Abstract
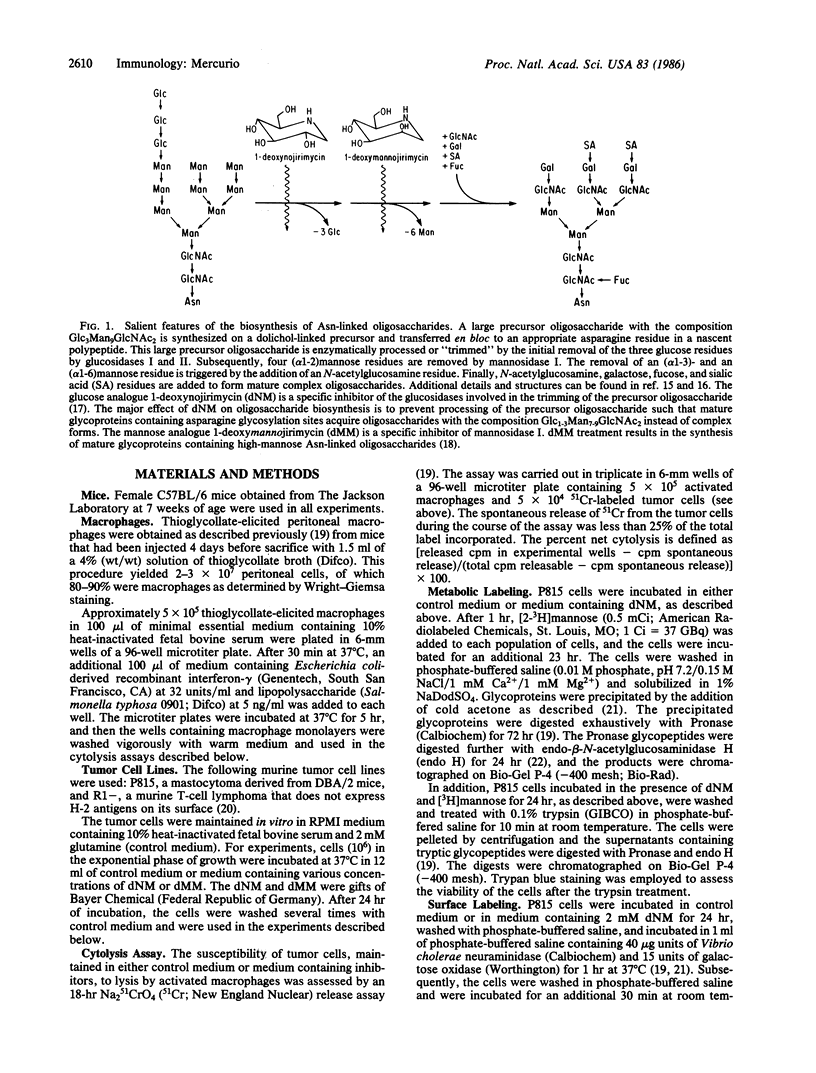
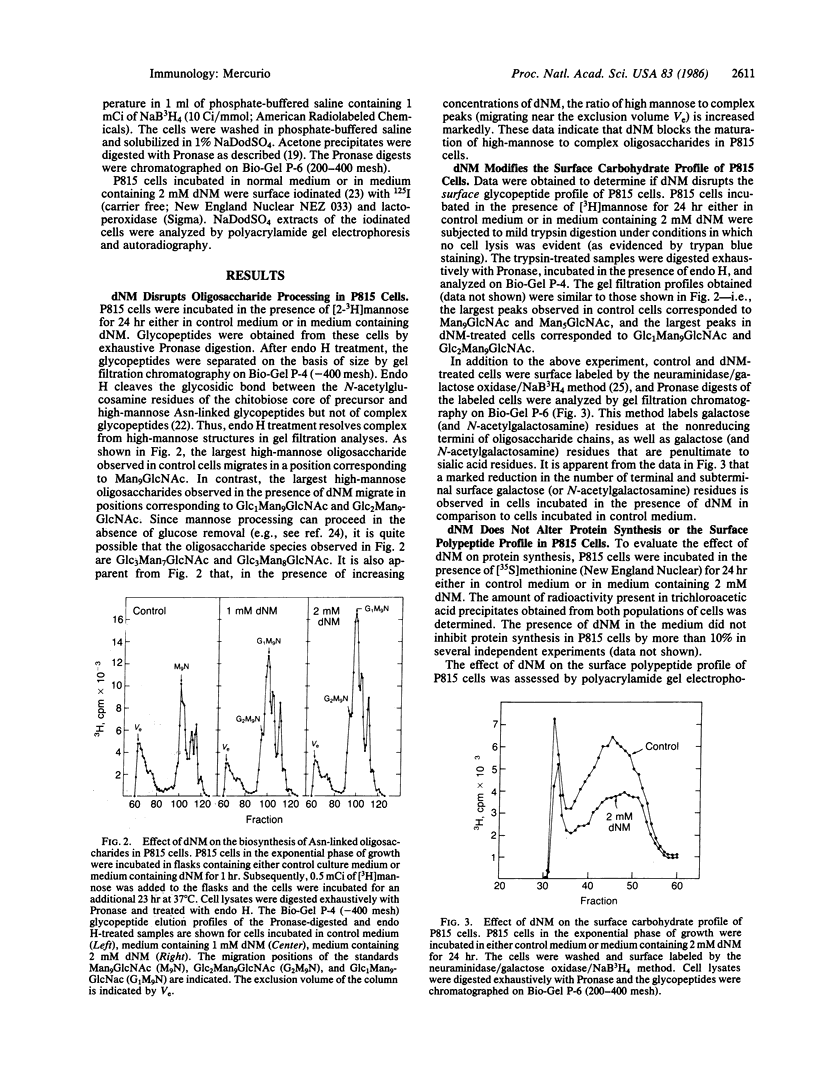
The components of tumor cell surfaces that participate in the recognition and lysis of these cells by activated macrophages have not been identified. One plausible hypothesis is that these components are specific carbohydrate structures. As an initial test of this hypothesis, I have made use of the oligosaccharide processing inhibitors 1-deoxynojirimycin (dNM) and 1-deoxymannojirimycin (dMM). dNM is an inhibitor of the glucosidases involved in the initial steps of oligosaccharide processing. dMM inhibits mannosidase I. P815 cells incubated in the presence of 1-2 mM dNM for 24 hr synthesized mature glycoproteins that contained glucosylated high-mannose asparagine-linked oligosaccharides instead of complex forms. The glucosylated oligosaccharides were present in trypsin digests of the cell surface. The dNM treatment resulted in a diminution in the amount of surface galactose residues as evidenced by neuraminidase/galactose oxidase/NaB3H4 labeling of surface glycopeptides. It did not, however, inhibit protein synthesis or alter the surface polypeptide profile of the tumor cells. P815 and R1- cells incubated in the presence of 1-3 mM dNM for 24 hr were considerably less sensitive to lysis by interferon-gamma-activated macrophages than were cells incubated in control medium. At a dNM concentration of 3 mM, a 71% inhibition of P815 cell lysis was observed. Similarly, P815 and R1- cells incubated in the presence of 2 mM dMM were also less sensitive to macrophage-mediated lysis than were control cells. The inhibitors did not affect cell viability, growth, or gross morphology. These observations suggest that complex asparagine-linked oligosaccharides on tumor cell surfaces may participate in recognition and lysis by activated macrophages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Adams D. O., Snyderman R. Do macrophages destroy nascent tumors? J Natl Cancer Inst. 1979 Jun;62(6):1341–1345. [PubMed] [Google Scholar]
- Baenziger J. U., Fiete D. Galactose and N-acetylgalactosamine-specific endocytosis of glycopeptides by isolated rat hepatocytes. Cell. 1980 Nov;22(2 Pt 2):611–620. doi: 10.1016/0092-8674(80)90371-2. [DOI] [PubMed] [Google Scholar]
- Brisson J. R., Carver J. P. The relation of three-dimensional structure to biosynthesis in the N-linked oligosaccharides. Can J Biochem Cell Biol. 1983 Sep;61(9):1067–1078. doi: 10.1139/o83-135. [DOI] [PubMed] [Google Scholar]
- Celis E., Chang T. W., Eisen H. N. Cyclical changes in susceptibility of a myeloma tumor (LPC-1) to immune destruction. III. Periodic production of a cell surface glycoprotein and changes in reactivity with cytotoxic T cells and anti-H-2d sera. J Immunol. 1979 Jun;122(6):2245–2250. [PubMed] [Google Scholar]
- Fidler I. J., Barnes Z., Fogler W. E., Kirsh R., Bugelski P., Poste G. Involvement of macrophages in the eradication of established metastases following intravenous injection of liposomes containing macrophage activators. Cancer Res. 1982 Feb;42(2):496–501. [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Legler G., Ploegh H. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature. 1984 Feb 23;307(5953):755–758. doi: 10.1038/307755a0. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Marino P. A., Adams D. O. Interaction of Bacillus Calmette--Guérin-activated macrophages and neoplastic cells in vitro II. The relationship of selective binding to cytolysis. Cell Immunol. 1980 Aug 15;54(1):26–35. doi: 10.1016/0008-8749(80)90186-0. [DOI] [PubMed] [Google Scholar]
- Marino P. A., Adams D. O. Interaction of Bacillus Calmette--Guérin-activated macrophages and neoplastic cells in vitro. I. Conditions of binding and its selectivity. Cell Immunol. 1980 Aug 15;54(1):11–25. doi: 10.1016/0008-8749(80)90185-9. [DOI] [PubMed] [Google Scholar]
- Marino P. A., Whisnant C. C., Adams D. O. Binding of bacillus Calmette-Guérin-activated macrophages to tumor targets. Selective inhibition by membrane preparations from homologous and heterologous neoplastic cells. J Exp Med. 1981 Jul 1;154(1):77–87. doi: 10.1084/jem.154.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Tucker R. W., Breuer A. C. Interaction of BCG-activated macrophages with neoplastic and noneoplastic cell lines in vitro: cinemicrographic analysis. Cell Immunol. 1975 May;17(1):30–42. doi: 10.1016/s0008-8749(75)80004-9. [DOI] [PubMed] [Google Scholar]
- Mercurio A. M., Robbins P. W. Activation of mouse peritoneal macrophages alters the structure and surface expression of protein-bound lactosaminoglycans. J Immunol. 1985 Aug;135(2):1305–1312. [PubMed] [Google Scholar]
- Mercurio A. M., Schwarting G. A., Robbins P. W. Glycolipids of the mouse peritoneal macrophage. Alterations in amount and surface exposure of specific glycolipid species occur in response to inflammation and tumoricidal activation. J Exp Med. 1984 Oct 1;160(4):1114–1125. doi: 10.1084/jem.160.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montreuil J. Spatial conformation of glycans and glycoproteins. Biol Cell. 1984;51(2):115–131. doi: 10.1111/j.1768-322x.1984.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Ogata S. I., Muramatsu T., Kobata A. New structural characteristic of the large glycopeptides from transformed cells. Nature. 1976 Feb 19;259(5544):580–582. doi: 10.1038/259580a0. [DOI] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Pan Y. T., Hori H., Saul R., Sanford B. A., Molyneux R. J., Elbein A. D. Castanospermine inhibits the processing of the oligosaccharide portion of the influenza viral hemagglutinin. Biochemistry. 1983 Aug 2;22(16):3975–3984. doi: 10.1021/bi00285a038. [DOI] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Saunier B., Kilker R. D., Jr, Tkacz J. S., Quaroni A., Herscovics A. Inhibition of N-linked complex oligosaccharide formation by 1-deoxynojirimycin, an inhibitor of processing glucosidases. J Biol Chem. 1982 Dec 10;257(23):14155–14161. [PubMed] [Google Scholar]
- Schlepper-Schäfer J., Holl N., Kolb-Bachofen V., Friedrich E., Kolb H. Role of carbohydrates in rat leukemia cell-liver macrophage cell contacts. Biol Cell. 1984;52(3):253–258. doi: 10.1111/j.1768-322x.1985.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Datema R. The lipid pathway of protein glycosylation and its inhibitors: the biological significance of protein-bound carbohydrates. Adv Carbohydr Chem Biochem. 1982;40:287–379. doi: 10.1016/s0065-2318(08)60111-0. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J. Evidence for the participation of saccharide-lipids in the synthesis of the oligosaccharide chain of ovalbumin. J Biol Chem. 1977 Feb 10;252(3):1007–1013. [PubMed] [Google Scholar]
- Tarentino A. L., Trimble R. B., Maley F. endo-beta-N-Acetylglucosaminidase from Streptomyces plicatus. Methods Enzymol. 1978;50:574–580. doi: 10.1016/0076-6879(78)50065-7. [DOI] [PubMed] [Google Scholar]
- Warren L., Buck C. A., Tuszynski G. P. Glycopeptide changes and malignant transformation. A possible role for carbohydrate in malignant behavior. Biochim Biophys Acta. 1978 Sep 18;516(1):97–127. doi: 10.1016/0304-419x(78)90005-7. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Ohkura T., Tachibana Y., Takasaki S., Kobata A. Comparative study of the oligosaccharides released from baby hamster kidney cells and their polyoma transformant by hydrazinolysis. J Biol Chem. 1984 Sep 10;259(17):10834–10840. [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Ohkura T., Kobata A. Enzymatic basis for the structural changes of asparagine-linked sugar chains of membrane glycoproteins of baby hamster kidney cells induced by polyoma transformation. J Biol Chem. 1985 Apr 10;260(7):3963–3969. [PubMed] [Google Scholar]
- Yusuf H. K., Pohlentz G., Sandhoff K. Tunicamycin inhibits ganglioside biosynthesis in rat liver Golgi apparatus by blocking sugar nucleotide transport across the membrane vesicles. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7075–7079. doi: 10.1073/pnas.80.23.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]




