Abstract
The mesopontine rostromedial tegmental nucleus (RMTg) is a newly discovered brain structure thought to profoundly influence reward-related pathways. The RMTg is prominently GABAergic, receives dense projections from the lateral habenula and projects strongly to the midbrain ventral tegmental area and substantia nigra compacta. It receives additional afferent connections from widespread brain structures and sends additional strong efferent connections to a number of non-dopaminergic brainstem structures and, to a lesser extent, the forebrain. Projection neurons of the RMTg have been shown to express Fos in response to aversive stimuli and/or reward omission and psychostimulant drug administration. This review will first recount how the RMTg was discovered and then describe in greater detail what is known about its neuroanatomical relationships, including afferent and efferent connections, neurotransmitters, and receptors. Finally, we will focus on what has been reported about its function.
Keywords: dopamine, ventral tegmental area, substantia nigra compacta, drug abuse, reward
Introduction
The midbrain ventral tegmental area (VTA) gives rise to the mesocorticolimbic dopaminergic system [1-6], which contributes to a broad range of functions, including but not limited to locomotor activation (e.g., [7]), stimulus and response reinforcement (e.g., [8]) and fear conditioning [9-11]. The VTA thus is essential to the neural processes that subserve adaptive behavior. Furthermore, the VTA is the substrate for the initiation of behavioral sensitization to psychostimulant drugs [12-20]. Dopamine neurons in the VTA respond to rewards and reward-predicting cues with increased firing and to omission of expected rewards with decreased firing [21-23]. In constrast, the lateral habenula (LHb), an epithalamic structure that projects to the VTA [24-26], decreases firing in response to rewards and reward-prediciting cues and increases firing in response to aversive stimuli or reward omission [27-31]. In view of these considerations, it long seemed that LHb firing must inhibit dopamine neurons. As it turns out, however, most of the direct LHb projections to the VTA are glutamatergic [32, 33], which suggested that inhibition of the VTA by the LHb should require the action of an inhibitory mediator interposed between the LHb and VTA. A study undertaken to determine if GABAergic neurons in the VTA have this role revealed that half of recorded VTA GABAergic neurons were activated by LHb stimulation, but only half of these with a sufficiently brief time course to be presumptive mediators of the proposed feed forward inhibition of VTA dopamine neurons [29]. Because the inhibitory effect on the VTA of LHb stimulation is profound (more than 95% of VTA dopamine neurons are inhibited), it seemed likely that additional brain structures are involved in mediating the inhibition.
The initial evidence for another such a mediator was reported in an abstract by Chou et al. [34], who showed that a cluster of cells in the paramedian tegmentum behind the VTA projects to the VTA and the substantia nigra pars compacta (SNc), influences fear-elicited behavior, and expresses GAD67 mRNA. Chou’s observations were foreshadowed by earlier reports noting that the densest accumulation of LHb efferent terminations in the ventral mesencephalon was not in the VTA but behind it [24, 25]. In a subsequent abstract, Jhou (same person as Chou in [34]) and Gallager [35] showed that these neurons are activated by aversive stimuli. Definitive papers from Jhou and colleagues [36, 37] showed that the structure in question is prominently GABAergic, receives dense projections from the LHb, and projects strongly to the VTA and SNc (see also [38]). Earlier, Scammell et al. [39], following administration of modafinil in rats had identified a cluster of Fos-expressing neurons, which they designated as “retroVTA”, due to its location behind the VTA. Barrot and colleagues [40] subsequently identified a cluster of neurons in the same part of the brain that expresses a long-lived splice variant of FosB (deltaFosB) following chronic forced administration of amphetamine and called it the “posterior tail of the VTA”. Further inspection of the region revealed that what might be the same cluster of neurons expresses Fos after self-administration of cocaine and projects to the VTA [41]. Finally, Jhou et al. [37] and Kaufling et al. [42] concluded that all of these studies describe the same structure, which Jhou et al. [37] named the mesopontine rostromedial tegmental nucleus (RMTg) and Barrot and colleagues [40, 42] designated as the tail of the VTA (tVTA). This GABAergic midbrain structure is now thought to convert excitation of the LHb into an inhibitory influence on the VTA [36, 43-45] through glutamatergic LHb neurons projecting to GABAergic RMTg neurons which, in turn, inhibit dopaminergic VTA/SNc neurons. These findings at the least suggest that the RMTg has a role as a modulator of reward and arousal, and this has been a sufficient stimulus to lead to further neuroanatomical and functional investigation of the structure.
Neuroanatomical Framework
The studies cited above that pose the RMTg as an inhibitory mediator interposed between the LHb and VTA bore numerous functional implications, but also begged a better understanding of the connectivity of the RMTg. At the present, the criteria for designating neurons as belonging to the RMTg include a major input from the LHb, projection to the VTA/SNc, GABAergic phenotype, and expression of Fos following certain aversive stimuli or administration of various psychostimulant drugs. Neurons fulfilling these criteria are concentrated in a rostrocaudally elongated cluster that protrudes into the caudal part of the VTA in the angle between the medial lemniscus and interpeduncular nucleus and ascends caudalward in relation to the crossing fibers of the superior cerebellar peduncle in a position just lateral to the median raphe nucleus (Figs. 1 and 2). As it turns out, additional neurons also fulfilling these criteria are scattered around the main cluster in a concentration that diminishes with increasing distance from the main focus [41], such that, despite the seemingly discrete appearance of the main focus of the RMTg (Figs. 1 and 2), precise neuroanatomical boundaries for it are difficult to establish. Furthermore, it is not known whether neuronal types other than GABAergic should be included within the RMTg. Thus, while the early studies into the cytoarchitecture and afferent and efferent connectivity of the RMTg may not as yet perfectly represent its true nature, they lay a foundation for continuing study.
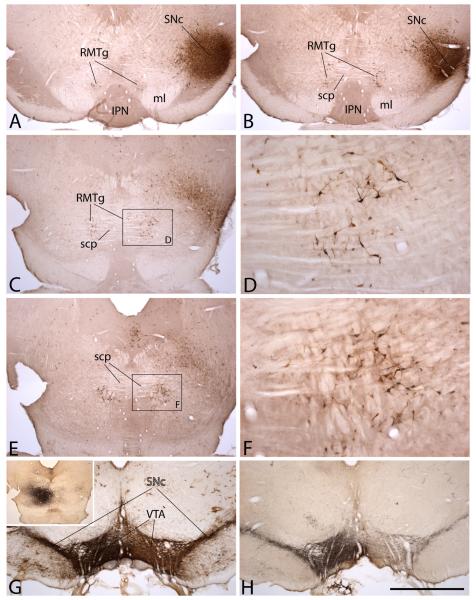
Figure 1.
Photomicrographs illustrating the rostromedial tegmental nucleus (RMTg) with the aid of retrogradely transported Fluorogold (FG, panels A-F) and anterogradely transported Phaseolus vulgaris-leucoagglutinin (PHA-L, panels G, H). FG was injected into the lateral extremity of the substantia nigra involving substantially both the compacta (SNc) and reticulata. Retrogradely labeled neurons can be seen in the RMTg in sequentially more caudal sections in A, B, C and E. The rostral tip of the RMTg (A) occupies the angle between the medial lemniscus (ml) and interpeduncular nucleus (IPN). Caudalward from there, RMTg labeling ascends in the paramedian tegmentum in relation to the crossing fibers of the superior cerebellar peduncle (scp). D and F show enlargements of retrogradely labeled RMTg neurons in the boxed areas in C and E, respectively. A PHA-L injection into the RMTg at a site approximating that shown in E (G, inset) gave rise to robust ipsilateral and moderate contralateral anterograde labeling that perfectly overlaps tyrosine hydroxylase immunoreactivity in the ventral tegmental area (VTA) and SNc (G). The section shown in G was processed for TH (brown) and PHA-L (black) immunoreactivity. The adjacent section in H was processed only for PHA-L immunoreactivity (black). Scale bar: 1 mm for all but D and F; 200 μm for D and F.
Figure 2.
Photomicrographs of sequentially more caudal sections showing the RMTg in preparations processed to exhibit the μ-opioid receptor (A, C, E, G and H) and somatostatin (B, D and F) immunoreactivities at levels comparable to those shown in Figure 1A, B, C and E. H is an enlargement of the box shown in C and illustrates Fos immunoreactive nuclei (brown dots), which are concentrated in the RMTg in rats, which, like the one from which these preparations were made, received an injection of psychostimulant drug prior to being sacrificed. Abbreviations: IPN – interpeduncular nucleus; ml – medial lemniscus; mlf – medial longitudinal fasciculus; MR – median raphe nucleus; RMTg – rostromedial tegmental nucleus; scp – superior cerebellar peduncle. Scale bar: 1 mm for A, -G; 250 μm for G.
The position particularly of the rostral extremity of the RMTg in relation to the caudal part of the VTA (Figs. 1A and 2A-D), has led some investigators to regard the RMTg as a caudal extension, or ‘tail of the VTA’ [38, 40, 42, 46] and necessitates, if possible, that grounds be established to distinguish the RMTg from the VTA both anatomically and functionally. As it turns out, this is not easily done. While dopamine neurons are gnomic for the VTA and substantia nigra [1, 6], neurons with other phenotypes, including GABAergic, are prominent therein as well [47, 48]. Insofar as RMTg neurons, at least for now, seem to be exclusively GABAergic, one can logically argue that the RMTg represents a subpopulation of VTA GABA neurons, but this position must also account for the observation that nearly all RMTg neurons express Fos and related immediate-early genes following psychostimulant administration (Figs. 2H and 3A and B), whereas psychostimulant-elicited Fos expression is relatively sparse in the main part of the VTA and SNc [49]. Neglecting psychostimulant-induced Fos, an equivalently reasonable proposition is that GABA neurons in the VTA/SNc complex are outlying neurons belonging to the RMTg. These are but speculations, however, and, while a persuasive means to distinguish the RMTg from the VTA/SNc is somewhat elusive, there seems also to be little reason to lump the two. For example, the observation that psychostimulant-induced Fos-expressing neurons at the rostral tip of the RMTg comingle with those of the caudal VTA, but do not show co-localization of tyrosine hydroxylase immunoreactivity [37] argues against ‘lumping’. Nor does anterograde axonal tracer injected into the RMTg robustly label projections to the ventral striatum, which, of course, is a connectional feature also gnomic for the VTA.
Figure 3.
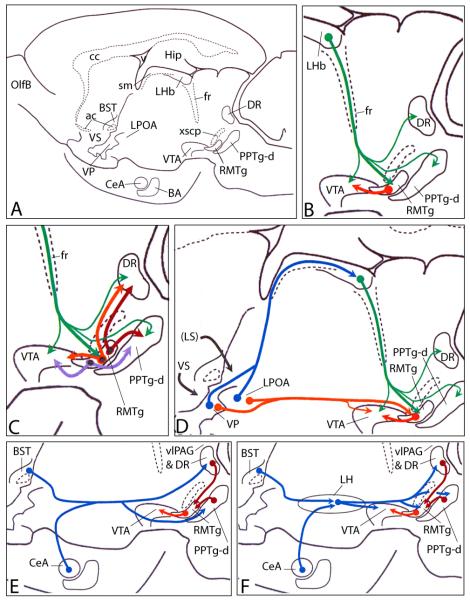
Diagrams indicating some putatively important sources of afferents to the rostromedial tegmental nucleus (RMTg). RMTg interconnections with structures giving rise to ascending modulatory projections, including the ventral tegmental area (VTA), dorsal raphe (DR) and medial dissipated part of the pedunculopontine tegmental nucleus (PPTg-d). Panel A shows an orientation diagram illustrating the rat brain in sagittal section with structures labeled that are relevant to the succeeding panels (see Additional Abbreviations below). The preparation is depicted as rotated slightly upward so that central (CeA) and basal (BA) nuclei of the amygdala are visible in the medial temporal lobe. To simplify the presentation, some structures are included in this single plane of section, although in actuality they do not lie with other included structures in the same mediolateral sagittal plane. (B) Enlargement of the region in (A) depicting the RMTg, lateral habenula (LHb), VTA, DR and PPTg-d showing the disynaptic connection via the RMTg that links the LHb and VTA. Note that the LHb also projects, less robustly, to the VTA, DR and PPTg-d. (C) Same region as shown in (B) illustrating RMTg neurons projecting to the VTA, DR and PPTg-d that exhibit Fos expression (denoted by black dot signifying Fos-immunoreactive nuclei) following administration of psychostimulant drugs. A significant number of such psychostimulant-induced Fos expressing neurons projects by axon collaterals to the VTA and DR, VTA and PPTg-d, and DR and PPTg-d. (D) Two trajectories available to ventral pallidum (VP) and lateral preoptic area (LPOA) neurons that project to the RMTg. Note that the dorsal diencephalic pathway via the LHb is disynaptic and thus would require a longer latency for the passage of signals. The ventral monosynaptic route passes via the medial forebrain bundle. (E and F) Whereas direct projections to the RMTg from extended amygdala structures, such as the CeA and bed nucleus of stria terminalis (BST), are scarce, major indirect routes via the DR and PPTg-d (E) and lateral hypothalamus (LH in F) provide putatively strong multisynaptic access to the RMTg. Robust projections to the VTA from the DR and PPTg are left out to simplify the presentation. Note that the lateral hypothalamic relay insinuates an additional synapse in the pathway. Additional abbreviations: ac – anterior commissure; cc – corpus callosum; fr – fasciculus retroflexus; Hip – hippocampus; OlfB – olfactory bulb; sm – stria medullaris; VS –ventral striatum; xscp – decussation of the superior cerebellar peduncle.
Further analysis of the RMTg revealed some additional details about its neurochemistry. As noted, Jhou et al. [37] found that more than 70% of RMTg neurons retrogradely labeled following tracer injections in the VTA exhibit mRNA for GAD67. Along similar lines, Kaufling et al. [42] estimated that 92% of neurons that expressed FosB/DeltaFosB after cocaine administration, thus representing the RMTg (or tVTA in their nomenclature), were GABAergic. An enrichment of immunoreactivity against the μ-opioid receptor is present in the location designated by psychostimulant-induced Fos expression as the RMTg (Fig. 2A, C, E and G; see also [38]) and endomorphin-1, a μ-opioid agonist, is self-administered into the RMTg more vigorously than into the VTA itself, and not into adjacent regions [50]. RMTg exhibits enriched somatostatin immunoreactivity (Fig. 2B, D and F) and, recently, Jhou has detected there an enrichment of the peptide nociceptin orphanin fq and its mRNA (personal communication). Although nitric oxide synthase (Nos) immunoreactivity is not visibly enriched in the RMTg, Nos-IR neurons in lateral and dorsal hypothalamus, ventral lateral geniculate nucleus, supramammillary region, dorsolateral column of the periaqueductal gray, pedunculopontine and laterodorsal tegmental nuclei, and pontomedullary reticular formation contained retrograde label following injections in the RMTg [37]. The functional implications of enrichment of these various peptides and macromolecules in the RMTg require further investigation.
Connectivity
Efferent connections of the RMTg were described with the aid of the anterograde tracer, PHA-L [37]. As mentioned, the densest RMTg efferent projection is to the VTA/SNc complex (Figs. 1G and H and 3). Complementary injection of retrograde tracer into the VTA revealed a topographical organization such that injections in the medial and lateral part of the midbrain dopaminergic complex retrogradely labeled more medial and lateral parts of the RMTg, respectively. The projection from RMTg to the nigral dopaminergic complex is strongest on the ipsilateral side of the brain, but does have a moderately robust contralateral component that is stronger and weaker in medial and lateral parts of the RMTg, respectively. Jhou et al. [36] have shown that many axons labeled following PHA-L injections into the RMTg exhibit close apposition to dopaminergic neurons in the VTA and SNc. The synaptic nature of this relationship was confirmed by Balcita-Pedicino et al. [51], who observed that 81% of axon terminals labeled following RMTg injections of PHA-L synapse onto tyrosine hydroxylase immunoreactive dendrites in the VTA/SNc complex.
Other robust outputs from the RMTg occupy the medial part (pars dissipata) of the pedunculopontine tegmental nucleus (PPTg), periaqueductal gray, including the dorsal raphe nucleus (DR), and pontine and medullary reticular formation (Fig. 3C). Varyingly moderate to sparse labeling was observed in numerous other structures, including in the ventral pallidum, diagonal band complex, lateral preoptic region, thalamic midline and intralaminar cell groups, lateral hypothalamus, entopeduncular nucleus, parafascicular thalamic nucleus, deep mesencephalic nucleus, laterodorsal tegmental nucleus, locus coeruleus and subcoeruleus complex, and deep cerebellar nuclei.
Lavezzi et al. [52] have studied the dense projections from the RMTg to the DR and pars dissipata of the pedunculopontine tegmental nucleus (PPTg-d), reporting that they, like RMTg projections to the VTA, are enriched in neurons that exhibit psychostimulant-induced Fos expression (Fig. 3C). Lavezzi et al. [52] further demonstrated RMTg neurons that project via collaterals to the VTA and PPTg-d, and others that project to the VTA and DR. In this regard, it should be recognized that both the PPTg-d [53] and DR [54] provide robust inputs to the midbrain dopaminergic complex. In addition, it is probably relevant to recall here that the PPTg-d lacks abundant cholinergic neurons and has been referred to as the midbrain extrapyramidal area [55, 56], insofar as it is a major target of projections descending from the entopeduncular nucleus (globus pallidus internal segment), subthalamic nucleus and substantia nigra reticulata [55-57], not to mention robust outputs from extended amygdala structures [58]. Consequently, the RMTg, by virtue of its dense inputs to this region, may exert additional influences on the basal ganglia circuitry distinct from its actions on the dopaminergic neurons of the VTA-SNc.
Interestingly, Aston-Jones and colleagues [59] described a GABA-expressing structure quite similar to the RMTg, but preferentially related to the locus coeruleus. Whether the RMTg is but one of a group of such compact structures that project robustly to brainstem structures giving rise to long ascending neuromodulatory projections and thus reflects a general feature of brainstem-forebrain organization begs further study.
Afferents of the RMTg have been described by Jhou et al. [37] and Kaufling et al. [42] following injections into the RMTg of FG and CTβ, respectively. These are listed in Table 1. However, of all of afferent connections of the RMTg noted, that from the LHb (Fig. 2B, considered in combination with the RMTg efferent projection to the VTA) is mainly responsible for the current keen interest in the RMTg, which at present is regarded as the main mediator of an inhibitory influence of the LHb on nigral dopamine neurons. The dense projections from the LHb to the RMTg as shown by both retrograde and anterograde tracing [37, 42] are mainly ipsilateral with moderate labeling in the contralateral RMTg and are topographically organized such that injections of PHA-L in medial and lateral LHb label mainly projections to the medial and lateral RMTg, respectively. Also, medial LHb injections give rise to a stronger contralateral RMTg projection [37]. Balcita-Pedicino et al. [51] used electron microscopy to demonstrate that 53% of axon terminals labeled following PHA-L injections in the LHb synapse onto GABAergic neurons in the RMTg.
Table 1.
Structures retrogradely labeled following injection of retrograde tracer into the RMTg (listed approximately from rostral to caudal).
| rostral association cortex | pedunculopontine tegmental nucleus |
| prelimbic cortex | laterodorsal tegmental nucleus |
| dorsal peduncular cortex | locus ceruleus |
| orbital cortex | parabrachial nucleus |
| Cingulate cortex | deep cerebellar nuclei |
| agranular insular cortex | Prerubral field |
| rostral claustrum | Retrorubral field |
| interstitial nucleus of the posterior limb of the anterior commissure |
intermediate layers of superior colliculus |
| pontine reticular nuclei | |
| lateral septum | medullary reticular formation |
| medial septum | nucleus ambiguus |
| vertical limb of the diagonal band | nucleus of the solitary tract |
| accumbens | dorsal vagal complex |
| ventral pallidum | |
| lateral preoptic area | |
| medial preoptic area | |
|
ventral bed nucleus of the stria terminalis |
|
| sublenticular region | |
| lateral hypothalamus | |
| dorsal hypothalamus | |
| entopeduncular nucleus | |
| hypothalamic paraventricular nucleus | |
| thalamic parafascicular nucleus | |
| tuber cinereum | |
| magnocellular preoptic nucleus | |
| zona incerta | |
| Supramammillary nucleus | |
| periaqueductal gray | |
| lateral habenula | |
| ventral tegmental area | |
| substantia nigra compacta | |
| substantia nigra reticulata | |
| Red nucleus |
Another fairly focal clustering of afferents within the RMTg was observed following PHA-L injections into various parts of the VTA/SN complex, including parts of the SN pars reticulata bordering on the pars compacta [37], suggesting the presence of a convergent set of inputs that reciprocates the dense outputs to the VTA/SN complex from the RMTg. Accordingly, numerous retrogradely labeled neurons were observed within the VTA/SN complex following injections of retrograde tracer into the RMTg and a majority of these did not exhibit co-localized tyrosine hydroxylase immunoreactivity, suggesting a non-dopaminergic, possibly GABAergic phenotype. The precise nature of this input to the RMTg remains to be determined.
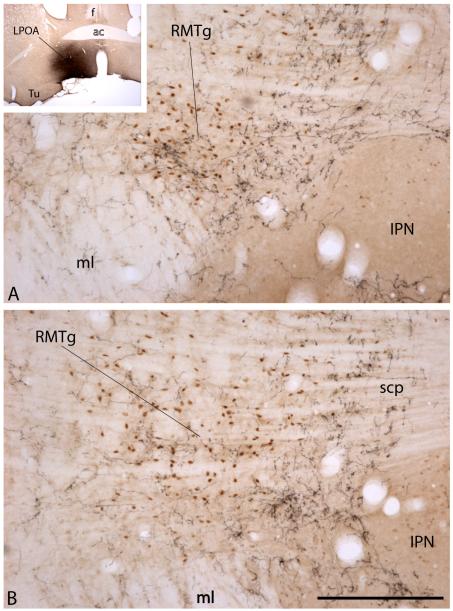
Finally, Zahm et al. [60] have reported that injections of anterogradely transported PHA-L into the lateral preoptic area produce a focal enrichment of labeled, highly branched and varicose axons in the RMTg (Figs. 3D and 4). The location of lateral preoptic area injection sites that produce such RMTg axonal terminations appears to be in a transition zone between the ventral pallidum and lateral preoptic area, which happens to be an area of basal forebrain with moderate numbers of glutamatergic neurons, among which some projecting to the VTA have been identified [32]. Whether there are glutamatergic projections from here to the RMTg remains to be determined. This area also gives rise to a dense, possibly glutamatergic, projection to the LHb [61], which might then serve as a relay in a disynaptic pathway from the lateral preoptic area to the RMTg.
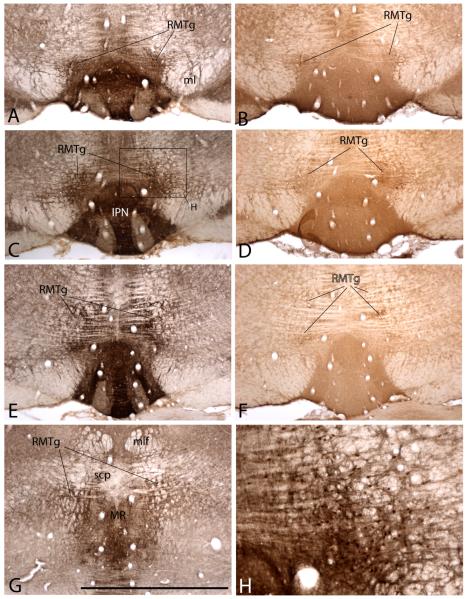
Figure 4.
Photomicrographs showing the RMTg at a rostral (A) and more caudal (B) level. Note the position of the collection of Fos–immunoreactive neuronal nuclei (brown dots) representing the RMTg, which occupy the angle between the medial lemniscus (ml) and interpeduncular nucleus (IPN) in A and lie above and lateral to the IPN in the more caudal section shown in (B). Levels shown in (A) and (B) are comparable to those in (A) and (C) in Figure 2. The RMTg is Fos immunoreactive in this preparation due to the fact that the rat from which the preparation was made was injected with the psychostimulant drug, methamphetamine (10 mg/kg), prior to being sacrificed. Ten days before sacrifice, the rat received an injection of anterogradely transported Phaseolus vulgaris-leucoagglutinin (PHA-L) into the lateral preoptic area (LPOA), resulting in anterograde labeling of a dense plexus of axons in and around the RMTg (black labeling). Note the horizontal banding pattern of the crossing fibers of the superior cerebellar peduncle (scp in B). Scale bar: 200 μm. Additional abbreviations: ac – anterior commissure; f – fornix; Tu – olfactory tubercle.
It should be noted that conclusions regarding the actual afferents of structures, particularly in brainstem, cannot be made on the basis of retrograde labeling alone because uptake by passing and damaged fibers can lead to spurious results. Furthermore, FG and CTβ are exceedingly sensitive tracers in the sense that even sparse projections can give rise to robust retrograde labeling. Jhou et al. [37] followed up on the retrograde labeling with complementary anterograde tracing, which confirmed a number of the projections identified with the retrograde tracers (Table 1). However, it should be noted that, in contrast to projections from the LHb, SN complex and, possibly, lateral preoptic area, which appear to be more concentrated in the RMTg than adjacent structures, the projections to the RMTg from most forebrain and brainstem structures do not terminate in a manner that distinguishes the RMTg from adjacent similary innervated structures, such as, e.g., the VTA, retrorubral area, paramedian tegmentum, mesencephalic reticular formation and pedunculopontine and laterodorsal tegmental nuclei. Until all structures projecting to the vicinity of the RMTg are evaluated with anterograde tracing, knowledge of the precise nature of its afferents will remain incomplete.
Electrophysiology
Studies in brain slices by Lecca et al. [45] revealed that RMTg neurons exhibit electrophysiological parameters similar to those previously recorded in vivo by Jhou et al. [37] and that EPSCs evoked in RMTg neurons by LHb stimulation are mediated by AMPA receptors. LHb stimulation caused spiking activity in 46.3% of RMTg neurons, whereas stimulation of the RMTg neurons resulted in suppression of VTA neuronal activity. These findings recall those reported in the monkey by Hikosaka and colleagues [43, 44] in which it was demonstrated that stimulation of the LHb excites neurons in the RMTg and stimulation of the RMTg inhibits DA neurons in the SNc. Taken together, the tract tracing, electron microscopic, and electrophysiological evidence provide strong support for the hypothesis that the RMTg mediates the inhibitory influence of the LHb on dopaminergic neurons in both the rat and monkey.
Interestingly, however, Hikosaka and colleagues [43, 44] observed that the latency to fire following a reward omission is slightly shorter for a significant number of RMTg as compared to LHb neurons, suggesting that RMTg afferents other than from the LHb may more promptly signal reward omission. From where these postulated afferents might arise is a good question, because, while many structures provide afferents to the RMTg, few exhibit the focused concentration of terminations within the RMTg characteristic of the LHb projection [37]. One exception, however, is a projection discussed above from the lateral preoptic area to the RMTg (Figs. 3D and 4; [60]), which appears to run parallel to a disynaptic pathway involving the lateral preoptic area, LHb and RMTg (Fig. 3D). This situation could explain the longer latency for LHb as compared to RMTg responses to reward omission, although it requires experimental evaluation.
RMTg and drugs of abuse
The robust expression of Fos and related immediate-early genes observed in the RMTg after administration of psychostimulants [37-42, 62] and strong projection of the RMTg to the DAergic midbrain region strongly implicate the RMTg in the actions of psychostimulant drugs of abuse and likely addiction. Interestingly, Kaufling et al. [38] have shown that strong FosB/DeltaFosB expression follows administration of stimulant drugs, including, methamphetamine, 3,4-methylenedioxymethamphetamine (Ecstasy), methylphenidate and caffeine, but not after drugs from other drug classes, such as ethanol, diazepam, gamma hydroxybutyric acid, morphine, ketamine, phencyclidine, delta-9-tetrahydrocannabinol, sodium valproic acid, or gabapentin. However, expression of Fos and other immediate-early genes does not necessarily imply that a neuron or structure has been excited, and the absence of an immediate-early gene response does not necessarily signify that a neuron or structure is unaffected by a particular drug [49]. Thus, an important goal would seem to be to more precisely define RMTg function in conditions of taking drugs of abuse.
Lecca et al. [45] have recorded the electrical activity of RMTg neurons after administration of various drugs of abuse. They have shown that systemic administration of morphine strongly inhibits RMTg neurons, producing a near 50% decrease in their mean spontaneous firing rate, and that this effect is dependent upon μ-opioid receptor activation. Thus, despite the lack of a Fos response following morphine administration [40, 46] a significant electrophysiological effect is nonetheless observed. Whereas infusions of cocaine elicit robustly enhanced RMTg Fos expression [41], Lecca et al. [45] recorded a decrease of about 18% in the firing rate of morphin-exposed RMTg neurons, indicating that Fos expression does not necessarily coincide with neuronal activation, but may instead signify only ongoing transcriptional regulation, in this case associated with neuronal inhibition. Intravenous administration of the cannabinoid CB1 receptor agonist, WIN55212-2 (WIN), produced a 55% decrease in the firing rate of RMTg neurons that was prevented by pre-incubation with the CB1 receptor antagonist SR141716A, demonstrating that the inhibition depends upon CB1 receptor stimulation. An increase in paired-pulse ratio (EPSC2/EPSC1) following administration of WIN suggests that WIN activation of presynatic CB1 receptor reduces the probability of glutamate release. Surprisingly, Lecca and colleagues found that i.v. administration of nicotine nearly doubled the firing rate of RMTg neurons. Pretreatment with the nicotinic acetylcholine (nACh) receptor antagonist, mecamylamine, blocked this effect but did not alter spontaneous activity. Nicotine-induced potentiation of RMTg neuron EPSC amplitude was attenuated by methyllycacoitine, which blocks cholinergic receptors containing an α7 subtype nAChR subunit, suggesting that α7 subunit-containing receptors are main components of glutamatergic presynaptic terminals that synapse onto RMTg neurons. Lecca et al. [45] suggest that these receptors may mediate a long-lasting excitation of the RMTg neurons. Because it is unknown if CB1 or nACh receptors are expressed by RMTg neurons, Lecca et al. [45] reason that cholinergic and cannabinoid modulation of RMTg neurons may occur presynaptically at glutamate-releasing afferent terminals, such as those projecting to the RMTg from the lateral habenula.
It is well-established that the VTA plays a prominent role in the initiation of behavioral sensitization [20, 62] and the sheer density of RMTg projections to the VTA/SNc [36, 37, 51] suggests a role in this process. Similarly, the PPTg has robust cholinergic [53, 63], glutamatergic [32, 64, 66], and GABAergic [64-67] projections to the VTA, and increasing evidence implicates PPTg involvement in the initiation of behavioral sensitization as well [68, 69]. The serotoninergic DR also projects strongly to the VTA [32, 54, 70] and has long been regarded as having a role in processes underlying drug abuse [71-73]. As noted in an earlier section of this paper, the RMTg projects strongly to the PPTg-d and DR [37]. RMTg neurons giving rise to these projections, like those projecting to the VTA, express Fos following psychostimulant drug administration [52]. Indeed, as noted, a subset of such neurons projects to both the VTA and the PPTg, as does another subset to the VTA and DR (Fig. 3C). In view of these connections, it seems reasonable to speculate that the RMTg has a role in a complex circuitry modulating the initiation of behavioral sensitization to psychostimulants. The perceived simplicity of the circuitry underlying these presumptions may be deceptive, however, in consideration of earlier reports of both decreases [74] and increases [75] in extracellular concentrations of serotonin in target structures of the DR following stimulation of LHb. As a further cautionary note, the possibility must be considered that RMTg projections to the PPTg may less exert influence on VTA-projecting PPTg neurons than on PPTg neurons with other functional consequences, such as, e.g., modulation of spinal motor neuron excitability [76]. Future study will be required to sort out these issues.
RMTg neuronal function and reward-related stimuli
The LHb increases neuronal firing in response to aversive stimuli or omission of reward [27-31] and because LHb projections to the ventral tegmental area are mainly glutamatergic [32-33] they can inhibit dopamine neurons only via an inhibitory relay. The neuroanatomical evidence that the RMTg is such a mediator has been described. Functional data relevent to this idea follow.
Jhou et al. [36] provided a detailed account of the role of RMTg neurons in encoding information signifying aversive stimuli and the accompanying behavioral responses. They observed that GABAergic RMTg neurons that project to the VTA increase Fos expression in response to unconditioned footshock (46% increase) or a tone previously paired with footshock (30% increase). They then examined whether aversive and appetitive stimuli elicit opposite responses in RMTg neurons, by comparing Fos expression in food-deprived as compared to fed rats. Thirty-two percent of VTA-projecting RMTg neurons expressed Fos in the food deprived group as compared to only 18% in the fed group. In the same study, electrophysiological recordings performed on RMTg neurons demonstrated that about half of presumptive RMTg neurons (55%) were excited by shock-predictive cues and, among these, most also were activated by an unconditioned shock (72%) and/or inhibited by sucrose. In contrast, the few RMTg neurons that were activated by sucrose-predictive cues were valence-reversed when presented with an unconditioned stimulus, such that they were inhibited by unanticipated sucrose and stimulated by unexpected shock. Studies done in monkeys by Hikosaka and colleagues [43-44] support the findings in the rat, demonstrating that RMTg neurons are inhibited by reward predictive cues and excited by omitted reward. Further studies by Hikosaka and colleagues [43, 44] demonstrated that RMTg neurons encode negative reward prediction errors, such that they increase their firing rate in response to cues predicting smaller, as compared to larger, rewards. These investigations reveal some functional heterogeneity in the RMTg, but that the majority of its neurons are activated by the presentation of conditioned or unconditioned aversive stimuli and generally do not respond to reward-predictive cues or the presentation of reward. This situation is the inverse of that recorded from DAergic neurons, which are generally inhibited by aversive stimuli and omitted rewards and activated by reward or reward-predictive stimuli [21-23, 30].
Lesions of the brainstem region that contains the RMTg caused a reduction in passive responses to fear, such as freezing and open-arm avoidance in the plus maze, but had no effect, and sometimes enhanced, active fear-elicited behaviors, such as treading or escaping [36, 77]. These results are inverse relative to those observed following dopamine depletion, which produces impairment of active fear-elicited behaviors and enhances passive fear-elicited behaviors [78]. Insofar as lesions of the ventrolateral periaqueductal gray (PAG) produce behavioral effects similar to those observed following lesions of the RMTg [79-80] and the PAG receives input from both the central nucleus of the amygdala [81] and the bed nucleus of stria terminalis [82], Jhou et al. [36] have speculated that these structures activate RMTg neurons in fear-arousing circumstances. This might happen by virtue of direct connections (Fig. 3E) and via relays in the lateral hypothalamus (Fig. 3F). Indeed, the effects of RMTg lesions on fear-elicited behaviors may reflect removal from the fear circuitry of influences that are mediated by the RMTg, but that arise in other structures known to be activated by fear-arousing stimuli, such as the amygdala, bed nucleus of stria terminalis, periaqueductal gray or LHb, that project to the RMTg (Fig. 3D-F). The situation is undoubtedly more complex, however, insofar as these considerations do not explain the reduced avoidance of elevated plus maze open arms following RMTg lesions, which implicates afferents from the septum [83, 84]. At present, suffice it to say that multiple afferent influences are likely integrated in the RMTg, which in turn modulates fear-related and, possibly, reward-related behaviors through both direct and indirect interconnections with brainstem arousal centers.
Clinical considerations
As a relatively newly described entity, the RMTg has yet to figure large in the clinical literature and one is able at present only to speculate regarding its clinical import based upon its robust and intimate connectional relationships with structures of well-known clinical consequence, such as, e.g., the midbrain dopaminergic complex, PPTg, and serotoninergic DR. For example, in view of the reported capacity of stimulation of the LHb to influence the activity of a number of structures known to impact affect, including dopaminergic neurons in the VTA/SNc complex, serotoninergic neurons in the DR and noradrenergic neurons in the locus coeruleus, Henn and colleagues speculated that inactivation of the LHb by deep brain stimulation (DBS) could have a therapeutic effect on treatment resistant depression [85]. In the single clinical trial reported at the time of this writing [86], a depressed patient so treated did indeed remit, but only following many weeks of habenular DBS. Upon cessation of habenular DBS the patient promptly relapsed into depression and, when habenular DBS was re-initiated soon after, a second remission occurred, but again only after a period of weeks of DBS. In view of the connectivity involving the LHb, RMTg, VTA/SNc, DR and other structures described herein, it seems probable that the RMTg played some role in the complex, delayed therapeutic response observed by Henn and colleagues.
A number of the pontomesencephalic structures with which the RMTg is innerconnected, such as the PPTg, locus coeruleus, DR, and VTA/SNc complex, are known to exhibit synucleinopathy and loss of neurons in Parkinson’s disease [87-90]. Indeed, Parkinson’s disease itself is no longer regarded by many scientists and clinicians as exclusively a disease of mesostriatal dopamine neurons, but rather one that is preceded by degenerative changes in the brainstem and limbic and olfactory areas associated with depression, anosmia and multiple autonomic symptoms that often precede the onset of Parkinsonian motor symptoms, sometimes by years [91-96]. Surely, the intimate interconnectivity of the RMTg with brainstem systems thought to contribute to this spectrum of pathology dictates that the RMTg should, at least conditionally, be included among them.
Finally, a role for the RMTg in the pathogenesis of addiction to psychostimulant and other classes of abused drugs has been noted at some length in other parts of this review.
Conclusions and future directions
The identification of the RMTg as a distinct GABAergic structure capable of directly influencing DAergic neurons of the VTA/SNc has created avenues for significant advances in our understanding of neural mechanisms of reward-related processing. Here, we have discussed the vast, convergent input to the RMTg from structures extending from the rostral pole of the frontal cortex to the medulla [37, 42], with particular emphasis on those arising in the lateral habenula and, less so, lateral preoptic area. RMTg efferent connections are strongest to the VTA/SNc complex, reflective of the modulatory effect this structure likely has on the dopaminergic projection system, but it also has significant projections to other VTA-projecting brainstem sites, such as the PPTg and DR [37], which would seem to imply that the RMTg is but a node in a complex neuromodulatory circuitry.
One of the hallmark characteristics of the RMTg is expression of Fos in response to psychostimulant drug administration. However, various drugs of abuse (morphine, cocaine, cannabinoids, nicotine) have now been shown to modulate RMTg activity [45, 46], some in the absence of detectable immediate-early gene expression. This evidence, when assimilated with that of the strong projections to the DAergic midbrain neurons, supports the hypothesis that the RMTg is a major modulator of the reward system under conditions of drug use. Future studies should be able to clarify how various drugs affect the RMTg, whether directly or indirectly. That potential therapeutic targets against drug addiction may emerge from such studies seems possible.
A majority of RMTg neurons are activated by aversive stimuli and reward omission and cues that predict them, while substantially fewer respond to the presentation of reward or reward-predictive cues. Lesions of the RMTg resulted in reduced passive fear behaviors, such as freezing and open-arm avoidance, but had little effect on active responses to fear. Taken together, the rapidly increasing numbers of observations on the RMTg suggest that it modulates responses to both appetitive and aversive stimuli, both of which happen to accompany administration of drugs of abuse [45, 97-100].
Acknowledgement
This work was supported by USPHS grant NIH NS-23805.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- [1].Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- [2].Andén NE, Carlsson A, Dahlström A, Fuxe K, Hillarp NÅ, Larsson K. Demonstration and mapping out of nigro-neostriatal dopaminergic neurons. Life Sci. 1964;3:523–530. doi: 10.1016/0024-3205(64)90161-4. [DOI] [PubMed] [Google Scholar]
- [3].Andén NE, Dahlström A, Fuxe K, Larsson K. Mapping out of catecholaminergic and 5-hydroxytryptamine neurons innervating the telencephalon and diencephalon. Life Sci. 1965;4:1275–1279. doi: 10.1016/0024-3205(65)90076-7. [DOI] [PubMed] [Google Scholar]
- [4].Andén NE, Dahlström A, Fuxe K, Larsson K, Olson L, Ungerstedt U. Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol Scand. 1966a;67:313–326. [Google Scholar]
- [5].Andén NE, Fuxe K, Hamberger B, Hökfelt T. A quantitative study on the nigro-striatal dopamine neuron system in the rat. Acta Physiol Scand. 1966b;67:306–312. doi: 10.1111/j.1748-1716.1966.tb03317.x. [DOI] [PubMed] [Google Scholar]
- [6].Björklund A, Lindvall O. Dopamine-containing systems in the CNS. In: Björklund A, Hökfelt T, editors. Classical Transmitters in the CNS. Handbook of Chemical Neuroanatomy. Part I. Vol. 2. Elsevier; Amsterdam: 1984. pp. 55–122. [Google Scholar]
- [7].Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- [8].Wise RA. Dopamine, learning and motivation. Nature Rev Neurosci. 2004;5:1–12. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- [9].Guarraci FA, Frohardt RJ, Kapp BS. Amygdaloid D1 dopamine receptor involvement in Pavlovian fear conditioning. Brain Res. 1999;827:28–40. doi: 10.1016/s0006-8993(99)01291-3. [DOI] [PubMed] [Google Scholar]
- [10].Guarraci FA, Frohardt RJ, Falls WA, Kapp BS. The effects of intra-amygdaloid infusions of a D2 dopamine receptor antagonist onPavlovian fear conditioning. Behav Neurosci. 2000;114:647–651. doi: 10.1037//0735-7044.114.3.647. [DOI] [PubMed] [Google Scholar]
- [11].Fadok JP, Dickerson TM, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. J Neurosci. 2009;29:11089–11097. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kalivas PW, Webber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol ExpTherap. 1988;245:1095–1102. [PubMed] [Google Scholar]
- [13].Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- [14].Hooks MS, Jones HG, Liem BJ, Justice JB., Jr Sensitization and individual differences to IP amphetamine, cocaine, or caffeine following repeated intracranial amphetamine infusions. Pharmacol Biochem Behav. 1992;43:815–823. doi: 10.1016/0091-3057(92)90413-a. [DOI] [PubMed] [Google Scholar]
- [15].Perugini M, Vezina P. Amphetamine administered to the ventral tegmental area sensitizes rats to the locomotor effects of nucleus accumbens amphetamine. J Pharmacol Exp Ther. 1994;270:690–696. [PubMed] [Google Scholar]
- [16].Cador M, Bjijou Y, Caihol S, Stinus L. D-amphetamine-induced behavioral sensitization: implication of a glutamatergic medial prefrontal cortex-ventral tegmental area innervation. Neurosci. 1995;94:705–721. doi: 10.1016/s0306-4522(99)00361-9. [DOI] [PubMed] [Google Scholar]
- [17].Cador M, Bjijou Y, Stinus L. Evidence of a complete independence of the neurobiological substrates for the induction and expression of behavioral sensitization to amphetamine. Neuroscience. 1999;65:385–395. doi: 10.1016/0306-4522(94)00524-9. [DOI] [PubMed] [Google Scholar]
- [18].Bjijou Y, Stinus L, Le Moal M, Cador M. Evidence for selective involvement of dopamine D1 receptors of the ventral tegmental area in the behavioral sensitization induced by intra-ventral tegmental area injections of D-amphetamine. J Pharmacol Exp Ther. 1996;277:1177–1187. [PubMed] [Google Scholar]
- [19].Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci. 1996;16:2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacol. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- [21].Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- [22].Schultz W. Multiple dopamine functions at different time courses. Ann Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- [23].Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- [25].Araki M, McGeer PL, Kimura H. The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res. 1988;441:319–330. doi: 10.1016/0006-8993(88)91410-2. [DOI] [PubMed] [Google Scholar]
- [26].Omelchenko N, Sesack SR. Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J Comp Neurol. 2006;494:863–875. doi: 10.1002/cne.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gao DM, Huffman D, Benabid AL. Simultaneous recording of spontaneous activities and nociceptive responses from neurons in the pars compacta of substantia nigra and in the lateral habenula. Eur J Neurosci. 1996;8:1474–1478. doi: 10.1111/j.1460-9568.1996.tb01609.x. [DOI] [PubMed] [Google Scholar]
- [29].Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- [31].Shumake J, Ilango A, Scheich H, Wetzel W, Ohl FW. Differential neuromodulation of acquisition and retrieval of avoidance learning by the lateral habenula and ventral tegmental area. J Neurosci. 2010;30:5876–5883. doi: 10.1523/JNEUROSCI.3604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brinschwitz K, Dittgen A, Madai VI, Lommel R, Geisler S, Veh W. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience. 2010;168:463–476. doi: 10.1016/j.neuroscience.2010.03.050. [DOI] [PubMed] [Google Scholar]
- [34].Chou TC, Baxter MG, Saper CB. A novel afferent to dopaminergic neurons regulates fear-induced freezing. Soc Neurosci Abstr. 2004;30:783.13. [Google Scholar]
- [35].Jhou TC, Gallagher M. Paramedian raphe neurons that project to midbrain dopamine neurons are activated by aversive stimuli. Soc. Neurosci.Abstr. 2007;33:425.5. [Google Scholar]
- [36].Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. (2009)b;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. γ-aminobyturic acid cells with cocaine-induced ΔFosB in the ventral tegmental area innervate mesolimbic neurons. Biol Psych. 2010a;67:88–92. doi: 10.1016/j.biopsych.2009.08.001. [DOI] [PubMed] [Google Scholar]
- [39].Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagi- sawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci. 2005;21:2817–2824. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- [41].Geisler S, Marinelli M, Degarmo B, Becker ML, Freiman AJ, Beales M, Meredith GE, Zahm DS. Prominent activation of brainstem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacol. 2008;33:2688–2700. doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- [43].Hong S, Hikosaka O. Negative reward signals from lateral habenula to dopamine neurons are mediated by a group of medial mesopontine neurons. Soc Neurosci Abstr. 2009:482.6. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hong S, Jhou TC, Smith MK, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011:11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, Muntoni AL, Pistis M. Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacol. 2011;36:589–602. doi: 10.1038/npp.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kaufling J, Waltisperger E, Bourdy R, Valera A, Veinante P, Freund-Mercier MJ, Barrot M. Pharmacological recruitment of the GABAergic tail of the ventral tegmental area by acute drug exposure. Br J Pharmacol. 2010b;161:1677–1691. doi: 10.1111/j.1476-5381.2010.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Olson VG, Nestler EJ. Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse. 2007;61:87–95. doi: 10.1002/syn.20345. [DOI] [PubMed] [Google Scholar]
- [48].Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zahm DS, Becker ML, Freiman AJ, Strauch S, Degarmo B, Geisler S, Meredith GE, Marinelli M. Fos after single and repeated self-administration of cocaine and saline in the rat: Emphasis on the basal forebrain and recalibration of expression. Neuropsychopharmacol. 2010;35:445–463. doi: 10.1038/npp.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jhou TC, Xu S, Ikemoto S. Self-administration of a mu-opioid agonist into the rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons and other arousal systems. Soc Neurosci Abstr. 2009c:683.1. [Google Scholar]
- [51].Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp. Neurol. 2010;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lavezzi HN, Parsley KP, Ariel M, Zahm DS. Fos expression in a projection from the rostromedial tegmental nucleus (RMTg) to the pars dissipata of the pedunculopontine tegmental nucleus (PPTg) following administration of methamphetamine in the rat. Soc Neurosci Abstr. 2010:491.4. [Google Scholar]
- [53].Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- [55].Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol. 1987;259:483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- [56].Steininger TL, Rye DB, Wainer BH. Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrpyramidal area in the albino rat. I. Retrograd tracing studies. J Comp Neurol. 1992;321:515–543. doi: 10.1002/cne.903210403. [DOI] [PubMed] [Google Scholar]
- [57].Spann BM, Grofova I. Nigropeduncuolopontine projection in the rat: an anterograde tracing study with Phaseolus vulgaris-leucoagglutinin (PHA-L) J Comp Neurol. 1991;311:375–388. doi: 10.1002/cne.903110308. [DOI] [PubMed] [Google Scholar]
- [58].Gastard M, Jensen SL, Martin JR, III, Williams EA, Zahm DS. The caudal sublenticular region/anterior amygdaloid area is the only part of the rat forebrain and mesopontine tegmentum occupied by magnocellular cholinergic neurons that receives outputs from the central division of extended amygdala. Brain Research. 2002;957:207–222. doi: 10.1016/s0006-8993(02)03513-8. [DOI] [PubMed] [Google Scholar]
- [59].Aston-Jones G, Zhu Y, Card JP. Numerous GABAergic afferents to locus ceruleus in the pericerulear dendritic zone: possible interneuronal pool. J Neurosci. 2004;24:2313–2321. doi: 10.1523/JNEUROSCI.5339-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zahm DS, Arends JJA, Parsley KP, Geisler S. Projection to the mesopontine rostromedial tegmental nucleus from the lateral preoptic area. Soc Neurosci Abstr. 2011:79.09. [Google Scholar]
- [61].Kowski AB, Geisler S, Krauss M, Veh RW. Differential projections from subfields in the lateral preoptic area to the lateral habenular complex of the rat. J Comp Neurol. 2008;507:1465–1478. doi: 10.1002/cne.21610. [DOI] [PubMed] [Google Scholar]
- [62].Colussi-Mas J, Geisler S, Zimmer L, Zahm DS, Bérod A. Activation of afferents to the ventral tegmental area in response to acute amphetamine: a double-labelling study. Eur J Neurosci. 2007;26:1011–1025. doi: 10.1111/j.1460-9568.2007.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Charara A, Smith Y, Parent A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J Comp. Neurol. 1996;364:254–266. doi: 10.1002/(SICI)1096-9861(19960108)364:2<254::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [65].Clements JR, Grant S. Glutamate-like immunoreactivity in neurons of the laterodorsal tegmental and pedunculopontine nuclei in the rat. Neurosci Lett. 1990;120:70–73. doi: 10.1016/0304-3940(90)90170-e. [DOI] [PubMed] [Google Scholar]
- [66].Jia H-G, Yamuy J, Sampogna S, Morales FR, Chase MH. Colocalization of γ-aminobutyric acid and acetylcholine in neurons in the laterodorsal and pedunculopontine tegmental nuclei in the cat: a light and electron microscopic study. Brain Res. 2003;992:205–219. doi: 10.1016/j.brainres.2003.08.062. [DOI] [PubMed] [Google Scholar]
- [67].Hui-Ling W, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic, and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Alderson HL, Faulconbridge LF, Gregory LP, Latimer MP, Winn P. Behavioural sensitisation to repeated d-amphetamine: effects of excitotoxic lesions of the pedunculopontine tegmental nucleus. Neuroscience. 2003;118:311–315. doi: 10.1016/s0306-4522(03)00152-0. [DOI] [PubMed] [Google Scholar]
- [69].Nelson CL, Wetter JB, Milovanovic M, Wolf ME. The laterodorsal tegmentum contributes to behavioral sensitization to amphetamine. Neuroscience. 2007;146:41–49. doi: 10.1016/j.neuroscience.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- [71].Robbins TW, Granon S, Muir JL, Durantou F, Harrison A, Everitt BJ. Neural systems underlying arousal and attention. Implications for drug abuse. Ann NY Acad Sci. 1998;846:222–237. [PubMed] [Google Scholar]
- [72].Jolas T, Nestler EJ, Aghajanian GK. Chronic morphine increases GABA tone on serotonergic neurons of the dorsal raphe nucleus: association with an up-regulation of the cyclic AMP pathway. Neuroscience. 2000;95:433–443. doi: 10.1016/s0306-4522(99)00436-4. [DOI] [PubMed] [Google Scholar]
- [73].Shin R, Ikemoto S. The GABAB receptor agonist baclofen administered in the median and dorsal raphe nuclei is rewarding as shown by intracranial self-administration and conditioned place preference in rats. Psychopharmacol. 2010;208:545–554. doi: 10.1007/s00213-009-1757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Nishikawa T, Scatton B. Inhibitory influence of GABA on central serotonergic transmission. Involvement of the habenulo-raphe pathways in the GABAergic inhibition of ascending cerebral serotonergic neurons. Brain Res. 1985;331:81–90. doi: 10.1016/0006-8993(85)90717-6. [DOI] [PubMed] [Google Scholar]
- [75].Kalen P, Strecker RE, Rosengren E, Bjorklund A. Regulation of serotonin release by the lateral habenula-dorsal raphe pathway in the rat as demonstrated by in vivo microdialysis: role of excitatory amino acids and GABA. Brain Res. 1989;492:187–202. doi: 10.1016/0006-8993(89)90901-3. [DOI] [PubMed] [Google Scholar]
- [76].Scarnati E, Florio T, Capozzo A, Confalone G, Mazzone P. The pedunculopontine tegmental nucleus: implications for a role in modulating spinal cord motoneuron excitability. J Neural Transm. 2010 doi: 10.1007/s00702-010-0532-2. [epub ahead of print] DOI 10.1007/s00702-010-0532-2. [DOI] [PubMed] [Google Scholar]
- [77].Jhou TC. Neural mechanisms of freezing and passive aversive behaviors. J Comp Neurol. 2005;493:111–114. doi: 10.1002/cne.20734. [DOI] [PubMed] [Google Scholar]
- [78].Lenard LG, Beer B. 6-Hydroxydopamine and avoidance: possible role of response suppression. Pharmacol Biochem Behav. 1975;3:873–878. doi: 10.1016/0091-3057(75)90120-3. [DOI] [PubMed] [Google Scholar]
- [79].LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:991–997. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- [81].Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons, and medulla oblongata in the cat. Brain Res Rev. 1978;56:27–78. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- [82].Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res. 1985;58:379–391. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- [83].Treit D, Menard J. Dissociations among the anxiolytic effects of septal, hippocampal, and amygdaloid lesions. Behav Neurosci. 1997;111:653–658. doi: 10.1037//0735-7044.111.3.653. [DOI] [PubMed] [Google Scholar]
- [84].Pezuk P, Aydin E, Aksoy A, Canbeyli R. Effects of BNST lesions in female rats on forced swimming and navigational learning. Brain Res. 2008;1228:199–207. doi: 10.1016/j.brainres.2008.06.071. [DOI] [PubMed] [Google Scholar]
- [85].Sartorius A, Henn FA. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypoth. 2007;69:1305–1308. doi: 10.1016/j.mehy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- [86].Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, Henn FA, Meyer-Lindenberg A. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psych. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- [87].Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J Neurol. 2000;247(Suppl 2):II3–10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- [88].Marui W, Iseki E, Nakai T, Miura S, Kato M, Ueda K, Kosaka K. Progression and staging of Lewy pathology in brains from patients with dementia with Lewy bodies. J Neurol Sci. 2002;195:153–159. doi: 10.1016/s0022-510x(02)00006-0. 2002. [DOI] [PubMed] [Google Scholar]
- [89].Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Seur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- [90].Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, III, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG, the Arizona Parkinson’s Disease Consortium Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mayeux R, Smith M, Goetz C. Does a long preclinical period occur in Parkinson’s disease? Neurology. 1991;41(Suppl 1):8–13. [PubMed] [Google Scholar]
- [92].Koller WC, Langston JW, Hubble JP, Irwin I, Zack M, Golbe L, Forno L, Ellenberg J, Kurland L, Ruttenber AJ, Spencer P, Tanner C, Tetrud J, Wicox T, Roman G, Mayeux R, Smith M, Goetz C. Does a long preclinical period occur in Parkinson’s disease? Neurology. 1991;41(Suppl 1):8–13. [PubMed] [Google Scholar]
- [93].Lang AE, Lozano AM. Parkinson’ Disease. First of two parts. New Eng J Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- [94].Ahlskog JE. Beating a dead horse. Dopamine and Parkinson disease. Neurol. 2007;69:1701–1711. doi: 10.1212/01.wnl.0000296942.14309.4a. [DOI] [PubMed] [Google Scholar]
- [95].Savica R, Rocca WA, Ahlskog JE. When does parkinsonism start? Arch Neurol. 2010;67:798–801. doi: 10.1001/archneurol.2010.135. [DOI] [PubMed] [Google Scholar]
- [96].Chaudhuri KR. The debate of motor versus non-motor aspects of Parkinson’s disease: Time for marriage. Basal Ganglia. 2011;1:47–48. [Google Scholar]
- [97].Wise RA, Yokel RA, DeWit H. Both positive reinforcement and conditioned aversion from amphetamine and from apo- morphine in rats. Science. 1976;191:1273–1275. doi: 10.1126/science.1257748. [DOI] [PubMed] [Google Scholar]
- [98].Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacol. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- [99].Simpson GR, Riley AL. Morphine preexposure facilitates morphine place preference and attenuates morphine taste aversion. Pharmacol Biochem Behav. 2005;80:471–479. doi: 10.1016/j.pbb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- [100].Hutchison MA, Riley AL. Adolescent exposure to nicotine alters the aversive effects of cocaine in adult rats. Neurotoxicol Teratol. 2008;30:404–411. doi: 10.1016/j.ntt.2008.04.004. [DOI] [PubMed] [Google Scholar]