Abstract
Objective
To determine the efficacy of pazopanib eye drops in the streptozotocin induced diabetic retinopathy rat model.
Methods
A 0.5 % w/v pazopanib suspension was prepared in phosphate buffered saline (PBS, pH 7.4) in the presence of 0.5 % w/v sodium carboxymethyl cellulose. Brown Norway rats were divided into three groups (n = 4) – (1) healthy, (2) diabetic, and (3) diabetic with treatment. The drug suspension was administered twice daily as eye drops to group 3 for 30 days. Efficacy parameters including the number of adherent leukocytes in the retinal vasculature (leukostasis), blood-retinal FITC-dextran leakage, and vitreous-to-plasma protein ratio were measured.
Results
Pazopanib suspension in the form of eye drops significantly reduced leukostasis (32 %), FITC-dextran leakage (39 %), and the vitreous-to-plasma protein ratio (64 %) in diabetic animals compared to untreated diabetic group.
Conclusion
Pazopanib eye drops can alleviate retinal complications of diabetic retinopathy.
Keywords: Pazopanib, Leukostasis, blood-retinal-barrier, diabetic macular edema
Introduction
Diabetic retinopathy (DR) is a chronic inflammatory, microvascular complication of diabetes, afflicting the back of the eye (Amrite et al.,; Cheruvu et al., 2009; Hernandez and Simo, 2007; Schwartz and Flynn, 2007). It is the principal cause of blindness among the working age population worldwide. DR manifests as diabetic macular edema (DME) in early stages and as proliferative diabetic retinopathy in late stages (Penn et al., 2008). While vascular leakage is the hallmark of DME, retinal neovascularization is characteristic of proliferative diabetic retinopathy. Tight blood-glucose control can prevent development of diabetic eye disease to certain extent. However, the benefits are less as patients under tight glycemic control still go on to develop DR, making it necessary for local therapeutic intervention. Laser photocoagulation and vitrectomy are important surgical tools in the treatment of DR. However, these techniques can result in visual field loss and damage to dark adaptation or color vision (Hernandez and Simo, 2007). In addition to these surgical techniques, inhibitors of vascular endothelial growth factor (VEGF) or anti-inflammatory agents such as corticosteroids have been assessed for their efficacy in preclinical models and clinical trials (Hernandez and Simo, 2007). The role of VEGF in DR is well established. VEGF is an important pro-inflammatory mediator in the pathogenesis of DR (Penn et al., 2008). Elevation in retinal VEGF mRNA levels has been seen consistently in animal models of DR from as early as 1 week after induction of diabetes (Qaum et al., 2001) to six months (Hammes et al., 1998). In early DR, VEGF induced vascular hyperpermeability might contribute to diabetic macular edema. In late stages of DR, the ability of VEGF to promote the mobilization and proliferation of vascular endothelium to form new blood vessels might contribute to retinal neovascularization in proliferative diabetic retinopathy (Penn et al., 2008). Pegaptanib (Macugen, OSI/Eyetech, USA), bevacizumab (Avastin, Genentech, Inc., USA), and ranibizumab (Lucentis, Genentech, Inc., USA) are examples of FDA approved anti-VEGF agents for the treatment of some of the diseases of the back of the eye such as the wet form of age-related macular degeneration or choroidal neovascularization. These agents are in phase II/III clinical trials for the treatment of DME.
Pazopanib hydrochloride (Votrient™; GlaxoSmithKline, USA) is a multi-tyrosine kinase inhibitor (TKI) recently approved by the FDA for the treatment of patients with advanced renal cell carcinoma (2010a). It has also been shown to be orally effective in laser induced CNV mouse model (Takahashi et al., 2009). This drug molecule has been designed to target growth factor receptors with integral tyrosine activities involved in angiogenesis; most notably, members of the VEGFR family. The 50% inhibitory concentrations (IC50) of pazopanib for VEGFR1, VEGFR2, and VEGFR3 are 10, 30, and 47 nM, respectively (2010b). The presence of elevated VEGF in DR suggests that VEGF inhibitors such as pazopanib may be therapeutically beneficial.
Despite the fact that this compound is sparingly soluble in water with predicted water solubility of < 8 μg/ml (Source: SciFinder Scholar 2008), it has high intestinal permeability and is classified as a Class 2 compound under the Biopharmaceutics Classification Scheme (2010a). That is, pazopanib is a compound with low solubility and high permeability. In addition to the oral route, pazopanib is also effective when administered in eye drops. Indeed, pazopanib eye drops are currently in clinical trials to treat age related macular degeneration (AMD) (NCT01134055, Source: www.clinicaltrials.gov). This becomes of importance when considering the current VEGF inhibitors, which, as proteins, must be introduced as intravitreal injections. The success in the use of pazopanib in the treatment of neovascular pathologies has propelled us, in this study, to examine its ability to provide therapeutic benefit to diabetic macular edema. Here we show that indeed pazopanib is efficacious in treating this early pathological manifestation of DR.
Materials and methods
Pazopanib free base was purchased from LC Laboratories (Product # 6706, Woburn, MA, USA). FITC-dextran (4.4 kDa), Triton X-100, sodium carboxymethyl cellulose (low viscosity, 50–200 cps, Cat # C5678), and streptozotocin (Cat. # S0130) were purchased from Sigma-Aldrich (St. Louis. MO, USA). Bovine serum albumin was purchased from Fermentas Life Sciences (Bradford kit; Glen Burnie, MD, USA). FITC-conjugated concanavalin A lectin was purchased from Vector Laboratories (Burlingame, CA, USA). Male Brown-Norway (BN; pigmented) rats weighing 200 to 250 g were purchased from Harlan Labs (Livermore, CA, USA).
Pazopanib suspension
Pazopanib was triturated with sodium carboxymethyl cellulose (0.5 % w/v) using a pestle and mortar and 5 mg/ml suspension was made after adding sufficient quantity of phosphate buffer saline (pH 7.4).
Diabetes Induction
BN rats weighing 200 – 250 g (Harlan labs) were acclimatized for at least two days prior to any experimental procedure. After overnight fasting for 12–16 h, an intraperitoneal injection of 30 mg/ml solution of streptozotocin in 10 mM citrate buffer (pH 4.5) was administered (60 mg/kg body weight) to induce diabetes. After 3–4 h of streptozotocin injection, animals were put on a regular diet and 24 h after streptozotocin injection, blood sample (5–10 μl) was collected via tail vein. The blood glucose levels in the animals were determined with a glucose monitor (One Touch; Life Scan Inc., Milpitas, CA). Animals with blood glucose levels greater than 250 mg/dL were considered diabetic (Rao et al., 2010). The animals were divided into three groups. Group 1: Healthy (n = 12), Group 2: Diabetic (n = 12) and Group 3: Diabetic + Treatment (n = 12). Treatment was started immediately after diabetes induction. Both eyes were dosed twice daily for 30 days with 0.5 % w/v pazopanib suspension (10 μl volume in each eye) and animals in all groups were sacrificed on day 31, 16–17 h after last dose on day 30. For each of the three assays, namely, FITC-Dextran leakage, vitreous-to-plasma protein ratio, and leukostasis, we used 4 animals from each of the three groups mentioned above. All animal experiments in this study were performed after receiving approval from the University of Colorado IACUC.
Retinal leukostasis
BN rats from each group were used for the assessment of adherent leukocytes. At 16 h after last dose, rats were sacrificed for ex-vivo retinal leukostasis assay. First, the animals were anesthetized with ketamine (80 mg/kg) and xylazine (12 mg/kg) administered intraperitoneally. Then, the chest cavity was carefully opened and animals were perfused with PBS (250 ml/kg body weight) for 6–7 minutes after inserting a 20G needle attached to a 50 ml syringe into the left ventricle. Animals were then perfused with a 40 μg/ml PBS (pH 7.4) solution of FITC-conjugated concanavalin A lectin (5 mg/kg, ~ 33 ml) to label the adherent leukocytes and the vascular endothelial cells. Animals were perfused again with similar volume of PBS as above to remove unbound lectin. Eyes were enucleated and fixed in 2% paraformaldehyde for 2 h. Retinas were carefully removed to prepare the flat mounts. Fluorescence microscope (Digital Eclipse C1; Nikon Inc., Melville, NY) under blue light (Ex 465–495, DM 505, BA 515–555) with a 20X objective was used to count the number of leukocytes adhered to the vessel walls (Rao et al., 2010). The count was compared between treated and untreated rats.
Blood retinal barrier leakage
Retinal FITC-Dextran Leakage
BN rats from each group were used for the assessment of FITC-dextran leakage. At 16 h after last dosing, rats were sacrificed for FITC-dextran leakage assay. Brief protocol for the assay and tissue sample processing is described below. First, the animals were anesthetized with ketamine (80 mg/kg) and xylazine (12 mg/kg) administered intraperitoneally. Then a 50 mg/ml PBS (pH 7.4) solution of FITC-dextran with a molecular weight of 4.4 kDa was administered (50 mg/kg body weight) intravenously via tail vein. Animals were euthanized with 150 mg/kg sodium pentobarbital after 10 minutes (circulation time for FITC-dextran) of tail vein injection. Blood samples (0.5–1 ml) were withdrawn from the heart in 2 ml Eppendorf tubes (SureLock Microcentrifuge Tubes, LIGHTLABS, USA) containing 50 μl of EDTA. The chest cavity was opened. Animals were perfused with PBS (500 ml/kg body weight) for 6–7 minutes after a 20G needle attached to a 50 ml syringe was inserted into the left ventricle. Eyes were enucleated and isopentane-dry ice bath was used to immediately snap-freeze the eyes before storing them at −80°C. Retina of each eye was isolated, weighed and homogenized in 500 μl of PBS (pH 7.4). Following homogenization, 500 μl of PBS containing 2% Triton X-100 was added to the homogenate. The mixture was vortexed at room temperature for 1 h. The homogenate was centrifuged at 15000 rpm (21,130 g) for 20 min and the supernatant was collected. The relative FITC-dextran fluorescence units in 1 ml of supernatant were measured using a spectrofluorometer set at an excitation wavelength of 483 nm and an emission wavelength of 538 nm. Fluorescence of blank PBS was also measured for subtraction from each sample reading. Final concentration of FITC-dextran was expressed as μg/g tissue. The standard curve was generated using known amounts of the FITC-dextran (3 ng/ml to 250 ng/ml). 20 μl (~20 mg) of plasma was diluted to 1 ml (50 times dilution) with PBS for quantification under the linear range of the standard samples. The dilution factor was taken into account for estimating the amount of FITC-dextran per microliter of blood sample. The amount of FITC-dextran leakage in to the ocular tissues was calculated using the following equation, after correcting for dilutions.
Vitreous-to-Plasma protein ratio
BN rats from each group were used for the assessment of vitreous to plasma protein ratio. At 16 h after last dosing, rats were sacrificed for determining the protein ratio. Rats were euthanized with 150 mg/kg sodium pentobarbital administered intraperitoneally. Eyes were enucleated and isopentane-dry ice bath was used to immediately snap-freeze the eyes before storing them at −80°C. Blood samples (0.5–1 ml) were withdrawn from the heart following cardiac puncture in 2 ml Eppendorf tubes (SureLock Microcentrifuge Tubes, LIGHTLABS, USA) containing 50 μl of EDTA. The above samples were centrifuged at 15,000 g at 4° C for 15 min to collect the plasma in the supernatant. Plasma samples were stored at −80 ° C. Ocular tissues including the retina and the vitreous from each eye were isolated and weighed. The vitreous was allowed to liquefy. The vitreous samples were centrifuged at 15,000 g at 4° C for 20 min. The supernatant of vitreous was collected (20 μl) in new Eppendorf tubes and weighed (weight range = 19–21 mg). The supernatant (20 μl) was diluted to 1 ml with PBS (pH 7.4) (50 times dilution). One hundred microliters of the above diluted material was mixed with 1 ml of Bradford reagent. Absorbance of the above 1 ml volume was measured at 595 nm. The plasma sample (20 μl) was also diluted to 1 ml with PBS (pH 7.4) (50 times dilution). One hundred microliters of the above diluted material was mixed with 1 ml of Bradford reagent. Absorbance of the above 1 ml volume was measured at 595 nm. The standard curve was generated using known concentrations of bovine serum albumin (25–500 μg/ml in PBS, pH 7.4). Hundred microliters of each standard was mixed with 1 ml of Bradford reagent. The amount of protein in plasma and vitreous was estimated from the standard curve after correcting for dilutions.
Results
Blood glucose levels and body weights
Blood glucose levels were not statistically different between diabetic untreated group and pazopanib treated group on the day of the measurement of the effect parameters. Average blood glucose levels in the above two groups were about 500 mg/dL. However, the non-diabetic, untreated group of animals (healthy) had blood glucose levels of 120 ± 20 mg/dL [n=4].
The average body weights ± s.d. (n=4) of rats in three groups – healthy, diabetic, and diabetic + treatment at the start of the study (day 0) were 209.17 ± 3.19, 217.33 ± 2.14, and 210.67± 7.12, respectively. The average body weights ± s.d. of rats in healthy, diabetic, and diabetic + treatment groups at the end of the study (day 31) were 242 ± 9.96, 208.5 ± 3.83, and 214 ± 4.34, respectively.
Retinal leukostasis
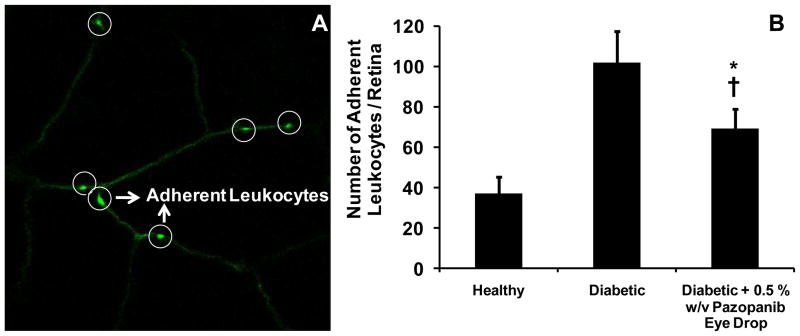
Leukocytes adhesion to the retinal vasculature among various groups of animals was counted under fluorescence microscope and is presented in Figure 1. In this study, the retinal vasculature of diabetic rats could be clearly distinguished from the healthy rats based on the quantity of adherent leukocytes. The quantity of adherent leukocytes in the pazopanib eye drops group was less than untreated diabetic animals and more than the healthy animals. Average leukocytes adhered to the retinal vasculature in healthy animals was 37.2 ± 7.8, whereas diabetic animals had an average value of 102.0 ± 15.6, approximately 3-fold higher than healthy animals. Animals treated with 0.5 % w/v pazopanib suspension demonstrated 69.5 ± 9.5 leukocytes adhered in their retinal vasculature, which was found to be significantly lower than diabetic animals. The rank order for the retinal leukostasis among various groups of animals was: diabetic > diabetic + treatment > healthy (Student’s t-test, p-value < 0.05).
Figure 1.
Panel A represents confocal microscopic image of retinal vasculature with adherent leukocytes (white circles). Panel B represents total number of adherent leukocytes in the entire retina isolated from the eyes of healthy (n = 4), diabetic (n = 4), diabetic + 5 % w/v pazopanib eye drop suspension (n = 4) groups. Data is expressed as mean ± SD. * Significantly different from healthy group, † Significantly different from diabetic group (Student’s t-test, p < 0.05).
Blood-retinal barrier leakage
Retinal FITC-Dextran Leakage
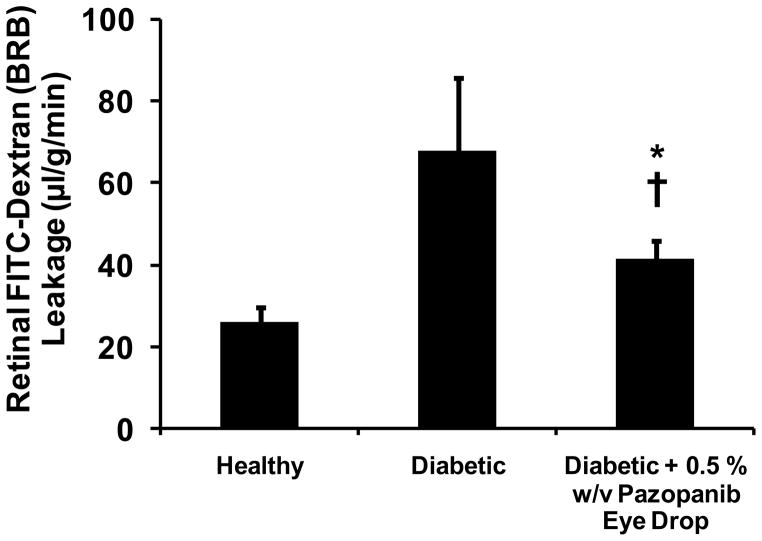
Blood-retinal barrier leakage assessed via FITC-dextran leakage assay is presented in Figure 2. Diabetic animals had approximately three times higher retinal barrier leakage with an average value of 67.9 ± 17.9 (μl/g per min of plasma) compared to healthy animals, with an average value of 25.9 ± 3.6 (μl/g per min of plasma). Animals treated with 0.5 % w/v pazopanib suspension demonstrated retinal barrier leakage with an average value of 41.5 ± 4.4 (μl/g per min of plasma), which was significantly lower than diabetic animals. The rank order for the retinal barrier leakage among various groups of animals was: diabetic > diabetic + treatment > healthy (Student’s t-test, p-value < 0.05).
Figure 2.
Inhibitory effects of pazopanib suspension administered in the form of eye drops on diabetes induced elevations in blood–retinal barrier leakage (n = 4). The BRB leakage was estimated on day 31, 16–17 h after last dose on day 30. Data is expressed as mean ± SD. * Significantly different from healthy group, † Significantly different from diabetic group (Student’s t-test, p-value < 0.05).
Vitreous-to-Plasma protein ratio
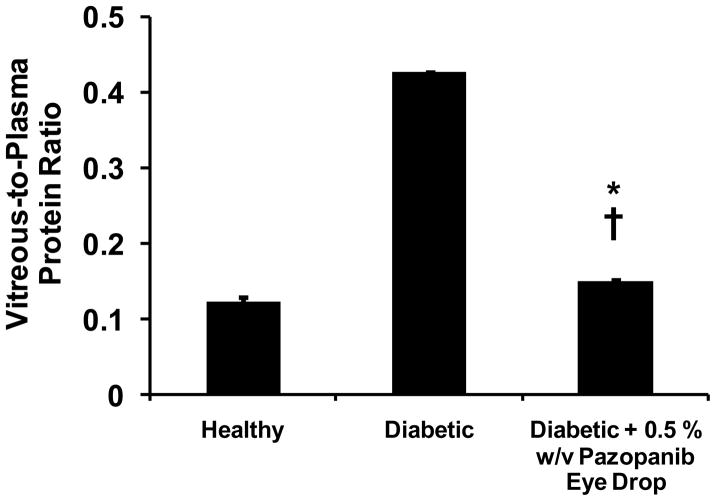
Blood-retinal barrier leakage assessed by means of vitreous-to-plasma protein ratio is presented in Figure 3. Healthy animals demonstrated an average vitreous-to-plasma protein ratio of 0.12 ± 0.006, whereas diabetic animals had an average value of 0.43 ± 0.002, approximately 3.5 fold higher than healthy animals. Animals treated with 0.5 % w/v pazopanib suspension demonstrated an average vitreous-to-plasma protein ratio of 0.15 ± 0.002, which was significantly lower than diabetic animals. The rank order for the vitreous-to-plasma protein ratio among various groups of animals was: diabetic > diabetic + treatment > healthy (Student’s t-test, p-value < 0.001).
Figure 3.
Vitreous-to-plasma protein ratio of healthy (n = 4), diabetic (n = 4), diabetic + 5 % w/v pazopanib eye drop suspension (n = 4) groups. Data is expressed as mean ± SD. * Significantly different from healthy group, † Significantly different from diabetic group (Student’s t-test, p < 0.05).
Discussion
In this study we have demonstrated for the first time the therapeutic efficacy of pazopanib in an STZ induced rat model of DR. Pazopanib, when applied as an eye drop suspension (0.5% w/v twice daily) is capable of reducing DR associated blood retinal barrier (BRB) leakage and retinal leukostasis. Thus far, this is the first study to demonstrate that a multi-tyrosine kinase inhibitor (designed to target VEGFR family members) is beneficial in treating DR.
VEGF is an important pro-inflammatory and angiogenic factor involved in the pathogenesis of DR. Elevated VEGF levels have been found in patients with DR (Penn et al., 2008; Smith et al., 1999) as well as in preclinical models of DR including STZ induced DR in rats (Amrite et al., 2006). In addition to the well known role of VEGF in angiogenesis (Penn et al., 2008), VEGF activity has been associated with numerous important pro-inflammatory events involved in DR such as elevation of the levels of inflammatory mediators such as prostaglandins (Amrite et al., 2006; Cheng et al., 1998), upregulation of ICAM1 on vascular endothelial cells that contributes to enhanced leukostasis (Lu et al., 1999; Miyamoto et al., 1999; Rao et al., 2010), and direct enhancement of vascular permeability, collectively leading to blood retinal barrier leakage (Rao et al., 2010). VEGF effects are mediated through the tyrosine kinase activities of the VEGFR family (Penn et al., 2008). Current therapies which inhibit VEGF work by targeting VEGF itself, rendering it unable to bind to its receptors. Anti-VEGF antibodies such as Ranibizumab (Lucentis) have been shown to decrease vascular leakage both in preclinical animal models and in clinical trials (2006). The efficacy of these therapeutics have done much to validate the role of VEGF in DR. However, one limitation of this treatment paradigm is that Ranibizumab and all similar therapeutics must be introduced via intravitreal injection. Pazopanib represents an interesting alternative to the direct targeting of VEGF. By virtue of its ability to inhibit all members of the VEGFR family, we can effectively lesion VEGF signaling and by virtue of its status as a small molecule, it can be administered in a non-invasive fashion.
Being a lipophilic drug, pazopanib might cross the various barriers primarily via passive diffusion. Following topical administration, transscleral delivery or systemic delivery can contribute towards drug levels in the back of the eye including retinal capillaries. Studies by Lee and Robinson (Lee and Robinson, 2001) showed that drugs injected in aqueous humor do not gain significant entry into the back of the eye as opposed to those administered in the subconjunctival space. Thus, corneal pathway is unlikely to contribute significant drug levels in the back of the eye. Our earlier studies indicated that transscleral delivery contributes about 54-fold greater levels (Ayalasomayajula and Kompella, 2004) to the back of the eye tissues when compared to systemic mode of administration due to local diffusion of the drug. Thus, conjunctival transport followed by transscleral delivery is a likely pathway for pazopanib delivery to the retina and retinal capillaries. In the transscleral pathway, drug diffuses across sclera and choroid-RPE, to reach retina and the embedded retinal capillaries. The relative contribution of transscleral and systemic pathways to the retinal delivery of pazopanib was not determined in this study. Prior studies indicated that pazopanib decreased VEGF release as well as VEGF mRNA levels in cultured human retinal pigment epithelial cells (RPE) (Yafai et al.). Further, it was reported that laser induced elevations in VEGF levels in the back of the eye tissues were reduced by pazopanib eye drops in a choroidal neovascularization animal model (Yafai et al.). Thus, inhibition of VEGF is one likely mechanism for the anti-vascular leakage effects observed in the current study. However, since we did not measure the levels of various angiogenic and inflammatory markers, the relative roles of various tyrosine kinases in the observed effects is unclear at this stage.
Numerous studies of the STZ induced DR model have demonstrated similarities with human DME including leukostasis, pericyte collapse, platelet aggregation, and blood retinal barrier breakdown (Penn et al., 2008). Measures of capillary non-perfusion and differences in leukostasis have been observed as early as 3 days post STZ administration (Miyamoto et al., 2000; Miyamoto et al., 1999; Rao et al., 2010). Given that leukostasis is mediated by the VEGF regulated protein, ICAM1, our measure of decreased leukostasis in pazopanib treated rats (Figure 1) is consistent with the putative inhibition of VEGF signaling.
In this study we found that pazopanib was effective in reducing BRB breakdown, a major cause of macular edema associated with DR, as measured both by FITC-dextran leakage (Figure 2) and by the vitreous-to-plasma protein ration (Figure 3). These techniques, along with others including Evans blue, FITC-albumin extravasation, and isotope dilution have been used to infer changes in the retinal vascular permeability (Miyamoto et al., 1999; Vinores, 1995). It has been demonstrated by several groups that FITC-dextran is a better predictor of BRB leakage as compared to Evans blue in terms of sensitivity and quantification (Atkinson et al., 1991; Ishida et al., 2003; Miyahara et al., 2004; Stitt et al., 2000). Further, selective breakdown of the BRB can be assessed by using FITC-dextrans with different molecular weights (4.4, 70 or 150 kDa). For example, Atkinson et al. (Atkinson et al., 1991) demonstrated that 4.4 kDa molecular weight dextran was not able to permeate through healthy BRB, whereas, the same molecule could easily penetrate through swollen optic discs, areas of macular edema and new retinal vessels. On the other hand, 150 kDa FITC-dextran could leak only through swollen optic discs but not through areas of macular edema and new retinal vessels. A similar observation was made by Tolentino et al. (Tolentino et al., 2000), with respect to choroidal neovascularization in cynomolgus monkeys. While 4.4 kDa dextran leaked rapidly from the CNV, 150 kDa dextran could not. Isotope dilution (Miyamoto et al., 2000; Miyamoto et al., 1999) and vitreous-to-plasma (Carmo et al., 2000) ratio are additional methods for measuring retinal vascular leakage. In our study, we selected FITC-dextran (4.4 kDa) leakage and vitreous-to-plasma protein ratio to assess the blood retinal barrier dysfunction. The extent of BRB break down in diabetic animals was 2.5- fold and 3.5-fold, respectively, based on FITC-dextran leakage and vitreous-to-plasma protein ratio assays. Since the vitreous-to-plasma protein ratio measures the protein concentrations in the vitreous and not the retina, slightly higher BRB break down assessed by this assay might be because of the leakage from blood aqueous barrier. In diabetic animals, after treatment with pazopanib, a decrease of 64 % in the vitreous-to-plasma protein ratio and 39 % decline in FITC-dextran leakage was observed.
Since pazopanib inhibits several members of the VEGFR family, (2010a; Takahashi et al., 2009), it is likely that the therapeutic effects of this drug is mediated, in part, through the inhibition of members of the VEGFR family. We cannot yet discount the inhibition of other kinases such as PDGF in this process. The role of PDGF in the pathology of DME is complex. PDGFR engagement, for example, has been shown to contribute to pericyte survival as well as the upregulation of VEGF (Paques et al., 1997; Penn et al., 2008). Unlike intravitreal injections currently being assessed for treating diabetic macular edema, the proposed approach offers a convenient eye drop therapy for treating vascular leakage associated with diabetic macular edema.
Since pazopanib is a multityrosine kinase inhibitor, its effects and side effects in healthy animals need to be determined in future studies. However, since pazopanib is already undergoing late stage clinical trials at doses similar to those assessed in this study, we anticipate that these doses will be safe for human use. Further, drug dosing was initiated soon after streptozotocin treatment, this study can be considered as an intervention studies. In future studies we will assess the ability of pazopanib to regress established disease.
Conclusion
Topical administration of 5 % w/v pazopanib suspension in the form of eye drops reduced DR pathologies including retinal leukostasis and blood-retinal-barrier breakdown in diabetic rats.
Highlights.
Pazopanib reduced retinal leukostasis by 32% in STZ-induced rodent model of DR.
Pazopanib reduced retinal vascular leakage by 39% in the above animal model.
Pazopanib suspension eye drops can potentially alleviate complications of DR.
Acknowledgments
This work was supported by National Institutes of Health grants EY018940 and EY017533. The authors would like to thank the University of Nebraska Medical Center for the graduate student research fellowship awarded to Ashish Thakur.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 2006. LUCENTIS (Brand Name Drug), FDA Application No. (BLA) 125156, Pharmacology Review.
- Australian Public Assessment Report for Pazopanib hydrochloride. GlaxoSmithKline Australia Pty Ltd; 2010a. Proprietary Product Name: Votrient. Submission No. PM-2009-01084-4. [Google Scholar]
- PRODUCT MONOGRAPH. Votrient (Pazopanib hydrochloride), Antineoplastic Agent. GlaxoSmithKline Inc; 2010b. Submission Control No: 128332. [Google Scholar]
- Amrite A, et al. Delivery of celecoxib for treating diseases of the eye: influence of pigment and diabetes. Expert Opin Drug Deliv. 7:631–45. doi: 10.1517/17425241003663236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrite AC, et al. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest Ophthalmol Vis Sci. 2006;47:1149–60. doi: 10.1167/iovs.05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson EG, et al. Molecular size of retinal vascular leakage determined by FITC-dextran angiography in patients with posterior uveitis. Eye (Lond) 1991;5(Pt 4):440–6. doi: 10.1038/eye.1991.71. [DOI] [PubMed] [Google Scholar]
- Ayalasomayajula SP, Kompella UB. Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharm Res. 2004;21:1797–804. doi: 10.1023/b:pham.0000045231.51924.e8. [DOI] [PubMed] [Google Scholar]
- Carmo A, et al. Effect of cyclosporin-A on the blood--retinal barrier permeability in streptozotocin-induced diabetes. Mediators Inflamm. 2000;9:243–8. doi: 10.1080/09629350020025764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, et al. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Invest Ophthalmol Vis Sci. 1998;39:581–91. [PubMed] [Google Scholar]
- Cheruvu NP, et al. Effect of diabetes on transscleral delivery of celecoxib. Pharm Res. 2009;26:404–14. doi: 10.1007/s11095-008-9757-2. [DOI] [PubMed] [Google Scholar]
- Hammes HP, et al. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes. 1998;47:401–6. doi: 10.2337/diabetes.47.3.401. [DOI] [PubMed] [Google Scholar]
- Hernandez C, Simo R. Strategies for blocking angiogenesis in diabetic retinopathy: from basic science to clinical practice. Expert Opin Investig Drugs. 2007;16:1209–26. doi: 10.1517/13543784.16.8.1209. [DOI] [PubMed] [Google Scholar]
- Ishida S, et al. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;44:2155–62. doi: 10.1167/iovs.02-0807. [DOI] [PubMed] [Google Scholar]
- Lee TW, Robinson JR. Drug delivery to the posterior segment of the eye: some insights on the penetration pathways after subconjunctival injection. J Ocul Pharmacol Ther. 2001;17:565–72. doi: 10.1089/10807680152729257. [DOI] [PubMed] [Google Scholar]
- Lu M, et al. VEGF increases retinal vascular ICAM-1 expression in vivo. Invest Ophthalmol Vis Sci. 1999;40:1808–12. [PubMed] [Google Scholar]
- Miyahara S, et al. Simvastatin inhibits leukocyte accumulation and vascular permeability in the retinas of rats with streptozotocin-induced diabetes. Am J Pathol. 2004;164:1697–706. doi: 10.1016/S0002-9440(10)63728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, et al. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1) Am J Pathol. 2000;156:1733–9. doi: 10.1016/S0002-9440(10)65044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96:10836–41. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paques M, et al. Growth factors and diabetic retinopathy. Diabetes Metab. 1997;23:125–30. [PubMed] [Google Scholar]
- Penn JS, et al. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–71. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaum T, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408–13. [PubMed] [Google Scholar]
- Rao VR, et al. Delivery of SAR 1118 to the retina via ophthalmic drops and its effectiveness in a rat streptozotocin (STZ) model of diabetic retinopathy (DR) Invest Ophthalmol Vis Sci. 2010;51:5198–204. doi: 10.1167/iovs.09-5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SG, Flynn HW., Jr Pharmacotherapies for diabetic retinopathy: present and future. Exp Diabetes Res. 2007:52487. doi: 10.1155/2007/52487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5:1390–5. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- Stitt AW, et al. Advanced glycation end products induce blood-retinal barrier dysfunction in normoglycemic rats. Mol Cell Biol Res Commun. 2000;3:380–8. doi: 10.1006/mcbr.2000.0243. [DOI] [PubMed] [Google Scholar]
- Takahashi K, et al. Suppression and regression of choroidal neovascularization by the multitargeted kinase inhibitor pazopanib. Arch Ophthalmol. 2009;127:494–9. doi: 10.1001/archophthalmol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolentino MJ, et al. Angiography of fluoresceinated anti-vascular endothelial growth factor antibody and dextrans in experimental choroidal neovascularization. Arch Ophthalmol. 2000;118:78–84. doi: 10.1001/archopht.118.1.78. [DOI] [PubMed] [Google Scholar]
- Vinores SA. Assessment of blood-retinal barrier integrity. Histol Histopathol. 1995;10:141–54. [PubMed] [Google Scholar]
- Yafai Y, et al. Anti-angiogenic effects of the receptor tyrosine kinase inhibitor, pazopanib, on choroidal neovascularization in rats. Eur J Pharmacol. 666:12–8. doi: 10.1016/j.ejphar.2011.05.016. [DOI] [PubMed] [Google Scholar]