Abstract
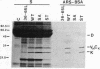
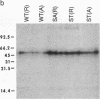
Structural analysis of 21 murine A/J antibodies specific for the hapten p-azobenzenearsonate (Ars), and bearing the major cross reactive idiotype (IdCRI), has revealed an invariant amino acid residue, serine, encoded by the variable-diversity gene segments junction of the heavy chain. To test whether this serine residue is essential for Ars binding, we changed it either to alanine or to threonine by oligonucleotide-directed mutagenesis of a heavy chain gene. Genes containing the mutations were separately introduced into mouse hybridoma cells producing the homologous light chain, and the resulting proteins were tested for antigen binding and idiotypic expression. Whereas the serine to threonine mutant retains full antigen binding activity, the serine to alanine mutant does not bind either to Ars-bovine serum albumin-Sepharose or to the Ars-tyrosine hapten. Both mutants show the same reactivity as wild type towards a series of anti-idiotypic antibodies. These results suggest that a hydroxyl group at the variable-diversity gene segments junction of A/J anti-Ars antibodies is essential for antigen binding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clevinger B., Schilling J., Hood L., Davie J. M. Structural correlates of cross-reactive and individual idiotypic determinants on murine antibodies to alpha-(1 leads to 3) dextran. J Exp Med. 1980 May 1;151(5):1059–1070. doi: 10.1084/jem.151.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Inhibitory and stimulatory effects of concanavalin A on the response of mouse spleen cell suspensions to antigen. II. Evidence for separate stimulatory and inhibitory cells. J Exp Med. 1973 Dec 1;138(6):1496–1505. doi: 10.1084/jem.138.6.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley T., Margolies M. N., Gefter M. L. The association of various D elements with a single-immunoglobulin VH gene segment: influence on the expression of a major cross-reactive idiotype. J Immunol. 1985 Feb;134(2):1236–1244. [PubMed] [Google Scholar]
- Hornbeck P. V., Lewis G. K. Idiotype connectance in the immune system. II. A heavy chain variable region idiotope that dominates the antibody response to the p-azobenzenearsonate group is a minor idiotope in the response to trinitrophenyl group. J Exp Med. 1985 Jan 1;161(1):53–71. doi: 10.1084/jem.161.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S., Kronvall G. The use of protein A-containing Staphylococcus aureus as a solid phase anti-IgG reagent in radioimmunoassays as exemplified in the quantitation of alpha-fetoprotein in normal human adult serum. Eur J Immunol. 1974 Jan;4(1):29–33. doi: 10.1002/eji.1830040108. [DOI] [PubMed] [Google Scholar]
- Kaartinen M., Griffiths G. M., Hamlyn P. H., Markham A. F., Karjalainen K., Pelkonen J. L., Mäkelä O., Milstein C. Anti-oxazolone hybridomas and the structure of the oxazolone idiotype. J Immunol. 1983 Feb;130(2):937–945. [PubMed] [Google Scholar]
- Kabat E. A. Antibody diversity versus antibody complementarity. Pharmacol Rev. 1982 Mar;34(1):23–38. [PubMed] [Google Scholar]
- Kuettner M. G., Wang A. L., Nisonoff A. Quantitative investigations of idiotypic antibodies. VI. Idiotypic specificity as a potential genetic marker for the variable regions of mouse immunoglobulin polypeptide chains. J Exp Med. 1972 Mar 1;135(3):579–595. doi: 10.1084/jem.135.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manser T., Huang S. Y., Gefter M. L. Influence of clonal selection on the expression of immunoglobulin variable region genes. Science. 1984 Dec 14;226(4680):1283–1288. doi: 10.1126/science.6334361. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Siekevitz M., Margolies M. N., Mudgett-Hunter M., Gefter M. L. Hybridoma proteins expressing the predominant idiotype of the antiazophenylarsonate response of A/J mice. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1120–1124. doi: 10.1073/pnas.77.2.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek K., Jeske D., Slaoui M., Leo O., Urbain J., Capra J. D. Complete amino acid sequence of heavy chain variable regions derived from two monoclonal anti-p-azophenylarsonate antibodies of BALB/c mice expressing the major cross-reactive idiotype of the A/J strain. J Exp Med. 1984 Oct 1;160(4):1070–1086. doi: 10.1084/jem.160.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbruch A., Zaiss S., Kappen C., Brüggemann M., Beyreuther K., Rajewsky K. Drastic change in idiotypic but not antigen-binding specificity of an antibody by a single amino-acid substitution. Nature. 1985 Jun 6;315(6019):506–508. doi: 10.1038/315506a0. [DOI] [PubMed] [Google Scholar]
- Rothstein T. L., Gefter M. L. Affinity analysis of idiotype-positive and idiotype-negative Ars-binding hybridoma proteins and Ars-immune sera. Mol Immunol. 1983 Feb;20(2):161–168. doi: 10.1016/0161-5890(83)90127-x. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Giusti A. M., Cook W. D., Scharff M. D. Single amino acid substitution altering antigen-binding specificity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1979–1983. doi: 10.1073/pnas.79.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schaffner W. Direct transfer of cloned genes from bacteria to mammalian cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2163–2167. doi: 10.1073/pnas.77.4.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon J., Gefter M. L., Manser T., Morrison S. L., Oi V. T., Ptashne M. Expression of a VHC kappa chimaeric protein in mouse myeloma cells. Nature. 1984 May 24;309(5966):364–367. doi: 10.1038/309364a0. [DOI] [PubMed] [Google Scholar]
- Sharon J., Kabat E. A., Morrison S. L. Studies on mouse hybridomas secreting IgM or IgA antibodies to alpha(1 to 6)-linked dextran. Mol Immunol. 1981 Sep;18(9):831–846. doi: 10.1016/0161-5890(81)90005-5. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Gefter M. L., Brodeur P., Riblet R., Marshak-Rothstein A. The genetic basis of antibody production: the dominant anti-arsonate idiotype response of the strain A mouse. Eur J Immunol. 1982 Dec;12(12):1023–1032. doi: 10.1002/eji.1830121208. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Huang S. Y., Gefter M. L. The genetic basis of antibody production: a single heavy chain variable region gene encodes all molecules bearing the dominant anti-arsonate idiotype in the strain A mouse. Eur J Immunol. 1983 Feb;13(2):123–132. doi: 10.1002/eji.1830130207. [DOI] [PubMed] [Google Scholar]
- Slaughter C. A., Capra J. D. Amino acid sequence diversity within the family of antibodies bearing the major antiarsonate cross-reactive idiotype of the A strain mouse. J Exp Med. 1983 Nov 1;158(5):1615–1634. doi: 10.1084/jem.158.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter C. A., Jeske D. J., Kuziel W. A., Milner E. C., Capra J. D. Use of JH4 joining segment gene by an anti-arsonate antibody that bears the major A-strain cross-reactive idiotype but displays diminished antigen binding. J Immunol. 1984 Jun;132(6):3164–3171. [PubMed] [Google Scholar]
- Takeo K., Kabat E. A. Binding constants of dextrans and isomaltose oligosaccharides to dextran-specific myeloma proteins determined by affinity electrophoresis. J Immunol. 1978 Dec;121(6):2305–2310. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. The strain A anti-p-azophenylarsonate major cross-reactive idiotypic family includes members with no reactivity toward p-azophenylarsonate. Eur J Immunol. 1981 Oct;11(10):832–839. doi: 10.1002/eji.1830111016. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]