Abstract
Human T lymphocytes are activated through either the antigen/major histocompatibility complex receptor (T3-Ti) or the T11 sheep erythrocyte-binding protein. Spectrofluorimetry and multiparameter flow cytometric techniques were utilized to examine the relationship of activation to alterations in cytoplasmic free calcium concentration, [Ca2+]i. T3-Ti receptor-triggered elevation in [Ca2+]i was found to be dependent in large part (approximately equal to 80%) on extracellular Ca2+ and to a much smaller extent (approximately equal to 20%) on mobilization of internal Ca2+ pools. Furthermore, T11-mediated increases in [Ca2+]i were entirely dependent on extracellular Ca2+. Though the kinetics of [Ca2+]i changes induced by monoclonal antibodies to T3-Ti and T11 differed, both pathways were otherwise similar, particularly with regard to effects on or mediated by the plasma membrane potential. Importantly, the T11 pathway was found to be functional in precursor T-lineage cells lacking the surface T3-Ti complex. These findings suggest that there may be a plasma membrane Ca2+ channel functionally or physically linked to the T11 structure or, alternatively, that there is a set of related T11- and T3-Ti-associated Ca2+ channels.
Full text
PDF
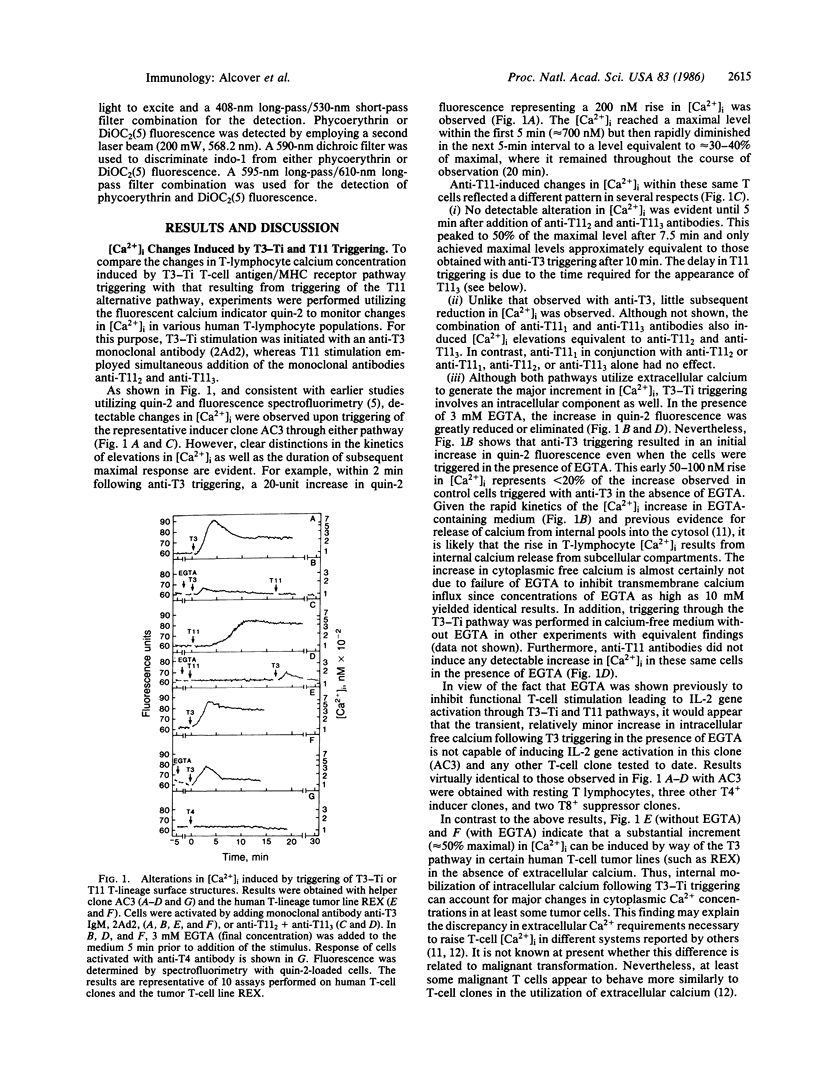
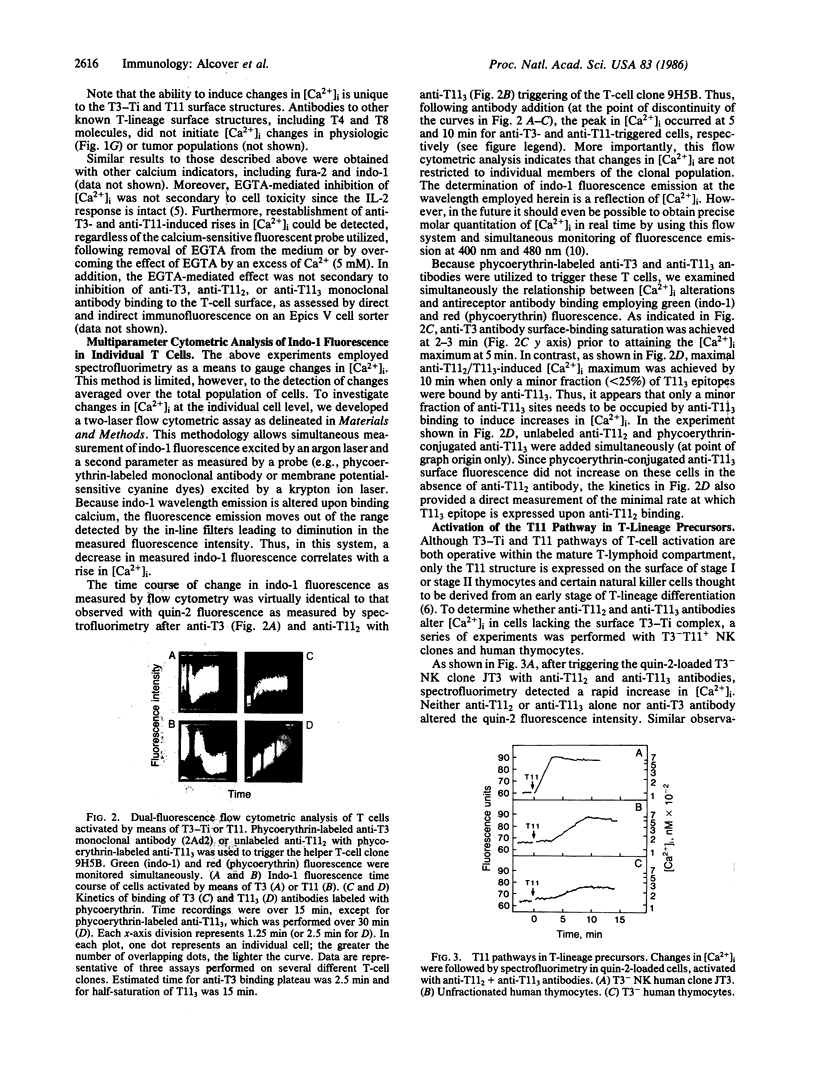
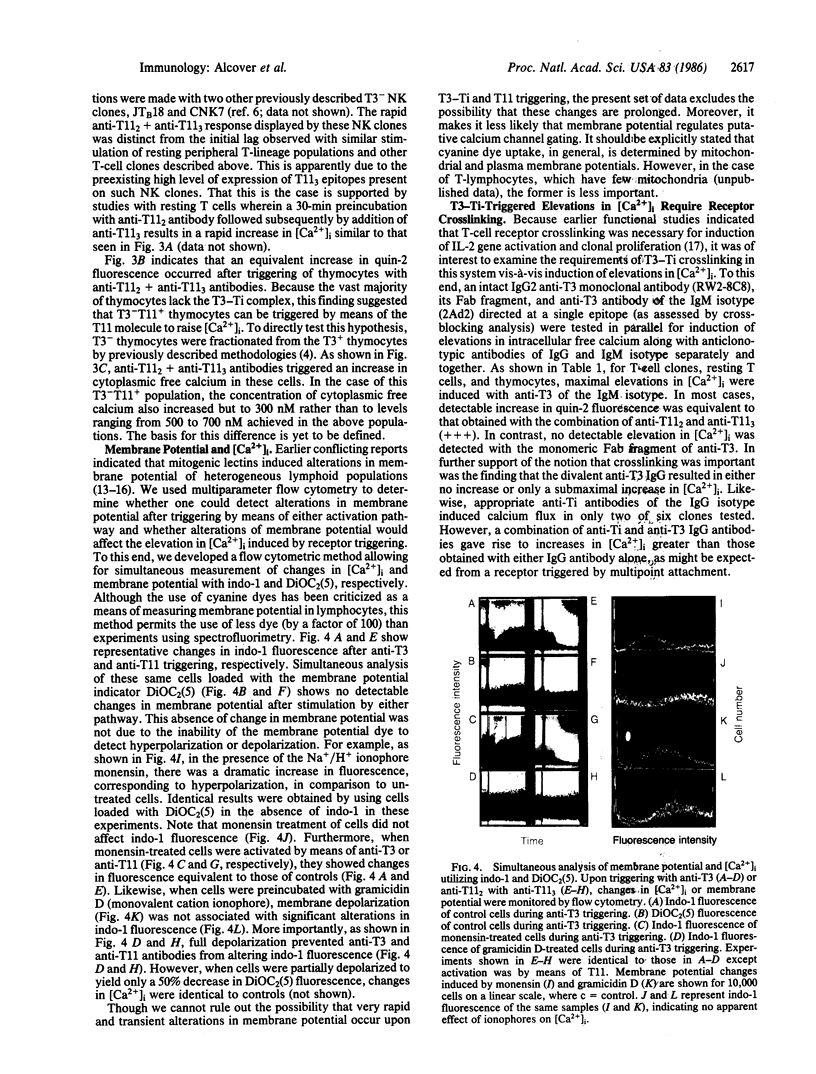

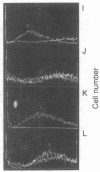
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Hussey R. E., Fitzgerald K. A., Protentis J. P., Meuer S. C., Schlossman S. F., Reinherz E. L. The human T cell receptor: appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 1983 Oct;34(3):717–726. doi: 10.1016/0092-8674(83)90528-7. [DOI] [PubMed] [Google Scholar]
- Bensussan A., Meuer S. C., Schlossman S. F., Reinherz E. L. Delineation of an immunoregulatory amplifier population recognizing autologous Ia molecules. Analysis with human T cell clones. J Exp Med. 1984 Feb 1;159(2):559–576. doi: 10.1084/jem.159.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., Kung P. C., Gingras S. P., Goldstein G. Does OKT3 monoclonal antibody react with an antigen-recognition structure on human T cells? Proc Natl Acad Sci U S A. 1981 Mar;78(3):1805–1808. doi: 10.1073/pnas.78.3.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber S. M., Brand M. D. Early plasma-membrane-potential changes during stimulation of lymphocytes by concanavalin A. Biochem J. 1983 Mar 15;210(3):885–891. doi: 10.1042/bj2100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer H., Blume A. J., Kaback H. R. Membrane potential changes during mitogenic stimulation of mouse spleen lymphocytes. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2200–2204. doi: 10.1073/pnas.77.4.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hussey R. E., Hodgdon J. C., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983 Jun 30;303(5920):808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hodgdon J. C., Hussey R. E., Protentis J. P., Schlossman S. F., Reinherz E. L. Antigen-like effects of monoclonal antibodies directed at receptors on human T cell clones. J Exp Med. 1983 Sep 1;158(3):988–993. doi: 10.1084/jem.158.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Molski T. F., Borgeat P., White J. R., Sha'afi R. I. Phorbol esters inhibit the fMet-Leu-Phe- and leukotriene B4-stimulated calcium mobilization and enzyme secretion in rabbit neutrophils. J Biol Chem. 1985 Feb 25;260(4):2125–2131. [PubMed] [Google Scholar]
- Oettgen H. C., Terhorst C., Cantley L. C., Rosoff P. M. Stimulation of the T3-T cell receptor complex induces a membrane-potential-sensitive calcium influx. Cell. 1985 Mar;40(3):583–590. doi: 10.1016/0092-8674(85)90206-5. [DOI] [PubMed] [Google Scholar]
- Ritz J., Campen T. J., Schmidt R. E., Royer H. D., Hercend T., Hussey R. E., Reinherz E. L. Analysis of T-cell receptor gene rearrangement and expression in human natural killer clones. Science. 1985 Jun 28;228(4707):1540–1543. doi: 10.1126/science.2409597. [DOI] [PubMed] [Google Scholar]
- Royer H. D., Acuto O., Fabbi M., Tizard R., Ramachandran K., Smart J. E., Reinherz E. L. Genes encoding the Ti beta subunit of the antigen/MHC receptor undergo rearrangement during intrathymic ontogeny prior to surface T3-Ti expression. Cell. 1984 Dec;39(2 Pt 1):261–266. doi: 10.1016/0092-8674(84)90003-5. [DOI] [PubMed] [Google Scholar]
- Sagi-Eisenberg R., Lieman H., Pecht I. Protein kinase C regulation of the receptor-coupled calcium signal in histamine-secreting rat basophilic leukaemia cells. Nature. 1985 Jan 3;313(5997):59–60. doi: 10.1038/313059a0. [DOI] [PubMed] [Google Scholar]
- Shapiro H. M., Natale P. J., Kamentsky L. A. Estimation of membrane potentials of individual lymphocytes by flow cytometry. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5728–5730. doi: 10.1073/pnas.76.11.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano R. F., Pratt J. C., Schmidt R. E., Ritz J., Reinherz E. L. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985 Oct 3;317(6036):428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Weiss M. J., Daley J. F., Hodgdon J. C., Reinherz E. L. Calcium dependency of antigen-specific (T3-Ti) and alternative (T11) pathways of human T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6836–6840. doi: 10.1073/pnas.81.21.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]