plant phylogenetics, systematics, population genetics and molecular biology [4,5].
Polygonum minus Huds., commonly known as kesum in Malaysia, is currently classified in the Polygonum section Persicaria. In 1967, Ridley [6] reported and described P. minus and another eight species of Polygonum from Malay Peninsula. No additional information on P. minus was available until Anjen et al. [7] and Freeman and Reveal [8] reported Polygonum kawagoeanum Makino and Persicaria minor (Huds.) Opiz, respectively, as being synonyms of P. minus. In 2010, we reported the chemical composition of essential oils in P. minus using two dimensional gas chromatography time of flight mass spectrometry (GC×GC-TOF MS) and found a significantly high level of aldehydes, especially decanal and dodecanal as the dominant aldehydes. Thus, P. minus appears to be more similar to Polygonum odoratum Lour., the Vietnamese coriander and Persicaria hydropiper L., known as the laksa plant in Singapore [9–11]. More recently, for the first time, we reported peltate glandular trichomes and conical brushlike clustered trichomes on both the adaxial and abaxial surfaces of P. minus and revealed potentially important distinctive features of the foliar micromorphology of the genus Polygonum [12]. Previously, Lerstern and Curtis [13] noted only capitate trichomes on the epidermis of P. minus, and they suggested trichome structure as a significant taxonomic feature in the genus Polygonum.
The taxonomic classification of the Polygonum genus has been debated since the work of Steward [14] and has presented a great taxonomic challenge [15]. Using cpDNA and nuclear ITS sequences, molecular phylogenetic studies within section Persicaria revealed that it is monophyletic and is most closely related to sections Tovara and Echinocaulon within the genus Polygonum [16]. To our knowledge, there has been no report on the genetic differentiation of P. minus individuals from Peninsular Malaysia. Therefore, in this study, we aim to highlight the potential impact of molecular genetic marker on selected P. minus accessions from Peninsular Malaysia based on the trnL-trnF and nuclear ribosomal ITS sequences in order to clarify the taxonomic status of P. kawagoeanum and P. minor as synonyms to P. minus.
2. Results and Discussion
PCR amplification of the trnL-trnF yielded a PCR product with a single-band of 380 bp in size, indicating the suitability of the universal PCR primers for P. minus. The trnL-trnF sequences among the four P. minus accessions were identical. This result implies that the trnL-trnF region from the plastid genome was not suitable to differentiate individuals of P. minus. The BLAST analysis against the GenBank database showed that the trnL-trnF sequences of P. minus had 100% similarity with sequences of Persicaria pubescens (EU197040.1), Persicaria hydropiper (EF653805.1) and Persicaria longiseta (EU109597.1). Therefore, the plastid DNA variation among members of the Persicaria section was low. However, plastid DNA variation has been successfully utilized in population genetics and biosystematic studies of other plants [17–19]. Okaura and Harada [20] reported the intraspecific variation in three non-coding regions of the plastid DNA in 21 Japanese beech (Fagus crenata Blume) populations and revealed the highly structured geographical distribution of cpDNA haplotypes.
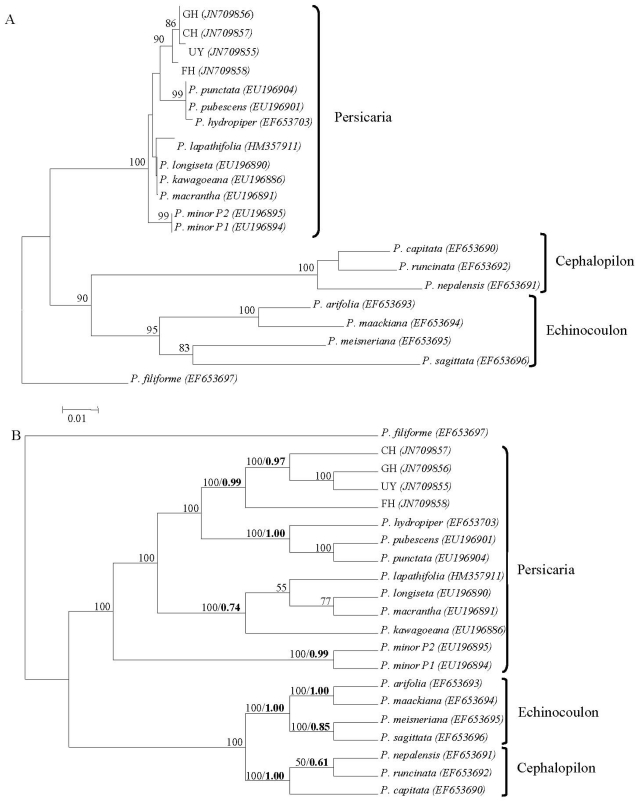
The amplification of the ITS region produced a specific DNA band with a size of 650 bp. No sequence heterogeneity was detected among DNA clones from the same individual. The ITS sequences of the P. minus accessions were deposited into the GenBank database (JN709855-JN709858). The ITS sequence divergence among the four P. minus accessions was 1%, and it was more variable compared to the trnL-trnF sequences of P. minus. Generally, the plant nuclear genome evolves faster than the chloroplast genome [21]. The phylogenetic relationships among the P. minus accessions and selected Persicaria species based on the ITS sequences are shown in the neighbor-joining (NJ) tree [Figure 1(A)]. In the maximum parsimony (MP) analysis, 22.92% sites within the ITS region were found to be parsimony-informative characters. The MP analysis produced 18 most parsimonious trees with a tree length of 359 steps. One of the most parsimonious trees is shown in Figure 1B. The phylogenetic characteristics of ITS sequences from the MP analysis are summarized in Table 1. The topology in the Bayesian inference was similar to the NJ and MP trees (Figure 1).
Figure 1.
(A) The optimal Neighbor-Joining tree based on the kimura-2-parameter model. (B) One of the most parsimonious trees and Bayesian posterior probability tree based on ITS sequences. The values above the branches indicate bootstrap support (1000 replicates). Bayesian posterior probability values are indicated in bold.
Table 1.
Phylogenetic characteristics of ITS sequences for maximum parsimony (MP) analysis.
| Characteristic | Value |
|---|---|
| Tree length | 359 |
| Consistency index (CI) | 0.7744 |
| Homoplasy index (HI) | 0.2256 |
| CI excluding uninformative characters | 0.7254 |
| HI excluding uninformative characters | 0.2746 |
| Retention index (RI) | 0.8247 |
| Rescaled consistency index (RC) | 0.6386 |
The genetic distances between the P. minus (CH, GH, UY and FH), P. kawagoeana, Persicaria minor P1 and P. minor P2 were determined based on kimura 2-parameter model in PAUP. Genetic distance values are shown in Table 2 as percentages.
Table 2.
Genetic distance percentages between Polygonum minus, P. kawagoeana, P. minor P1 and P. minor P2.
| Species | Polygonum minus | P. kawagoeana | P. minor P1 | P. minor P2 |
|---|---|---|---|---|
| Polygonum minus | - | 0.95 | 1.65 | 16.5 |
| P. kawagoeana | 0.95 | - | 0.9 | 0.9 |
| P. minor P1 | 1.65 | 0.90 | - | 0.0 |
| P. minor P2 | 1.65 | 0.90 | 0.0 | - |
The tree topologies from the NJ, MP and Bayesian analyses were generally congruent with each other. The Polygonum taxa formed monophyletic lineages according to section Persicaria (P. punctata, P. pubescens, P. hydropiper, P. lapathifolia, P. longiseta, P. kawagoeana, P. macrantha and P. minor), section Cephalopilon (P. capitata, P. runcinata and P. nepalensis) and section Echinocoulon (P. arifolia, P. maackiana, P. meisneriana and P. sagittata). All four of the P. minus accessions [Cameron Highlands (CH), Genting Highland (GH), Fraser’s Hill (FH) and Ulu Yam (UY)] from Peninsular Malaysia formed a clade that was strongly supported by bootstrap value and posterior probabilities (Figure 1). The MP tree topology showed that the Peninsular Malaysian P. minus clade formed a close relationship to a clade containing P. pubescens, P. hydropiper and P. punctata (Figure 1).
Although Persicaria minor and Polygonum kawagoeanum are treated as synonyms of Polygonum minus [7,8], these three taxa did not cluster together in any of the constructed trees (Figure 1). The three species were found to have great genetic distances (Table 2). Polygonum minus had the highest genetic distance from P. kawagoena and P. minor. Therefore, P. minus of Peninsular Malaysia is exclusive and unique, and it might be distinct from the synonymous P. minor and P. kawagoeanum.
3. Experimental Section
3.1. Plant Materials
Fresh leaves of wild-type Polygonum minus were collected in June 2009 from Genting Highland, Cameron Highlands and Fraser’s Hill in Pahang and Ulu Yam in Selangor. The morphology and anatomy of the wild accessions were examined and confirmed as P. minus as described in our previous study of the plant [12]. Accessions of Genting Highland (GH) and Cameron Highlands (CH) have large leaves, while accessions of Fraser’s Hill (FH) and Ulu Yam (UY) have small leaves. Voucher specimens were deposited in the Herbarium Universiti Kebangsaan Malaysia (UKMB), Bangi, Malaysia.
3.2. Genomic DNA Isolation
P. minus genomic DNA was isolated from fresh leaf tissue according to the methods of Doyle and Doyle [22] and Cullings [23]. The quantification of genomic DNA was achieved using UV–Visible spectroscopy (Varian, Australia) by measuring the absorbance at A260, A280 and A320 nm. DNA purity was determined by the A260/A280 absorbance ratio and tested by running the genomic DNA samples on a 1% agarose gel stained with 0.25 μg/mL ethidium bromide. The gel was visualized and photographed under UV light using a Fujifilm LAS-3000 imager.
3.3. PCR Amplification
PCR amplification was performed in a 50 μL volume containing 0.2 mM of each dNTP, 1× PCR reaction buffer (1.5 mM MgCl2), 1.0 μM of each primer, 1.25 U Taq DNA polymerase (Promega) and 0.5 μg/50 μL template DNA. The trnL-trnF region was amplified with primers 5′-GGT TCA AGT CCC TCT ATC CC-3′ and 5′-ATT TGA ACT GGT GAC ACG AG-3′ [24]. The primers ITS-leu 5′-GTC CAC TGA ACC TTA TCA TTT AG-3′ [25] and ITS4 5′-TCC TTC CGC TTA TTG ATA TGC-3′ [26] were used to amplify the ITS region. Amplification of the trnL-trnF and ITS regions was carried out using the following thermal cycle profile: primary denaturation for 5 min at 94 °C, followed by 30 cycles of 1 min at 95 °C, 1 min at 55 °C and 1 min at 72 °C, and a final extension of 5 min at 72 °C.
3.4. PCR Product Purification, Cloning and Sequencing
The PCR products of trnL-trnF and ITS were checked on a 1% agarose gel. The PCR products were then purified using the Gel Extraction Kit (Qiagen, Valencia, CA, USA). For ITS, the PCR products were further cloned by using the PGEM-T Easy Vector System (Promega, Madison, WI, USA). Minipreps of plasmid DNA were made with the Qiagen Miniprep Kit, followed by restriction analysis to identify the clones for sequencing. Ten positive clones were selected for sequencing using the SP6 Promoter Primer and T7 Promoter Primer to check for sequence heterogeneity. DNA cycle sequencing was performed using the ABI Prism Dye Terminator Cycle Sequencing Ready Reaction kit and analyzed with the ABI PRISM 3100 Genetic Analyzer (Perkin-Elmer, Waltham, MA, USA).
3.5. Data Analyses
The ITS sequences for the four P. minus accessions of Peninsular Malaysia (GH, CH, FH and UY) and 16 other Polygonum species acquired from the GenBank database [Persicaria pubescens (EU196901), Persicaria hydropiper (EF653703), Persicaria punctata (EU196904), Persicaria lapathifolia (HM357911), Persicaria longiseta (EU196890), Persicaria macrantha (EU196891), Persicaria kawagoeana (EU196886.1), Persicaria minor P1 (EU196894), Persicaria minor P2 (EU196895), Persicaria capitata (EF653690), Persicaria runcinata (EF653692), Persicaria nepalensis (EF653691), Persicaria arifolia (EF653693), Persicaria maackiana (EF653694), Persicaria meisneriana (EF653695) and Persicaria sagittata (EF653696)] were used in phylogenetic analyses. The obtained nucleotide sequences were aligned by the ClustalX Multiple Sequence Alignment programme [27] using the default settings. The maximum parsimonious (MP) and neighbor-joining (NJ) trees were then generated using PAUP* 4.0b10 [28], and the Bayesian inference was done by MrBayes 3.1 [29]. The genetic distances were determined based on the kimura 2-parameter model using the default settings in PAUP* 4.0b10 and were used in generating the NJ tree. The MP analysis was conducted using a heuristic search with the TBR branch-swapping algorithm, and gaps were treated as missing data. Bootstrap analyses with 1000 replicates were conducted to obtain confidence in the NJ and MP trees. Consistency and retention indices (CI and RI, respectively) of the MP tree were generated using PAUP* 4.0b10. Modeltest 3.7 [30] was used to select the substitution model that best fit the data using the AIC criterion. The best model was subsequently used for Bayesian analysis in MrBayes 3.1 (TVM + G, with a proportion of invariable sites of 0 and a gamma distribution shape parameter of 0.1092). Bayesian inference was made by running two simultaneous metropolis-coupled Monte-Carlo Markov chains for 2,000,000 generations with an average standard deviation of split frequencies of 0.003639. The consensus topology tree of 9001 was produced by omitting the first 1000 trees of 10,000 (burning) with a tree that was sampled for every 100 generations. Polygonum filiforme (EF653697) was used as an outgroup taxon in the phylogenetic analyses.
4. Conclusions
In P. minus, the ITS region from the nuclear genome was more variable than the trnL-trnF region from the plastid genome. The ITS sequences were informative enough to infer the phylogenetic relationships of P. minus and the related Polygonum taxa. The topology of the NJ, MP and Bayesian consensus trees showed that P. minus from Peninsular Malaysia did not form a cluster with P. kawagoeana and P. minor, which were previously reported to be synonyms to P. minus. These findings suggest that further investigation is needed to clarify the taxonomic status of these three taxa.
Acknowledgements
This research was supported by grant 07-05-MGI-GMB004 from the Ministry of Science, Technology and Innovation, Malaysia. The authors would like to thank Syed-Shabthar for his help with data analysis.
References
- 1.Arif I.A., Bakir M.A., Khan H.A., Al Farhan A.H., Al Homaidan A.A., Bahkali A.H., Al Sadoon M., Shobrak M. A brief review of molecular techniques to assess plant diversity. Int. J. Mol. Sci. 2010;11:2079–2096. doi: 10.3390/ijms11052079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golkar P., Arzani A., Rezaei A.M. Genetic variation in safflower (Carthamus tinctorious L.) for seed quality-related traits and inter-simple sequence repeat (ISSR) markers. Int. J. Mol. Sci. 2011;12:2664–2677. doi: 10.3390/ijms12042664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin B.G., Sanderson M.J., Porter J.M., Wojciechowski M.F., Campbell C.S., Donoghue M.J. The ITS region of nuclear ribosomal DNA: A valuable source of evidence on angiosperm phylogeny. Ann. Mo. Bot. Gard. 1995;82:247–277. [Google Scholar]
- 4.Olmstead R.G., Plamer J.D. Chloroplast DNA systematics: A review of methods and data analysis. Am. J. Bot. 1994;81:1205–1224. [Google Scholar]
- 5.Kuzoff R.K., Sweere J.A., Soltis D.E., Soltis P.S., Zimmer E.A. The phylogenetic potential of entire 26S rDNA sequences in plants. Mol. Biol. Evol. 1998;15:251–263. doi: 10.1093/oxfordjournals.molbev.a025922. [DOI] [PubMed] [Google Scholar]
- 6.Ridley H.N. The Flora of the Malay Peninsula. L. Reeve & Co.; Ashford, UK: 1967. p. 11. [Google Scholar]
- 7.Anjen L., Grabovskaya-Borodina A.E., Hong S.-P., McNeil J., Ohba H., Park C.-W. Polygonum Linnaeus. Flora China. 2003;5:278–315. [Google Scholar]
- 8.Freeman C.C., Reveal J.L. Flora of North America. Vol. 5. Oxford University Press; New York NY, USA: 2005. Polygonaceae; pp. 216–601. [Google Scholar]
- 9.Hunter M.V., Brophy J.J., Ralph B.J., Bienvenu F.E. Composition of Polygonum odoratum Lour, from southern Australia. J. Essent. Oil Res. 1997;9:603–604. [Google Scholar]
- 10.Jiang J. Volatile composition of the laksa plant (Polygonum hydropiper L.), a potential source of green note aroma compounds. Flavour Fragr. J. 2005;20:455–459. [Google Scholar]
- 11.Baharum S.N., Bunawan H., Ghani M.A., Mustapha W.A.W., Noor N.M. Analysis of the chemical composition of the essential oil of Polygonum minus Huds. using two-dimensional gas chromatography-time-of-flight mass spectrometry (GC-TOF MS) Molecules. 2010;15:7006–7015. doi: 10.3390/molecules15107006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunawan H., Talip N., Noor N.M. Foliar anatomy and micromorphology of Polygonum minus Huds. and their taxonomic implications. Aust. J. Crop Sci. 2011;5:123–127. [Google Scholar]
- 13.Lersten N.R., Curtis J.D. Foliar anatomy of Polygonum (Polygonaceae): Survey of epidermal and selected internal structure. Plant Syst. Evol. 1992;182:71–106. [Google Scholar]
- 14.Steward A.N. Contributions from the Gray Herbarium of Harvard University. Vol. 5. Gray Herbarium of Harvard University; Cambridge, MA, USA: 1930. The Polygonaceae of Eastern Asia; pp. 1–129. [Google Scholar]
- 15.Haraldson K. Anatomy and taxonomy in Polygonaceae subfam. Polygonoideae Meisn. emend. Jaretzky. Symb. Bot. Ups. 1978;22:1–95. [Google Scholar]
- 16.Kim S.-T., Donoghue M.J. Molecular phylogeny of Persicaria (Persicarieae, Polygonaceae) Syst. Bot. 2008;33:77–86. [Google Scholar]
- 17.Fujii N., Ueda K., Watano Y., Shimizu T. Intraspecific sequence variation in chloroplast DNA of Primula cuneifolia Lebed. (Primulaceae) J. Phytogeogr. Taxon. 1995;43:15–24. [Google Scholar]
- 18.Levy F., Antonovics J., Boynton J.E., Gillham N.W. A population genetic analysis of chloroplast DNA in Phacelia. Heredity. 1996;76:143–155. doi: 10.1038/hdy.1996.22. [DOI] [PubMed] [Google Scholar]
- 19.Wolf P.G., Murray R.A., Sipes S.D. Species-independent, geographical structuring of chloroplast DNA haplotypes in a montane herb Ipomopsis (Polemoniaceae) Mol. Ecol. 1997;6:283–291. [Google Scholar]
- 20.Okaura T., Harada K. Phylogeographical structure revealed by chloroplast DNA variation in Japanese beech (Fagus crenata Blume) Heredity. 2002;88:322–329. doi: 10.1038/sj.hdy.6800048. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe K.H., Li W.H., Sharp P.M. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc. Natl. Acad. Sci. USA. 1987;84:9054–9058. doi: 10.1073/pnas.84.24.9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle J.J., Doyle J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987;19:11–15. [Google Scholar]
- 23.Cullings K.W. Design and testing of a plant-specific PCR primer for ecological and evolutionary studies. Mol. Ecol. 1992;1:233–240. [Google Scholar]
- 24.Taberlet P., Gielly L., Patou G., Bouvet J. Universal primers for amplification of three non-coding regions of chroloplast DNA. Plant Mol. Biol. 1991;17:1105–1109. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- 25.Baum D.A., Small R.L., Wendel J.F. Biogeography and floral evolution of Baobabs (Adansonia, Bombacaceae) as inferred from multiple data sets. Syst. Biol. 1998;47:181–207. doi: 10.1080/106351598260879. [DOI] [PubMed] [Google Scholar]
- 26.White T., Bruns T., Lee S., Taylor J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Academic Press; San Diego, CA USA: 1990. pp. 315–322. [Google Scholar]
- 27.Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swofford D.L. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4. Sinauer Associates; Sunderland, MA, USA: 2000. [Google Scholar]
- 29.Ronquist F., Huelsenbeck J.P. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 30.Posada D., Crandall K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]