Abstract
Thrombopoietin (TPO) and its receptor c-Mpl are essential in the regulation of the hematopoietic stem and progenitors cells as well as for the differentiation of megakaryocytes into mature platelets. Once TPO binds to its receptor, an intracellular signaling process is initiated through Janus kinase (JAK-2)-induced phosphorylation of the c-Mpl intracellular domain. Although some protein mediators that transmit the effects of TPO have been identified, many remain undiscovered. Using an unbiased approach with peptide microarrays that contained virtually every Src Homology (SH)2 and Phosphotyrosine Binding (PT B) domains in the human genome, we discovered a previously unreported interaction between c-Mpl at phospho-Tyrosine631 (pY631) and Tensin2, a protein for which limited information is available. Confirming the findings of the microarrays, we discovered that Tensin2 co-precipitates with a pY631 bearing peptide. Furthermore, we found that Tensin2 becomes phosphorylated in a TPO-dependent manner. The functional consequence of Tensin2 was tested via knockdown of Tensin2, which dramatically decreased TPO-dependent cellular proliferation of UT7-TPO cell line as well as their activation of Akt signaling. These studies affirm the use of these arrays as an unbiased screening tool of proteinprotein interactions. We conclude that Tensin2 is an important new mediator in TPO/c-Mpl pathway and has a positive affect on cellular growth, at least in part through its effect on the PI3K/Akt signaling.
Key words: tensin2, thrombopoietin, c-Mpl, signal transduction, cellular proliferation
Introduction
Hematopoiesis is the process of maturation and differentiation of bone marrow stem and progenitor cells into the diverse range of cells found in the blood: leukocytes, important in immunity, erythrocytes, which shuttle oxygen and carbon dioxide to and from nearly all tissues and platelets, essential in blood clotting in response to injury. Thrombopoietin (TPO) and its receptor c-Mpl are primary regulators of hematopoiesis, promoting the growth, survival, migration and maturation of megakaryocytes, their precursors, the hematopoietic stem and progenitor cells, and their progeny, mature blood platelets.1 Like many cytokine receptors that promote cell proliferation and differentiation in response to cognate ligand(s), c-Mpl initiates intracellular signaling by inducing the activation of intracellular kinases and adapter molecules, which ultimately impact the function of transcription factors, mitochondrial membrane permeability and other critical processes.2
The basic intracellular mechanisms by which c-Mpl function is initiated are clear; upon binding its ligand, c-Mpl becomes phosphorylated on several tyrosine residues within its intracellular domain, which is at least in part mediated by the cytoplasmic signaling kinase Janus Kinase 2 (JAK2). The resultant phosphotyrosine (pY) residues serve to tether Src Homology 2 (SH2) domain containing proteins, allowing them to be modified, which stimulates or inhibits their downstream effector functions.1 The SH2 domain is a sequence context-dependent phosphotyrosine-binding module composed of approximately 100 amino acids and is highly conserved in structure.3,4 Most SH2-domain containing proteins bind pY containing peptides with sub-micromolar affinity, and the off rate kinetics of SH2 domain interactions are extremely rapid.5 Consequently, these transient interactions present a challenge for the use of traditional methods, such as co-immunoprecipitation to detect protein-protein interactions as such technique is better suited to detect more prolonged and stable interactions between proteins.
Although several downstream pathways of TPO signaling have been described, all the details of their activation have not been uncovered. For example, though it is clear that phosphoinositol-3-kinase (PI3K) is activated by TPO binding to c-Mpl, a direct interaction between the p85 regulatory subunit of PI3K and c-Mpl has never been demonstrated. While candidate gene approaches have identified several downstream mediators of TPO signal transduction, including PI3K, mitogen activated protein kinase and protein kinase C, other strategies appear necessary to identify the additional components that determine the specific outcomes of TPO signaling, especially in detecting rapid interactions such as those with SH2 domain containing mediators.
Using an expansive and unbiased approach, we report here, for the first time, the results of pY-mediated interactions between c-Mpl and nearly every human genomic SH2 and phosphotyrosine-binding (PTB) domain containing proteins. Furthermore, we show a previously never described interaction between c-Mpl pY631 and Tensin2 (also termed TENC1 or C1-TEN) and demonstrate Tensin2's effect on TPO-dependent cellular proliferation, a novel mode of action for Tensin2, likely in part due to its effect on the PI3K/Akt pathway. Y631 of c-Mpl is a site of known phosphorylation, to which important signaling mediator such as STAT5 has been shown to bind to in response to TPO signaling.6 Tensin2 is a relatively newly described protein for which there is very limited information. It has been implicated in focal adhesion as well as migration.7 Furthermore, overexpression of Tensin2 in cancer cells has been thought to be associated with more aggressive behaviors, including venous invasion as well as tumor microsatellite formation.8
Results
Protein microarrays highlight a previously unrecognized interaction between the SH2 domain of Tensin2 and pY631 of c-Mpl.
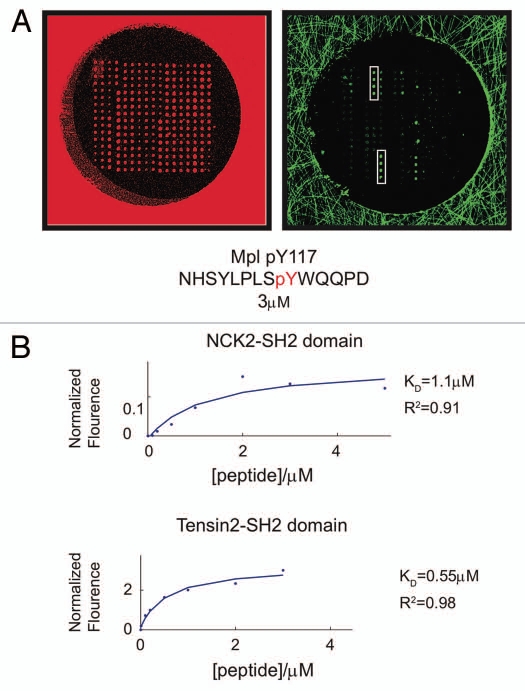
We used microarrays comprising 133 human SH2 and PTB protein domains to identify and quantify the interactions between pY631 and potential binding partners (for a complete list of SH2 and PTB domain containing proteins printed on the arrays, see Sup. Table 1). To represent the phosphorylated tyrosine at position 631, a pY-containing peptide was synthesized with sequence that corresponds to nine residues upstream and all five residues downstream of pY631 (NHSYLPLS(pY)WQQPD). Eight different concentrations of the 5,6-tetramethylrhodamine (5,6-TAMRA) labeled peptide were then used to probe replicate arrays in parallel. The fluorescence intensity at each spot was quantified and fit to an equation that described saturation binding, as previously described in reference 9. This allowed us to measure the equilibrium dissociation constants (KD) of each SH2 or PTB domain interaction with the peptide. Any given SH2 or PTB domain was deemed a “hit” if the brightest spot was two fold higher than the background fluorescence and if the data fit to equation (1) with an R2 of 0.9 or better and a KD < 2 µM.
| (1) |
Of the 133 domains printed on the microarrays, only two SH2 domains met these criteria. One was the adaptor protein NCK2, which bound the peptide with a KD of 1.1 µM. The other was Tensin2 and bound with sub-micromolar affinity (Fig. 1). As Tensin2 exhibited tighter binding and a better fit (R2 = 0.98), its interaction was further explored.
Figure 1.
Measuring the binding affinities of SH2 and PTB domains for a phosphopeptides derived from c-Mpl pY631 using protein microarrays. (A) Fluorescence images of SH2 and PTB domain microarrays in one well of a 96-well microtiter plate, obtained using a 633 nm and 543 nm lasers. The fluorescence in the 633 nm channel (red) arises from a trace amount of Cy5-labeled BSA that was added to each protein before arraying. The fluorescence in the 543 nm channel (green) arises from the 5,6-TAMRA-labeled phosphopeptide and represents a binding event between the peptide and the immobilized SH2 or PTB domains. Spots containing the SH2 domains of Tensin2 and NCK2 are highlighted with a white rectangle. (B) Saturation binding curves obtained by probing SH2/PT B domain microarrays with eight different concentrations of the phosphopeptides. Plots show fluorescence as a function of peptide concentration for the two highest intensity interactions on the arrays. The data were fit to equation (1).
Tensin2 interacts with c-Mpl at pY631 site in a cellular context.
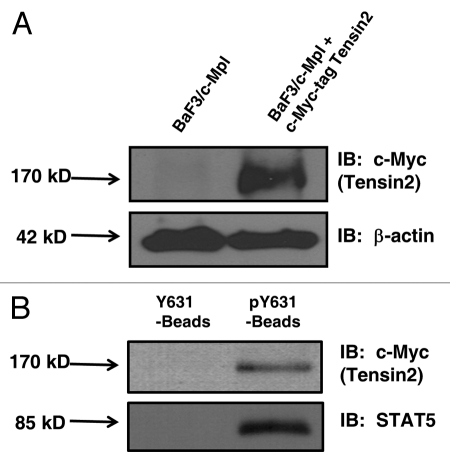
Given the absence of reliable Tensin2 antibodies, the presence of Tensin2 mRNA was assessed using reverse transcriptase PCR in murine megakaryocytes and the megakaryocytic leukemia cell line UT7-TPO, which revealed the presence of Tensin2 mRNA (Sup. Fig. 1). To validate the interaction seen on the array between c-Mpl and Tensin2 at a cellular level, we engineered a BaF3/c-Mpl cells to express Tensin2 fused at its N-terminus to a c-Myc tag (Fig. 2A). To circumvent the rapid off-rate nature of the interactions between pY and SH2 domain containing proteins, Neutravidin-biotin-labeled peptide beads, consisting of the sequence surrounding c-Mpl pY631 was synthesized and used to precipitate c-Myc-Tensin2. A non-phosphorylated version of the peptide beads (Y631) was used as negative control. Western blotting for STAT5, based on previous studies that established STAT5 as a binding partner for c-Mpl pY631,6 served as positive control. We observed significant binding between the c-Mpl pY631 peptide and c-Myc-tagged Tensin2 (Fig. 2B) when compared to control beads. This study confirmed our protein microarray results and affirmed the interaction between Tensin2 and c-Mpl at pY631.
Figure 2.
Western blots of engineered BaF3 cells (2a) and pY631 peptide bead co-precipitation of Tensin2 (2b). (A) BaF3/c-Mpl cells were engineered to express Tensin2 fused with a c-Myc tag. (B) BaF3/c-Mpl/c-Myc-tagged Tensin2 cells were lysed and co-precipitated with biontinylated Neutravidin beads as control or with biotinylated pY631 oligo-peptide Neutravidin beads. Precipitated material was analyzed by western blotting to c-Myc tagged Tensin2 or STAT5.
Tensin2 is phosphorylated in a TPO-dependent manner.
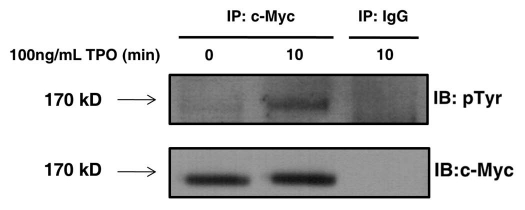
For additional evidence of an interaction between c-Mpl and Tensin2, we assessed Tensin2 for biochemical modification upon TPO administration. Given that numerous binding partners of c-Mpl receptor become phosphorylated upon stimulation with TPO, we assessed for changes in the state of phosphorylation of Tensin2. We stimulated BaF3/c-Mpl/c-Myc-tagged Tensin2 cells with TPO, immune-purified Tensin2 using an anti-c-Myc antibody and probed for tyrosine phosphorylation by western blot. We observed that upon stimulation with TPO, Tensin2 was phosphorylated on one or more tyrosine residues within 10 min of stimulation (Fig. 3).
Figure 3.
Tensin2 is phosphorylated in a TPO-dependent fashion. BaF3/c-Mpl/c-Myc-tagged Tensin2 cells were starved of serum and then stimulated with 100 ng/mL of TPO for various time points. Cells were then lysed and immune-purified with anti-c-Myc antibody or mouse IgG as control. Immune precipitates were analyzed by western blotting.
Knockdown of Tensin2 reduces cellular proliferation at a TPO dose-dependent manner.
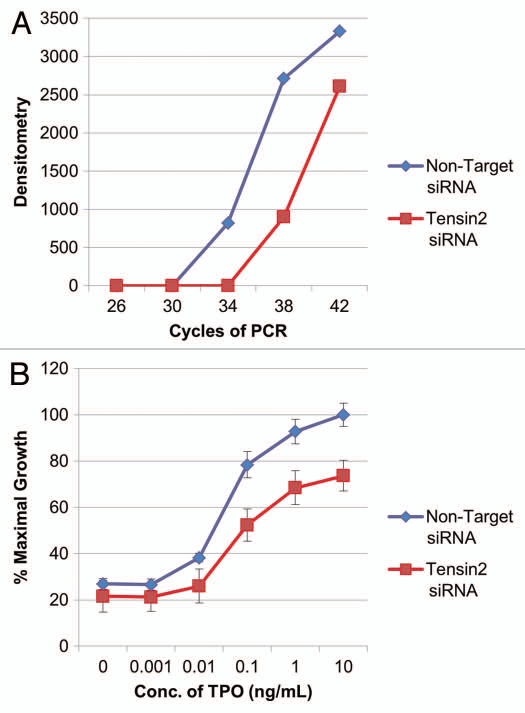
To investigate the biological pertinence of Tensin2 within known TPO-dependent cellular functions, we assessed the proliferation rate of UT7-TPO cells at various concentration of TPO with the loss of Tensin2 function. UT7-TPO cells endogenously express c-Mpl and Tensin2, providing a useful model system to explore the effects of Tensin2 on TPO/c-Mpl function. UT7-TPO cells were treated with either small interfering RNA (siRNA) specific to Tensin2 or with non-target siRNA as negative control. We found that cells treated with Tensin2-specific siRNA reduced the level of Tensin2 mRNA by at least 16-fold when assessed by semi-quantitative RT-PCR (Fig. 4A). When these cells were grown with various concentrations of TPO for 40–48 hours, we discovered that cells treated with Tensin2-specific siRNA had a significant reduction in their growth rate when compared to their controls (Fig. 4B).
Figure 4.
Tensin2 affects TPO-dependent cellular growth. (A) Graphic representation of downregulation of Tensin2 mRNA levels using siRNA specific to Tensin2. After ∼36 hr of treatment with Tensin2 specific mRNA or non-target siRNA as control, semi-quantitative PCR was performed on UT7-TPO cells using various numbers of PCR cycles. Semi-quantitative PCR of GAPDH mRNA was performed as RNA loading control. Densitometry was performed and graphically represented above after normalizing for loading. (B) MTT assay comparing the Tensin2 siRNA treated UT7-TPO cells versus negative control at various TPO concentrations grown for 40–48 hours. Tensin2 siRNA treated cells had a significant reduction in cellular proliferation at all doses of TPO.
Knockdown of Tensin2 reduces TPO-induced Akt activation.
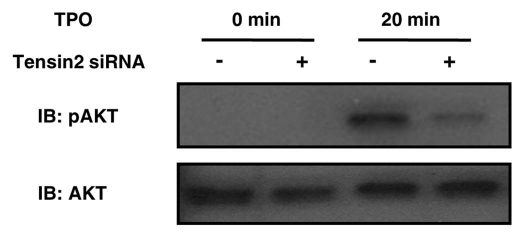
To dissect and map a potential downstream impact of Tensin2, we explored the functional consequences of loss of Tensin2 function. The PI3K/Akt pathway was examined in detail as our and others' prior studies had demonstrated an important effect of PI3K signaling on cell proliferation and because previous studies had suggested an effect of Tensin2 on PI3K function.10,11 UT7-TPO cells, which endogenously express c-Mpl, Tensin2, PI3K and Akt, were treated with either siRNA specific to Tensin2 or with non-target siRNA as negative control. When these cells were monitored for TPO-induced Akt activation, we noticed a significant decrease of Akt activation (i.e., phosphorylation of Akt) in the cells treated with Tensin2 specific siRNA (Fig. 5), densitometry revealing a 74% reduction in the phosphorylation of Akt when normalized with total Akt (not shown).
Figure 5.
Tensin2 is involved in TPO-mediated activation of Akt. siRNA-treated UT7-TPO cells were starved of serum and then stimulated with 5 ng/mL (of TPO ) for 20 min. Cells were then lysed and analyzed by western blotting for pAkt and total Akt. TPO-mediated phosphorylation of Akt was substantially diminished in the Tensin2 knockdown cells.
Tensin2 may interact with PI3K via a consensus PI3K binding motif.
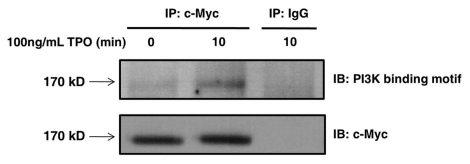
The TPO-dependent phosphorylation of tyrosine on Tensin2 led us to speculate that Tensin2 may play an important role in recruiting downstream signaling molecules. An extensive examination of the sequence of the Tensin2 revealed Y328 to lie within a consensus binding sequence motif YXXM. When phosphorylated, the sequence motif pYXXM is reported to serve as a binding site for the SH2 domains of the regulatory subunit of PI3K, the p85.12 Unlike the erythropoietin receptor, which directly interacts with p85 subunit of PI3K,13 a direct interaction between c-Mpl and PI3K has never been described. Thus, we investigated the potential role of Tensin2 in PI3K activation via its consensus binding sequence motif. We stimulated BaF3/c-Mpl/c-Myc-tagged Tensin2 cells with TPO and then immune purified Tensin2 using an anti-c-Myc antibody. We then determined whether Tensin2 was phosphorylated at its consensus PI3K binding sequence using an antibody that recognizes (pY)XXM. We observed that upon stimulation with TPO, Tensin2 was modified to contain phosphotyrosine at the consensus PI3K binding sequence motif (Fig. 6).
Figure 6.
Tensin2 is phosphorylated at its PI3K consensus binding motif in a TPO-dependent fashion. BaF3/c-Mpl/c-Myc-tagged Tensin2 cells were starved of serum and then stimulated with 100 ng/mL of TPO for various time points. Cells were then lysed and immune-purified with anti-c-Myc antibody or mouse IgG as control. Immune precipitates were analyzed by western blotting.
Discussion
In the current study, we found that (1) Tensin2 is present in both murine megakaryocytes and the megakaryocytic leukemia cell line UT7/TPO at the level of specific mRNA, (2) Tensin2 directly interacts with c-Mpl and that Tensin2 is phosphorylated in a TPO-dependent manner and (3) loss of Tensin2 function significantly decreases both TPO-dependent cellular proliferation and Akt signaling. These new findings strongly suggest that the interaction of Tensin2 with c-Mpl is an important event in TPO mediated cellular growth, at least in part through its effect on PI3K/Akt pathway.
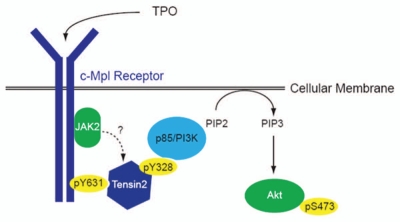
Due to the lack of a reliable anti-Tensin2 antibody, we were unable to detect the presence of Tensin2 at the level of the protein. Despite that, the strong evidence of TPO-induced biochemical modifications of Tensin2 as well as Tensin2's biological modulation of cellular proliferation and Akt signaling gives us confidence of the presence and importance of Tensin2 in the megakaryocyte lineage. In addition, we have presented evidence for a potential new path of activation for Akt, i.e., through Tensin2's recruitment of PI3K. Here, we have illustrated the proposed pathway graphically (Fig. 7).
Figure 7.
Proposed role of Tensin2 in TPO/c-Mpl signaling of PI3K/Akt. Upon binding of TPO to its receptor, c-Mpl, the intracellular domain of c-Mpl become phosphorylated, one site being at Y631. Tensin2 is then recruited to that site and is phosphorylated. Once phosphorylated, it may act to recruit the p85 subunit of PI3K, which then goes on to activate Akt and affects downstream functions including cell growth.
In our studies, we showed using two different methods that Tensin2 and c-Mpl directly interact with one another. First, in using the novel peptide microarrays, we were able to identify Tensin2 as a candidate-signaling mediator of TPO effects. Although the use of protein domain microarrays to uncover novel interactions has been previously described in reference 14, (and MacBeath and Meyer labs unpublished data), our studies are the first in using these assays to expand upon an established pathway, specifically to fill in the details of protein-protein interaction. Secondly, in using a peptide co-precipitation method as the confirmatory method, we have demonstrated not only the two proteins' interaction but also reaffirmed the use of the novel microarrays as an unbiased screening tool to discover new candidates of interaction. Despite numerous attempts, we were unable to consistently show this interaction using co-immunoprecipitation approach. To date, very few direct interactions between SH2 domain proteins and c-Mpl have been reported, a situation most likely due to the known rapid off-rate characteristic of SH2 domain containing proteins and their phosphotyrosine peptide docking sites;5 such binding kinetics make any experiments that require prolonged incubation, such as co-immunoprecipitation assays, problematic for “capturing” such interactions. In fact, the washing steps of the peptide microarrays experiments were rapid for that very reason. Furthermore, co-immunoprecipitation often requires exceptionally high levels of expression of both proteins, which does not pertain for the proteins under study.
Given the reduction of TPO-induced cellular growth and Akt signaling in the Tensin2 knock down experiments, we hypothesize that Tensin2's effect on proliferation is at least partially through its modulation of the Akt pathway, likely by creating a physical link between c-Mpl and the PI3K regulatory subunit, p85. The molecular details by which Tensin2 mediates its functional effects have never been fully described. As we have shown, the likely mechanism in which Tensin2 recruits the PI3K regulatory unit, p85, is by phosphorylation at Y328 of Tensin2's consensus PI3K binding sequence motif YXXM. Although our study focused on TPO-induced modification of Tensin2, it is possible that Tensin2 may be modified in a similar fashion by other receptors and may be a mediator of PI3K/Akt in their pathways.
There have been no studies showing a direct interaction between c-Mpl and PI3K. We (Miyakawa and colleagues) 15 have previously suggested adapter proteins as a mean of PI3K activation when stimulated by TPO. Our current study brings to light this theory and establish Tensin2 as one such potential adaptor. Given that the siRNA-mediated knock down of Tensin2 is unlikely to completely eliminate the protein, our studies argue strongly that Tensin2 may play a significant, but unlikely a solitary role as a mediator between TPO-induced c-Mpl signaling and the PI3K/Akt pathway.
The Tensin family of proteins has previously been described to be involved in focal adhesions and cellular motility, and Tensin2 specifically has been implicated as involved in cellular migration.6,16 In contrast to the results reported here, Tensin2 has also been previously reported to act as a negative regulator of the Akt pathway.10 However, we believe that study was performed in less than ideal conditions to assess for the specific effects on cellular signaling. Unlike the results reported here where we have employed a specific hematopoietic growth factor to assess downstream signaling effects, the study of Hafizi and collegues10 utilized serum, which may have led to multiple effects simultaneously, both inhibitory and stimulatory. In addition, Tensin2 was overexpressed in those cells, which may have disrupted the normal physiologic interactions. Given the use of a loss-of-function approach in our studies as well as the use of a specific ligand with a known receptor, we believe that our studies reflect more focused and physiologic conditions.
It is clear that cellular signal transduction resembles that of a complex electronic circuit with multiple branching and converging pathways. Given the likely large number of mediators in such a complex circuit, candidate gene approach is unlikely to yield all of the components that mediate the specific outcomes of each ligand. Our use of the protein domain microarrays suggests that this approach is an excellent tool in identifying new candidates for further study, especially of those with rapid and transient interactions that have been so difficult to verify previously. For the first time, we have used this novel and unbiased assay to identify a new protein that interacts with c-Mpl, Tensin2. Upon further study of the protein, we have determined that Tensin2's interaction with c-Mpl has a significant effect on TPO-dependent cellular proliferation as well as signaling of the PI3K/Akt pathway. Our work also suggests that Tensin2 may have these effects by directly interacting with PI3K p85 subunit. This work adds to our growing understanding of the molecular signals that mediate thrombopoiesis, but also suggests a novel mechanism of PI3K/Akt activation, which may be relevant in many cellular contexts outside of hematopoiesis and thrombopoiesis.
Materials and Methods
Fabrication and processing of protein microarrays.
Purified recombinant SH2 and PTB protein domains were spotted at a concentration of 40–200 µM onto 112.5 mm × 74.5 mm × 1 mm glass substrates, chemically modified to display aldehyde functionalities (Erie Scientific Company). The domains were spotted using a NanoPrint microarrayer (TeleChem International, Inc.,) at an approximate volume of 1 mL per spot. 96 microarrays (two different sets of 48 identical arrays) were fabricated in an 8 × 12 pattern on the glass, with a pitch of 9 mm. Each array consisted of a 16 × 17 pattern of spots, with a center-to-center spacing of 250 µm. All proteins were spotted in quadruplicate. Following an hr of incubation, the glass was attached to the bottom of a bottomless 96-well microtiter plate (Greiner Bio-one) using an intervening silicone gasket (Grace Bio-Labs). The arrays were stored at −80°C. Immediately before use, the plates were quenched with Buffer A (20 mM HEPES, 100 mM KCl, 0.1% Tween-20, pH 7.8) containing 1% BSA (w/v) for 30 min at room temperature, followed by several rinses with Buffer A. Arrays were probed with eight different concentrations of 5(6)-TAMRA-labeled phosphopeptides, dissolved in Buffer A: 5 µM, 3 µM, 2 µM, 1 µM, 500 nM, 200 nM, 100 nM and 10 nM. Following an hr of incubation at room temperature, the peptide solution was removed and the arrays were washed with 150 µL Buffer A for 10 s. The arrays were rinsed briefly with ddH2O and spun upside down in a centrifuge for 1 min to remove residual water. Protein microarrays were scanned at 10 µm resolution using a Tecan LS400 microarray scanner (Tecan). A small amount of Cyanine-5 labeled BSA was added to each of the spotted protein domains to establish a reference of their location. Cyanine-5 fluorescence was imaged using a 633 nm laser, while 5(6)-TAMRA fluorescence was imaged using a 543 nm laser. Images were analyzed using Array-Pro Analyzer 4.5 (Tecan). Spots were identified based on the cyanine-5 image and the mean 5(6)-TAMRA fluorescence of each spot calculated from the 5(6)-TAMRA image. The resulting data were imported into Matlab R2008a (Mathworks) and concentration-dependent measurements fit to equation (1) for each domain-peptide pair.
Chemicals and reagents.
Recombinant human (rh) TPO was a gift from Don Foster (Zymogenetics). Biotinylated Y631 and pY631 oligo-peptides were purchased from GenScript USA. Neutravidin agarose resin was purchased from Thermo Scientific (PI-29200). The following antibodies: cytoplasmic specific monoclonal anti c-Myc (9E10, sc-40), anti STAT5 (C-17, sc-835), anti phosphotyrosine (PY20, sc-508), goat anti-rabbit-horseradish peroxidase (HRP) (sc-2004), goat anti-mouse-HRP (sc-2005) and normal mouse IgG (sc-2025) were purchased from Santa Cruz Biotechnology. Protein A/G PLUS-Agarose beads (sc-2003) was purchased from Santa Cruz Biotechnology. Antibodies specific for Akt (#9272) and pAkt (193H12, #4358) were purchased from Cell Signaling Technologies. Antibody specific for phosphotyrosine 4G10 (05-321) was purchased from Millipore. Antibody specific to phospho-tyrosine containing PI3K binding motif was purchased from Active Motif (48675). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide, MTT (M5655), was purchased from Sigma.
Plasmid construct.
Wild-type Tensin2 plasmid, which was cloned into pCMV-Myc mammalian expression vector, was a gift by Drs. B. Dahlbäck and S. Hafizi. Its nucleotide sequence was compared to the sequence listed on Pubmed by DNA sequencing performed by Eton Bioscience Inc.
Cell lines and culture conditions.
To engineer BaF3/c-Mpl/c-Myc-tagged Tensin2 clones, a MSCV Neomycin construct and a pCMV-Myc-tagged Tensin2 construct were co-transfected at a ratio of 1:10, using an Amaxa nucleofector according to the manufacturer's protocol, into a previously engineered BaF3/c-Mpl cell line.6 BaF3/c-Mpl, BaF3/c-Mpl/c-Myc-tagged Tensin2 and UT7-TPO cell lines were maintained as previously described in reference 17.
pY631 oligo-peptide co-precipitation.
BaF3/c-Mpl/c-Myc-tagged Tensin2 cells were lysed and the amount of total cellular protein was quantified as previously described in reference 17. Approximately 2,000 µg in 300 µL of lysis buffer were incubated overnight on a rotator at 4°C with 50 µL of biotinylated Y631 oligo-peptide beads (1 mg/mL) as control or with 50 µL of biotinylated pY631 oligo-peptide beads (1 mg/mL). The beads were centrifuged then washed five times with the lysis buffer. The supernatant was removed and the beads were re-suspended in NaDodSO4 polyacrylamide loading buffer. After heating to 95°C for 5 min, the suspension was centrifuged and the supernatant loaded onto a 7.5% graded NaDodSO4 polyacrylamide gel and the size fractionated proteins transferred to membranes by western blotting.
Western blotting.
All cells were lysed and total cellular protein prepared for western blotting as previously described in reference 17.
Reverse transcriptase PCR and semi-quantitative PCR.
Primers for Tensin2 were generated using the Primer3 program. Murine megakaryocytes and UT7-TPO cells were lysed using RNeasy Mini Kit from Qiagen (Cat. # 74104). cDNA was prepared from the RNA lysate and reverse transcriptase PCR was performed using Taq Polymerase from Qiagen (Cat. # 201203). Semi-quantitative PCR was performed using the various numbers of PCR cycles as indicated. PCR of GAPDH was used as a control for RNA loading.
RNA interference.
Tensin2 specific siRNA SMART pool (M-009977-00) of four different Tensin2 specific siRNA sequences and the negative control (D-001810-10-10) were purchased from Dharmacon. BaF3/c-Mpl/c-Myc-tagged Tensin2 and UT7-TPO cells were transfected using an Amaxa nucleofector according to the manufacturer's protocol. Efficiency of siRNA knock-down was determined by western blot analysis for Myc-tagged Tensin2 after 24, 48 and 72 h for the BaF3/c-Mpl/c-Myc-tagged Tensin2 cells. Efficiency of siRNA in UT7-TPO cells was analyzed using semi-quantitative PCR as described above. Maximal knockdown occurred 24–72 h after transfection.
Proliferation assay.
24 hours after UT7-TPO cells were treated either with Tensin2 specific siRNA or negative control, approximately 5,000 cells per well in cytokine-free media were placed into 96-well plates. Various concentrations of TPO were added and incubated at 37°C for 40–48 hours. 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) was added at 1 mg/mL and incubated for at least 5 hours in 37°C. The cells were then lysed and formazan granules were solubilized with 100 µL of MTT lysis buffer (89.6% Isopropanol/10% Triton-X/0.4% concentrated HCl) overnight at 37°C. Absorbance at 570 nm was determined on a plate reader. Each experiment was performed in triplicate and proliferation was expressed as a percentage of maximal growth of UT7-TPO cells treated with negative control siRNA.
pAkt signaling assay.
UT7-TPO cells were transfected with the Tensin2 specific siRNA. After 36 hr, cells were starved in 0.5% BSA media for 12 hr. After starvation, cells were treated with rhTPO at 5 ng/mL and cells were lysed after 20 min of stimulation. Doses of rhTPO used were based on preliminary dose response studies of Akt phosphorylation, which revealed the concentration resulting in half the maximal phosphorylation.
Detection of pY and PI3K binding motif on Tensin2.
BaF3/c-Mpl/c-Myc-tagged Tensin2 cells were starved for 12 h in 0.5% BSA media. After starvation, cells were treated with rhTPO at 100 ng/mL for maximal stimulation. The cells were then lysed after 10 min of stimulation. Approximately 1,500 µg of protein lysate were then immuno-precipitated with 3 µg of the c-Myc antibody or control (mouse immunoglobulin) overnight on a rotator at 4°C after a pre-clear step using mouse immunoglobulin. The Protein A/G PLUS-Agarose beads were then added for 2 h on a rotator at 4°C. The beads were centrifuged then washed five times with the lysis buffer. The supernatant was removed and the beads were re-suspended in NaDodSO4 polyacrylamide loading buffer. After heating to 95°C for 5 min, the suspension was centrifuged and the supernatant loaded onto a 7.5% NaDodSO4 polyacrylamide gel and the size fractionated proteins transferred to membranes by western blotting.
Acknowledgments
We would like to thank Drs. B. Dahlbäck and S. Hafizi for their gift of the pCMV-c-Myc-wild-type Tensin2 plasmid.
Abbreviations
- TPO
thrombopoietin
- JAK2
janus kinase 2
- pY
phosphotyrosine
- SH2
Src homology 2
- PI3K
phosphoinositol-3-kinase
- siRNA
small interfering RNA
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- HRP
horseradish peroxidase
- PTB
phosphotyrosine binding
Support and Financial Disclosure Declaration
This research was supported in part by NIH grants T32 DK069263-10 (A.S.J.), R01 DK49855 (K.K.) and Tower Research Grant (A.S.J.). G.M. is a stockholder in and advisor to Merrimack Pharmaceuticals and Makoto Life Sciences.
Supplementary Material
References
- 1.Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034–2045. doi: 10.1056/NEJMra052706. [DOI] [PubMed] [Google Scholar]
- 2.Kaushansky K. Molecular mechanisms of thrombopoietin signaling. J Thromb Haemost. 2009;7:235–238. doi: 10.1111/j.1538-7836.2009.03419.x. [DOI] [PubMed] [Google Scholar]
- 3.Machida K, Mayer BJ. The SH2 domain: Versatile signaling module and pharmaceutical target. Biochim Biophys Acta. 2005;1747:1–25. doi: 10.1016/j.bbapap.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Filippakopoulos P, Muller S, Knapp S. SH2 domains: Modulators of nonreceptor tyrosine kinase activity. Curr Opin Struct Biol. 2009;19:643–649. doi: 10.1016/j.sbi.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felder S, Zhou M, Hu P, Urena J, Ullrich A, Chaudhuri M, et al. SH2 domains exhibit high-affinity binding to tyrosine-phosphorylated peptides yet also exhibit rapid dissociation and exchange. Mol Cell Biol. 1993;13:1449–1455. doi: 10.1128/mcb.13.3.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drachman JG, Kaushansky K. Dissecting the thrombopoietin receptor: Functional elements of the Mpl cytoplasmic domain. Proc Natl Acad Sci USA. 1997;94:2350–2355. doi: 10.1073/pnas.94.6.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Duncan IC, Bozorgchami H, Lo SH. Tensin1 and a previously undocumented family member, tensin2, positively regulate cell migration. Proc Natl Acad Sci USA. 2002;99:733–738. doi: 10.1073/pnas.022518699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yam JW, Ko FC, Chan CY, Yau TO, Tung EK, Leung TH, et al. Tensin2 variant 3 is associated with aggressive tumor behavior in human hepatocellular carcinoma. Hepatology. 2006;44:881–890. doi: 10.1002/hep.21339. [DOI] [PubMed] [Google Scholar]
- 9.Jones RB, Gordus A, Krall JA, MacBeath G. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature. 2006;439:168–174. doi: 10.1038/nature04177. [DOI] [PubMed] [Google Scholar]
- 10.Hafizi S, Ibraimi F, Dahlback B. C1-TEN is a negative regulator of the Akt/PKB signal transduction pathway and inhibits cell survival, proliferation and migration. FASEB J. 2005;19:971–973. doi: 10.1096/fj.04-2532fje. [DOI] [PubMed] [Google Scholar]
- 11.Hafizi S, Gustafsson A, Oslakovic C, Idevall-Hagren O, Tengholm A, Sperandio O, et al. Tensin2 reduces intracellular phosphatidylinositol-3,4,5-trisphosphate levels at the plasma membrane. Biochem Biophys Res Commun. 2010;399:396–401. doi: 10.1016/j.bbrc.2010.07.085. [DOI] [PubMed] [Google Scholar]
- 12.Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 13.Mayeux P, Dusanter-Fourt I, Muller O, Mauduit P, Sabbah M, Druker B, et al. Erythropoietin induces the association of phosphatidylinositol-3′-kinase with a tyrosine-phosphorylated protein complex containing the erythropoietin receptor. Eur J Biochem. 1993;216:821–828. doi: 10.1111/j.1432-1033.1993.tb18203.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaushansky A, Gordus A, Budnik BA, Lane WS, Rush J, MacBeath G. System-wide investigation of ErbB4 reveals 19 sites of Tyr phosphorylation that are unusually selective in their recruitment properties. Chem Biol. 2008;15:808–817. doi: 10.1016/j.chembiol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyakawa Y, Rojnuckarin P, Habib T, Kaushansky K. Thrombopoietin induces phosphoinositol-3-kinase activation through SHP2, Gab and insulin receptor substrate proteins in BAF3 cells and primary murine megakaryocytes. J Biol Chem. 2001;276:2494–2502. doi: 10.1074/jbc.M002633200. [DOI] [PubMed] [Google Scholar]
- 16.Yam JW, Ko FC, Chan CY, Jin DY, Ng IO. Interaction of deleted in liver cancer 1 with tensin2 in caveolae and implications in tumor suppression. Cancer Res. 2006;66:8367–8372. doi: 10.1158/0008-5472.CAN-05-2850. [DOI] [PubMed] [Google Scholar]
- 17.Hitchcock IS, Chen MM, King JR, Kaushansky K. YRRL motifs in the cytoplasmic domain of the thrombopoietin receptor regulate receptor internalization and degradation. Blood. 2008;112:2222–2231. doi: 10.1182/blood-2008-01-134049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.