Abstract
Objective
Cough is a typical side effect of angiotensin-converting enzyme (ACE) inhibitors, though its frequency quantitatively varies among the different compounds. Data on the incidence of cough with the lipophilic third-generation ACE inhibitor zofenopril are scanty and never systematically analyzed. The purpose of this paper is to give an overview on the epidemiology, pathophysiology, and treatment of ACE inhibitor-induced cough and to assess the incidence of cough induced by zofenopril treatment.
Methods
Published and unpublished data from randomized and postmarketing zofenopril trials were merged together and analyzed.
Results
Twenty-three studies including 5794 hypertensive patients and three studies including 1455 postmyocardial infarction patients exposed for a median follow-up time of 3 months to zofenopril at doses of 7.5–60 mg once-daily were analyzed. The incidence of zofenopril-induced cough was 2.6% (range 0%–4.2%): 2.4% in the hypertension trials (2.4% in the double-blind randomized studies and 2.4% in the open-label postmarketing studies) and 3.6% in the doubleblind randomized postmyocardial infarction trials. Zofenopril-induced cough was generally of a mild to moderate intensity, occurred significantly (P < 0.001) more frequently in the first 3–6 months of treatment (3.0% vs 0.2% 9–12 months), and always resolved or improved upon therapy discontinuation. Zofenopril doses of 30 mg and 60 mg resulted in significantly (P = 0.042) greater rate of cough (2.1% and 2.6%, respectively) than doses of 7.5 mg and 15 mg (0.4% and 0.7%, respectively). In direct comparison trials (enalapril and lisinopril), incidence of cough was not significantly different between zofenopril and other ACE inhibitors (2.4% vs 2.7%).
Conclusion
Evidence from a limited number of studies indicates a relatively low incidence of zofenopril-induced cough. Large head-to-head comparison studies versus different ACE inhibitors are needed to highlight possible differences between zofenopril and other ACE inhibitors in the incidence of cough.
Keywords: cough, ACE inhibitor, zofenopril, arterial hypertension, myocardial infarction, heart failure
Introduction
Zofenopril calcium, a prodrug of the active compound zofenoprilat, is a highly lipophilic third-generation angiotensin-converting enzyme (ACE) inhibitor, characterized by a high degree of tissue penetration and long-term cardiac ACE inhibition.1–4 Zofenopril, alone or in combination with a diuretic, has been successfully and safely employed in the treatment of acute myocardial infarction,5–7 heart failure,8,9 and essential hypertension.1–3,10–17
Lipophilicity confers zofenopril a high potency (five to six times more potent than captopril and twice as potent as enalaprilat and fosinoprilat, but three times less potent than ramiprilat),18,19 significant tissue selectivity (inhibition of cardiac ACE is 90% 1 hour after administration, 60% after 8 hours, and 45% after 24 hours),20 rapid onset (in hypertensive patients maximal blood pressure reductions are observed 2 hours after receiving zofenopril), and long duration of action (approximately 70% of the peak blood pressure response is maintained after 24 hours).10,11 The lipophilicity of zofenopril might potentially be associated with a low risk of coughing, but a systematic evaluation of this adverse event in patients treated with this drug has never been done.
The objective of the present paper was thus to specifically assess the incidence and characteristics of cough induced by zofenopril treatment by analyzing individual data of published and unpublished double-blind randomized and open-label postmarketing studies based on this drug. The results of this systematic review will be preceded by an ample overview and update on the epidemiology, pathophysiology, and treatment of ACE inhibitor-induced cough based on a thorough search of publications in the most popular electronic databases (PubMed, Embase, Cochrane library).
ACE inhibition and cough
General feature of ACE inhibitor-induced cough
ACE inhibitors are, together with beta-blockers and inhaled agents, the most common cause of reported cases of iatrogenic cough, with a rate of 75%.21 First cases of cough associated with ACE inhibitors were reported in the early 1980s with captopril.22,23 Since then cough has been reported as a typical side effect with all ACE inhibitors and it is likely to be a class side effect of these drugs; cough is not dose related and it is already observed with the lowest dose of the various active principles.24–27 Though it is considered a typical class side effect of ACE inhibitors, it is well known that incidence of cough may quantitatively vary among the different ACE inhibitors.28
ACE inhibitor-associated cough may take weeks or even months to develop, and it is reversible upon discontinuation of the drug. In first reports, time of onset of cough after starting an ACE inhibitor usually ranged from 3 days to 12 months.29 Although cough usually resolves within 1 day to 4 weeks of the cessation of therapy with the offending drug, in subgroups of individuals cough has been shown to linger for up to 3 months.24,25,29,30 After stopping treatment, recovery from cough is usually rapid and complete and there is no indication of long-term effects.24
The cough is typically dry, nonproductive, tickling, paroxysmal, often bothersome, associated with scratching sensation in the throat, and it is frequently worse when lying down.31,32 It is an annoying although harmless side effect,33 but it is persistent.34 Sometimes cough may be severe enough to cause some deterioration in well-being: cough may disturb sleep and wake up patients, usually early in the morning, it may cause vomiting, voice changes, and urinary incontinence, or may be debilitating.24,35,36 Cough may also lead to medication change and unnecessary diagnostic evaluation, and it may be associated with some depression and a tendency for increased fatigue: in one study this adverse drug reaction was associated with increased worry or discomfort in 49% of patients and with lower patient satisfaction.37
Cough is in the majority of cases benign, and only limited cases of lung damage, such as interstitial infiltrates, have been associated to treatment with an ACE inhibitor.38 Asthmatic patients do not seem to be at increased risk for ACE inhibitor-induced cough (14%–16% incidence of cough reported in patients with asthma treated with an ACE inhibitor versus 13% of patients without a prior history of bronchospasm).25,39,40
ACE inhibitor-induced cough may be often misdiagnosed, though awareness of this side effect has increased among physicians due to the widespread use of ACE inhibitors and the consequent increased chance of occurrence of such side effect.31
If the clinician fails to recognize the relationship between ACE inhibitor treatment and cough, the patient may be subjected to extensive and unnecessary evaluations, diagnostic tests, and consultations. One simple diagnostic procedure may be to stop treatment and to check whether cough recurs after treatment withdrawal and rechallenge: indeed, one randomized controlled trial demonstrated that 30% of patients did not develop a cough on third trial.30 Treatment withdrawal in this case must be done with caution, particularly in patients with moderate to severe hypertension or under multiple drug treatment, since abrupt discontinuation of successful antihypertensive therapy can result in a return of blood pressure to pretreatment levels. However, this blood pressure increase is usually gradual with ACE inhibitors, particularly in mild hypertensive subjects, and it rarely causes a withdrawal syndrome.
Epidemiology
Cough is thought to be a class effect, but there is considerable variation in estimates of the occurrence of coughing due to ACE inhibitors in the various systematic reviews and meta-analyses, probably due to the method used for its investigation.24,25,34,41–55 These differences reflect study design (postmarketing or general population studies versus randomized controlled studies), method of ascertaining the presence of cough (spontaneous reports, systematic inquiry, patient self-reporting), method of measuring occurrence of the phenomenon, and the size of the study.25,56,57 The complex pathophysiology of the phenomenon may also explain why this adverse effect is variable in occurrence (3%–35% in the various studies).31
Postmarketing surveillance and general population studies report a lower (0%–3%) incidence of ACE inhibitor-induced cough as compared to randomized controlled studies.25,42,58–63 The higher observed rates of cough found in randomized controlled trials (13% to 25%–35%) are probably due to the fact that in such studies patients were queried systematically for the symptom.
Risk of coughing has been found in some cases to be lower with newer ACE inhibitors probably because of an increased awareness of this symptom with time rather than for real differences in pharmacological effects. A conservative estimate of the incidence of ACE inhibitor-induced cough might be 5%–10% of treated patients.35
Cough due to ACE inhibitor treatment is common not only in hypertensive-treated patients but also in heart failure-treated patients. In 670 patients hospitalized for symptomatic chronic heart failure, of which 74% were receiving an ACE inhibitor alone or in combination with other drugs, cough due to an ACE inhibitor was reported in approximately 2% of subjects. 46 In a meta-analysis of 9668 patients with chronic heart failure or left ventricular dysfunction, about 2% of patients were withdrawn from the study because of cough, which represented 5% of all causes of treatment withdrawal.64
Patients treated with ACE inhibitors for chronic heart failure seem to cough more frequently than those treated for hypertension. In a prospective study involving 268 patients, cough was developed during the 1 year of follow-up by 14% of patients with hypertension and by 26% of patients with chronic heart failure, probably because of a lower threshold for cough in the latter group.57
Cough due to ACE inhibitors occurs more frequently in women (relative risk [RR]: 2–2.5 compared to men),24,65–67 elderly (RR: 1.5–2.0 compared to younger people),53 non-smokers (RR: 3.0 compared to smokers),25,66 and patients of Chinese or Asiatic origin (RR: 2.7 compared to white people).68,69 A study developed and validated a predicting model for risk of development of ACE inhibitor-induced cough within 6 months from starting treatment by using information available at first prescription for 567 patients.70 In the total cohort of subjects, 13% developed ACE inhibitor-induced cough. Independent predictors of cough were older age (adjusted odds ratio [OR]: 2.1 for age 60–69 years), female gender (OR: 2.3), East Asian origin (OR: 4.3), no history of previous ACE inhibitor use (OR: 2.4), and history of cough due to another ACE inhibitor (OR: 29). This model could help in avoiding the prescription of ACE inhibitors in patients at higher risk of developing cough.
Pathophysiology
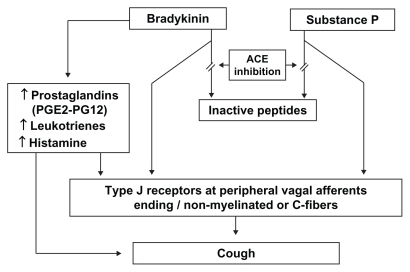
A possible pathophysiological mechanism for ACE inhibitorassociated cough is depicted in Figure 1. The pathogenesis of the phenomenon is not completely known, but cough is thought to be related to a cascade of effects that begins with the accumulation of kinins and then involves arachidonic acid metabolism and nitric oxide generation.71 ACE is synonymous with kininase II and bradykinin dehydrogenase, the enzyme responsible for bradykinin breakdown. ACE inhibition thus blocks this pathway and leads to accumulation of bradykinin in the airways, a substance recognized as a bronchoconstrictor. Bradykinin has many local effects, including the release of histamine, and also interferes with locally-produced neurotransmitters such as substance P and neuropeptide Y.25 Both bradykinin and substance P may interfere with type I receptors at peripheral nerve endings, perhaps mediated by unmyelinated or vagal afferent C-fiber, which have irritant effects on the bronchial mucosa.72 The increased cough response to the specific C fiber stimulant capsaicin, a local irritant on mucosal membranes, both in patients who cough and in normal volunteers, is a demonstration that in presence of an ACE inhibitor the cough reflex is increased. This sensitization of the cough reflex may potentiate other causes of chronic cough.73
Figure 1.
Pathophysiology of angiotensin-converting enzyme inhibitor-induced cough.71,72
Abbreviations: ACE, angiotensin-converting enzyme; PG, prostaglandin.
Other possible mechanisms for bronchoconstriction are direct action on smooth muscle and/or indirect inflammatory activity, like local edema.74 Bradykinin may also activate local release of histamine from mast cells, which might be a mediator of the ACE inhibitor-induced cough and be part of the tussive effect.33,62 The indirect demonstration that ACE inhibitor-induced cough is linked to accumulation of kinins, is proved by the fact that ACE is able to degrade kinins and that ACE inhibitor protussive effect is reduced by kinin antagonists.74
Local effects on prostaglandin synthesis have also been suggested, since prostaglandins act locally as inflammatory agents. Besides directly interacting with C fibers, bradykinin may also cause bronchoconstriction indirectly by release of arachidonic acid derivatives such as leukotrienes and prostaglandin E2 and I2.25,75,76 Prostaglandin E2 stimulates the unmyelinated afferent sensory C fibers (as does bradykinin through type J receptors), resulting in cough. Treatment with a prostaglandin synthetase inhibitor (eg, indomethacin) may alleviate the cough in affected patients.
Bradykinin has been shown to induce the production of nitric oxide, and there is some evidence that this product, which is subject to regulation by other pathways, may promote cough through pro-inflammatory mechanisms.71
Thus, there are suggested pharmacological reasons for the cough, but it is not clear why relatively few patients experience this side effect. It is thought that the mechanism responsible for ACE inhibitor-related cough might be an increased sensitivity of the cough reflex, probably a genetic polymorphism.77 Some evidence has suggested that the therapeutic effect of ACE inhibitors may involve the activation of bradykinin receptors and that bradykinin receptor gene polymorphism is associated with the cough that is related to ACE inhibitors.78,79 Subjects with ACE inhibitor-induced cough demonstrate increased cough reflex sensitivity to experimental stimulation with capsaicin, which resolves after the discontinuation of therapy with the inciting drug.73,77,80
More recently, hypothesis of genetic predisposition to ACE inhibitor cough has been supported by a study in which patients with a history of developing this side effect showed a lower activity of an enzyme called aminopeptidase P. This enzyme has an important role in degrading bradykinin and its reduced activity may be partially explained by genetic variation.81
Treatment
Numerous small studies have evaluated various drugs, such as cromolyn, baclofen, theophylline, sulindac, and local anesthetics use82–86 as potential therapies for ACE inhibitor-induced cough, but none of them seem to be resolutive. Also, antitussive drugs, such as antihistamines, are relatively ineffective in patients with cough following treatment with ACE inhibitors, although useful in cases of chronic cough due to other cause or idiopathic chronic cough.33 This is probably related to the specific mechanism responsible for ACE inhibitor-related cough, which is only marginally affected by treatment with antitussive drugs, acting on the symptoms, but not on the specific cause of cough.86
The only appropriate intervention for ACE inhibitor-induced cough is the cessation of therapy with the offending agent. Commonly, in half of the patients developing cough following treatment with ACE inhibitor, the drug has to be discontinued.32 Only very rarely may a dose reduction lead to improvement and indeed this confirms evidence that incidence of cough does not substantially vary with dose.33,87 Cough is usually remitting within a few days (1–4 days) after the agent is discontinued, but can rarely take as long as 4–12 weeks to subside.25,34
According to European and American guidelines,88,89 in patients with persistent or intolerable ACE inhibitor-induced cough, therapy should be switched to an angiotensin II antagonist with which the incidence of associated cough appears to be similar to that for the control drug, or to an appropriate agent of another drug class.31 Indeed, consistent fair-to-good quality evidence has shown that ACE inhibitors are associated with a risk of cough as much as three times higher than that of angiotensin II antagonists.42,90
As indicated by current guidelines,88,89 for patients whose cough resolves after the cessation of ACE inhibition therapy and for whom there is a compelling reason for treatment with these agents, a repeat trial of ACE inhibitor therapy may be attempted. One randomized double-blind, parallel-group controlled trial demonstrated that about 30% of patients with ACE inhibitor-induced cough who had been challenged and dechallenged twice did not develop cough after a third trial of ACE inhibitor therapy.91
Association of an angiotensin II antagonist to an ACE inhibitor is not a useful option for reducing the risk of cough. In the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study, the rate of discontinuation of study medication due to cough was similar in the 8576 patients treated with ramipril (4.2%) and in the 8502 patients treated with combination of ramipril and telmisartan (4.6%).92 In a meta-analysis of nine trials, involving 18,160 patients with heart failure or left ventricular dysfunction, risk of cough in the 9199 patients receiving combination therapy was not significantly different from that of patients receiving an ACE inhibitor alone (RR: 0.84; 95% confidence interval 0.65–1.09).93 Also, the combination of low-dose ACE inhibitors with other antihypertensive drugs does not substantially reduce the risk of cough.87
Zofenopril and cough
Methodology of the review
Individual published and unpublished data from double-blind randomized and open-label postmarketing therapeutic trials based on zofenopril treatment were retrieved from the manufacturer and merged together in an electronic database.1–7,10–17 In all studies, the prevalence of cough was monitored and assessed at each visit by the physician objectively, by physical examination, or subjectively by asking the patient a non-leading question or by patient’s spontaneous reporting. No specific questionnaire was used to assess the features of ACE inhibitor-induced cough. The physician assigned a severity category to cough, according to the following scale: (a) mild (an easily tolerated cough, causing minimal discomfort and not interfering with everyday activities), (b) moderate (a sufficiently discomforting cough, interfering with normal everyday activities), and (c) severe (cough preventing normal everyday activities). Data analysis was carried out by SPSS Statistical Package (v 11.5; IBM Corporation, Armonk, NY). Analysis of variance was used for comparison of continuous variables and a chi-square test for comparison of proportions. Data are summarized as mean values (±standard deviation) or as absolute and relative (%) frequencies.
General characteristics of the studies, with reference to published and unpublished data, study design, study drug doses, and inclusion criteria, are reported in Table 1. The database included 5794 treated hypertensive patients and 1455 treated postmyocardial infarction patients. A total of 7249 subjects had been exposed to zofenopril in dosages 7.5–60 mg once-daily from as early as 1 month to 1 year (median follow-up time 3 months). According to analysis of zofenopril studies, the overall incidence of cough was of 190 patients out of 7429 (2.6%, range 0%–4.2%). In the following paragraphs, a detailed report on the incidence of cough for hypertension and postmyocardial infarction studies by study design, treatment duration, and dosages will be shown.
Table 1.
Details of placebo or active drug controlled studies in which occurrence of cough with zofenopril was assessed in hypertensive and postmyocardial infarction patients
| Study | Zofenopril daily dose (mg) | Associated drug and daily dose (mg) | n | Control drug and daily dose (mg) | Study design | Inclusion criteria | Age range (years) | Treatment duration (weeks) |
|---|---|---|---|---|---|---|---|---|
| Study no. 21974-2310 | 7.5, 15, 30, or 60 | Hydrochlorothiazide 25 | 301 | Placebo | DBR PL PG |
DBP 95–110 mmHg | 19–79 | 8 |
| Study no. 21974-2610 | 7.5, 15, 30, or 60 | Hydrochlorothiazide 25 | 260 | Placebo (hydrochlorothiazide 25) | DBR ACT/PL PG |
DBP 95–114 mmHg | 23–86 | 8 |
| Study no. SZQ0210 | 7.5, 15, or 30 | None | 141 | Placebo | DBR PL PG |
Elderly DBP 95–115 mmHg SBP 170–220 mmHg |
65–86 | 8 |
| Study no. SZQ0310 | 15 or 30 | None | 88 | Nifedipine 80 | DBR ACT PG |
Elderly DBP 95–115 mmHg SBP 170–220 mmHg |
63–92 | 8 |
| Study no. SQZ0510 | 30 or 60 | None | 57 | Placebo | DBR PL PG |
DBP 95–110 mmHg | 27–71 | 6 |
| Study no. 21974-1610 | 30 or 60 | Hydrochlorothiazide 25 | 192 | Atenolol 50 or 100, hydrochlorothiazide 25 | DBR ACT PG |
DBP 95–110 mmHg | 23–81 | 12 |
| Study no. 21974-1710 | 30 or 60 | Nadolol 40 | 166 | Hydrochlorothiazide 25 or 50, nadolol 40 | DBR ACT PG |
DBP 95–110 mmHg | 22–81 | 12 |
| Study no. 21974-2010 | 30 or 60 | Prazosin 2, chlorthalidone 25 | 133 | Propranolol 80 or 160, prazosin 2, chlorthalidone 25 | DBR PL PG |
DBP 95–115 mmHg | 24–77 | 12 |
| Study no. 21974-1810 | 15, 30 or 60 | None | 41 | Enalapril 2.5, 5, or 10 | DBR PL PG |
DBP 95–110 mmHg | 27–79 | 12 |
| Study no. 21974-1110 | 30 or 60 | Chlorthalidone 25 | 373 | Placebo | DBR PL PG |
DBP 92–110 mmHg | 19–84 | 48 |
| Study no. 21974-2810 | 30 or 60 | Chlorthalidone 25 | 185 | Enalapril 5 or 10, chlorthalidone 25 | DBR ACT PG |
Renal impairment DBP 95–110 mmHg |
21–76 | 52 |
| Study no. ZOF01MEN10 | 7.5, 15, 30, or 60 | None | 168 | Placebo | DBR PL PG |
DBP 95–114 mmHg | 26–66 | 6 |
| Parati et al11 | 15, 30, or 60 | Hydrochlorothiazide 12.5 or 25 | 282 | None | DBR PG |
DBP 95–110 mmHg | 18–75 | 12 |
| Omboni et al1 | 30 | Hydrochlorothiazide 12.5 | 350 | Hydrochlorothiazide 12.5 | DBR ACT PG |
DBP 95–115 mmHg | 18–75 | 36 |
| Omboni et al1 | 30 | Hydrochlorothiazide 12.5 | 376 | None | DBR PG |
DBP 90–100 mmHg | 18–70 | 8 |
| Nilsson12 | 30 or 60 | None | 147 | Atenolol 50 or 100 DBR | ACT PG |
DBP 95–115 mmHg | 18–75 | 12 |
| Narkiewicz13 | 30 or 60 | None | 165 | Losartan 50 or 100 DBR | ACT PG |
DBP 95–110 mmHg | 18–75 | 12 |
| Mallion14 | 30 or 60 | None | 155 | Enalapril 20 or 40 | DBR ACT PG |
DBP 95–115 mmHg | 23–70 | 12 |
| Farsang15 | 30 or 60 | None | 152 | Amlodipine 5 or 10 | DBR ACT PG |
DBP 95–110 mmHg | 18–75 | 14 |
| Leonetti et al16 | 30 or 60 | None | 114 | Candesartan 8 or 16 | DBR ACT PG |
DBP 90–109 mmHg SBP 140–179 mmHg |
18–65 | 12 |
| Malacco et al17 | 30 or 60 | None | 91 | Lisinopril 10 or 20 | DBR ACT PG |
Elderly DBP 90–110 mmHg |
65–90 | 12 |
| Study no. PMS-1 (CRF23012)10 | 30 or 60 | None | 1,303 | None | OL | DBP ≥ 90 mmHg | 18–75 | 31 |
| Study no. PMS-2 (CRF23022)10 | 30 or 60 | None | 554 | None | OL | Diabetes mellitus DBP ≥ 90 mmHg |
18–75 | 19 |
| SMILE-15 | 7.5, 15, 30, or 60 | None | 772 | Placebo | DBR PL PG |
<24 hours nonthrombolyzed acute myocardial infarction | 18–80 | 6 |
| SMILE-26 | 7.5, 15, 30, or 60 | None | 504 | Lisinopril 2.5 to 10 mg | DBR ACT PG |
<48 hours thrombolyzed patients with acute myocardial infarction | 18–75 | 6 |
| SMILE-37 | 30 or 60 | None | 179 | Placebo | DBR PL PG |
Postmyocardial infarction with preserved left ventricular ejection fraction (>40%) | 18–75 | 24 |
Abbreviations: ACT, active control; DBP, diastolic blood pressure; DBR, double blind randomized; OL, open label; PG, parallel group; PL, placebo control; SBP, systolic blood pressure; SMILE, Survival of Myocardial Infarction Long-term Evaluation.
Results
Cough under zofenopril in hypertension trials
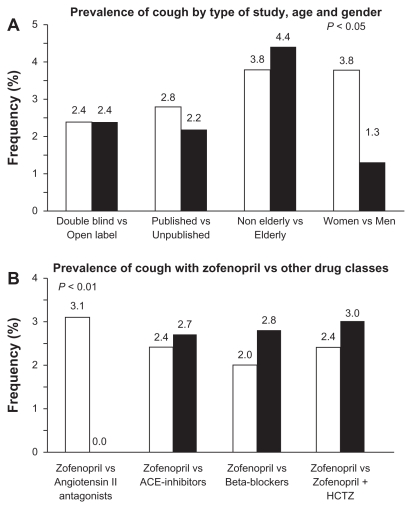
In the zofenopril hypertension studies, drug-related cough was experienced by 137 (2.4%) of the 5794 patients receiving zofenopril regimen: there was no significant difference in the incidence of cough between double-blind randomized studies (93 patients, 2.4%) and open-label studies (44 patients, 2.4%; P = 0.987), or the published and unpublished studies (2.8% vs 2.2%, P = 0.153) (Figure 2A).1,10–17 Expectedly, in placebo controlled studies, cough was reported significantly (P = 0.035) more often with zofenopril (4.1%) than with placebo (1.6%). Elderly patients (≥65 years) did not experience cough more frequently than nonelderly (4.4% vs 3.8%; P = 0.496), and cough did not occur in patients under 40 years of age (Figure 2A). Significantly more women than men experienced cough (3.8% vs 1.3%, P = 0.042) (Figure 2A).
Figure 2.
Prevalence (%) of cough under zofenopril in hypertensive patients (A) according to study design, age, and gender and (B) versus other drugs, including angiotensin II antagonists, other angiotensin-converting enzyme inhibitors, beta-blockers, and combination of zofenopril with hydrochlorothiazide.
Note: P values refer to between-group differences.1,10–17
Abbreviations: ACE, angiotensin-converting enzyme; HCTZ, hydrochlorothiazide.
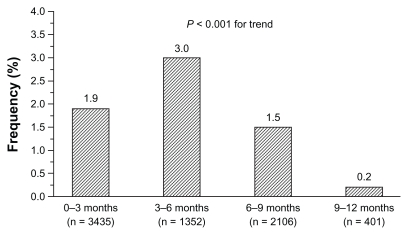
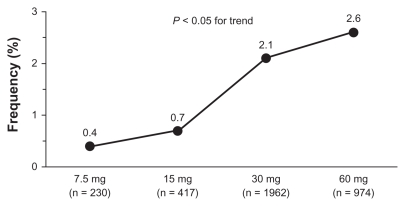
Cough was generally mild to moderate and tended to occur significantly more often (P < 0.001) in the first 6 months of treatment. There was no evidence of an increased incidence of cough during long-term trials, once the relative length of observation was taken into account. As reported in Figure 3, incidence of cough was 1.9% in trials lasting up to 3 months, 3.0% in trials lasting more than 3 months and up to 6 months, 1.5% in trials lasting more than 6 months and up to 9 months, and only 0.2% in long-term trials with duration up to 12 months. The occurrence of cough showed dose dependency, with doses of 30 mg and 60 mg resulting in significantly (P = 0.042) greater frequency of events (2.1% and 2.6% of treated patients, respectively) than doses of 7.5 mg (0.4%) and 15 mg (0.7%) (Figure 4). Neither respiratory tract disease antedating zofenopril therapy nor concomitant use of other medications appears to predispose patients to zofenopril-associated cough. Of the 2535 patients for which information on pretreatment with ACE inhibitor was available, 2.5% developed cough during treatment with zofenopril.
Figure 3.
Incidence (%) of drug-related cough stratified by observation period during zofenopril treatment of 5794 hypertensive patients.
Note: Numbers in brackets refer to the number of patients evaluated per treatment period.1,10–17
Figure 4.
Prevalence (%) of drug-related cough by zofenopril dose in hypertensive patients.
Note: Numbers in brackets refer to the number of patients evaluated per treatment period.1,10–17
Among the patients with zofenopril-associated cough, 23.8% discontinued treatment due to this side effect, 38.1% reported resolution of the cough without interruption of zofenopril, 26.2% had a persistent cough to the end of the study without discontinuing zofenopril. Cough resolved or improved upon discontinuation from therapy for all patients in whom the outcome was reported, and in the majority of patients it either disappeared during treatment continuation or was mild enough to allow the continuation of zofenopril treatment until the planned study conclusion.
Trials directly comparing safety of zofenopril with that of other ACE inhibitors reported a slightly, but not significantly (P = 0.846), lower occurrence of cough under zofenopril as compared to enalapril or lisinopril (2.4% vs 2.7%) (Figure 2B). The same was observed with beta-blockers, such as atenolol or propranolol (2.0% vs 2.8%, P = 0.688) (Figure 2B). The rate of cough during zofenopril was significantly (P = 0.009) higher than that observed during treatment with angiotensin II antagonist losartan (7/165 treated patients, 4.7% vs none under losartan) and not significantly (P = 0.145) greater than under candesartan (2/114 treated patients, 1.8% vs none under candesartan) (Figure 2B).
Combination with hydrochlorothiazide does not seem to significantly increase the chance of coughing in zofenopril-treated patients. In three double-blind randomized studies, including 1008 patients with mild to moderate essential hypertension who received zofenopril 15–60 mg (410 patients) or zofenopril 15–60 mg plus hydrochlorothiazide 12.5–25 mg (598 patients), the overall incidence of cough was 2.8%: 2.4% in the zofenopril monotherapy group (82.9% of patients receiving the usual dose of 30 mg) and 3.0% in the zofenopril plus hydrochlorothiazide group (76.8% of patients were receiving the usual proposed dose of zofenopril 30 mg plus hydrochlorothiazide 12.5 mg) (P = 0.588) (Figure 2B).1,3
Cough under zofenopril in postmyocardial infarction studies
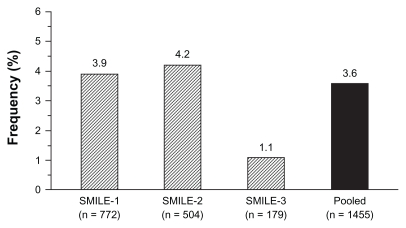
As mentioned above, zofenopril has also been largely employed in double-blind randomized trials in patients with myocardial infarction. The overall rate of cough in these trials was 3.6% (53/1455 treated patients) (Figure 5).
Figure 5.
Rate (%) of patients with zofenopril-related cough in the postinfarction Survival of Myocardial Infarction Long-term Evaluation (SMILE) studies.5–7
In the Survival of Myocardial Infarction Long-term Evaluation study group-1 (SMILE-1) study,5 1556 patients were enrolled within 24 hours after the onset of symptoms of acute myocardial infarction and randomly assigned to double-blind treatment with either placebo or zofenopril for 6 weeks. The initial dose of zofenopril was 7.5 mg and it could be progressively doubled up to 60 mg. At the end of the study the target daily dose of 60 mg was achieved in 78.8% of subjects. In the 772 patients treated with zofenopril, the major adverse side effect and main reason for discontinuation of treatment was hypotension: drug-related cough was reported by 30 patients (3.9%) and did not lead to study withdrawal in any patient. The rate of cough was slightly, but not significantly (P = 0.088), higher in the control group (5.7%) treated with placebo.
The SMILE-1 study was followed by the SMILE-2 study,6 which blindly assessed the efficacy of zofenopril at doses ranging from 7.5–60 mg daily against lisinopril 2.5–10 mg daily in 1204 thrombolyzed patients with acute myocardial infarction, in whom treatment was started within 48 hours from the onset of symptoms. During the 6 weeks of study treatment, drug-related cough was reported by 21 of the 504 patients treated with zofenopril (4.2%) and by 17 of the 520 patients treated with lisinopril (3.3%), with no statistically significant difference between the two treatment groups (P = 0.448).
In the SMILE-1 and SMILE-2 studies, the short follow-up period did not allow assessment of tolerability on long-term treatment. This was possible in the SMILE-3 (SMILE-ischemia) study,7 which enrolled 349 postmyocardial patients with preserved left ventricular ejection fraction treated for 6 months with zofenopril 30–60 mg or placebo. In the 179 patients treated with zofenopril, drug-related cough in response to treatment occurred in two patients (1.1%), which showed no significant difference (P = 0.579) to the rate observed in the placebo group (one patient, 0.6%).
Discussion
The aim of this paper was to produce evidence on drugrelated cough under zofenopril in clinical trials. This information is scanty because some data were never published or systematically analyzed before. An analysis of original double-blind randomized or open-label postmarketing studies based on zofenopril treatment confirmed that the use of such an ACE inhibitor may be associated with the occurrence of this side effect.
Though it was beyond the scope of this review to compare the incidence of cough under zofenopril with that observed with other ACE inhibitors, it must be recognized that the incidence of cough under zofenopril was relatively low (2.4%). This is probably related to its pharmacological features, in particular its potentially lower ability to induce local production of kinins and prostaglandins than other ACE inhibitors. This has been shown in some experimental and animal studies summarized and discussed in the following paragraphs.
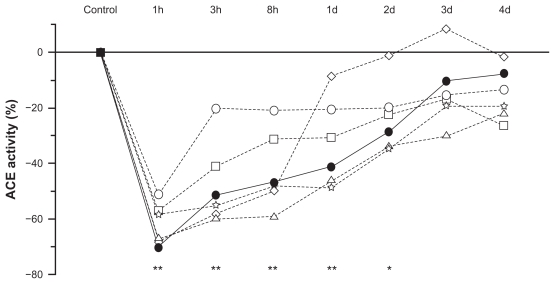
According to ex vivo animal studies, normalized oral doses of zofenopril, captopril, enalapril, fosinopril, lisinopril, and ramipril have equivalent effects on serum ACE, but the strength and duration of ACE inhibition with zofenopril is not the same in different tissues, particularly in the lungs.18 For instance, at the heart level, where ACE inhibition may prevent ischemic damage, zofenopril produces a more striking and long-lasting ACE inhibition than ramipril and enalapril, and to a lesser extent than captopril and fosinopril. As a matter of fact, 8 hours after administration, ACE inhibitor activity in the heart is approximately 60% with zofenopril, close to 0% with ramipril and enalapril, and about 30% with captopril and fosinopril.18 After 24 hours, cardiac ACE is inhibited by approximately 45% with zofenopril, while the effects of equivalent oral doses of other ACE inhibitors have virtually disappeared by this time (1%–10%). As shown in an ex vivo study performed in rats, in the lung zofenopril is distinguished for its lower ACE inhibition potency in the long term compared to other ACE inhibitors (Figure 6) and to other districts (eg, 40% less potent in the lung than in the heart).18,94 Though direct evidence on the characteristics of bradykinin production is lacking, this finding might at least partially explain the relatively low risk of coughing observed with zofenopril.
Figure 6.
Changes (%) in the inhibition of lung angiotensin-converting enzyme activity as a function of time (hours and days) after administration of equivalent oral doses of zofenopril (full circles), captopril (open circles), enalapril (open squares), ramipril (open triangles), lisinopril (open stars), and fosinopril (open diamonds) in 42 rats (ex vivo study).
Notes: Asterisks refer to the statistical significance at each time point versus control for zofenopril (**P < 0.01; *P < 0.05). Redrawn with permission.18
Abbreviations: ACE, angiotensin-converting enzyme; d, days; h, hours.
Indeed, the power of zofenopril to stimulate the production of prostaglandins, either directly or by inhibiting bradykinin metabolism, is lesser than other ACE inhibitors, as reported in animal studies. In anesthetized open-chest dogs, improvement of myocardial contractile function after left anterior descending coronary artery occlusion associated with enalapril treatment was largely reversed by indomethacin, known to inhibit myocardial prostaglandin synthesis (segment shortening reduced by 69% ± 12% at 2 hours after treatment/reperfusion to 38% ± 12% at 2 hours after indomethacin; P < 0.01).95 However, the drug had no effect on the improved contractile function associated with zofenopril (segment shortening was enhanced by 75% ± 10% at 2 hours after reperfusion and was maintained at 72% ± 8% 2 hours after indomethacin), indicating a different mechanism of action for the two drugs.95 Such an effect has also been recently demonstrated at the lung level. In one study, 2 weeks of oral treatment of guinea pigs with zofenopril (10 mg/kg/die) did not change the number of cough episodes following 0.1 M of citric acid aerosol as compared to control (15.0 ± 1.8 vs 16.0 ± 1.4), while this number was significantly (P < 0.05) increased after treatment with ramipril (21.0 ± 1.8 and 24.0 ± 2.5 at 3 mg/kg/die and 10 mg/kg/die, respectively).96 In another experimental study performed in rabbits, the animals were exposed to 1 M of nebulized citric acid for 3 minutes following 30 minutes of treatment with intravenous saline, zofenoprilat, or ramiprilat, the active metabolites of the prodrugs zofenopril and ramipril, at equieffective blood pressure reducing doses.97 Both saline and zofenoprilat did not induce any significant difference in the number of cough episodes (13.1 ± 1.3 and 15.8 ± 4.3 vs 15.2 ± 2.3 and 16.1 ± 4.9, respectively), while ramiprilat induced a significant increase in number of coughs (34.9 ± 3.5 vs 21.1 ± 2.6; P < 0.01). These two studies support the hypothesis of a difference in the protussive activity of zofenopril and ramipril, probably due to a difference in the activation of cyclooxygenase-2, which reflects on a different ability to induce accumulation of bradykinin and prostaglandin synthesis. Indeed, an indirect support to this hypothesis is given by a study in which ramipril was reported to induce increase in cyclooxygenase-2 expression in lungs of mice and in cultures of human endothelial cells.98 Ramipril-induced cough has also been associated with increased (by 36%) of bradykinin levels in the bronchoalveolar lavage fluid and with a marked increase (by 232%) in prostaglandin EM, a metabolite of prostaglandin E2.96 In the same experimental condition, zofenopril was employed for comparison and the compound did not enhance bradykinin and prostaglandin EM as compared to controls. Also, captopril was found to increase prostaglandin E2 production in hypertensive patients.99 This lower stimulation of kinin production by zofenopril might be explained by its lower ACE inhibition potency at lung level in the long term compared to captopril and moreover to ramipril (Figure 6). However, evidence is needed in direct comparative studies in humans to fully support this hypothesis.
Conclusion
ACE inhibitors are widely administered to treat numerous medical conditions, but their use may be associated with a dry cough that can lead to treatment discontinuation. Though this adverse drug reaction has been observed with all ACE inhibitors, its incidence may vary among the various agents.
According to analysis of individual data from several double-blind randomized and open-label studies, zofenopril seems to have a relatively low incidence of cough, which might be related to its limited ACE inhibitor potency at the lung level in the long term, responsible for a lesser accumulation of bradykinin and a reduced synthesis of prostaglandins in this tissue, as found in animal studies.
Large head-to-head comparative clinical trials with other ACE inhibitors in humans are still needed to demonstrate whether the incidence of cough under zofenopril may or may not significantly differ in respect to other ACE inhibitors. Notwithstanding this consideration, some important clinical implications of the findings must be highlighted. First, the results provide further evidence to the existing evidence, namely that dry cough is not an uncommon phenomenon during treatment with all ACE inhibitors. Physicians should be aware of this relationship when treating their hypertensive and cardiac patients, and probably should pay more attention to patients’ medical histories to prevent or reduce the risk of occurrence of such an adverse event. Second, results of the analysis suggest that not all ACE inhibitors have the same chance of inducing dry cough, probably because of potentially different pharmacological features. This aspect should be taken into account when a potential risk of dry cough exists and when treatment with an ACE inhibitor is unavoidable. Third, there is no proven effective treatment of ACE inhibitorinduced cough. Physicians should understand that when a patient is experiencing ACE inhibitor-related cough, the only possibility to avoid this effect is to stop treatment.
Acknowledgment
The authors would like to thank Istituto Luso Farmaco d’Italia for providing the original published and unpublished data necessary for completing this review and financially supporting this study with a research grant. The funding source did not participate in the literature search and did not influence or comment on planned methods, data analysis, and the draft report.
Footnotes
Disclosure
The authors have occasionally served as scientific consultants for Istituto Luso Farmaco d’Italia, manufacturer of zofenopril.
References
- 1.Omboni S, Malacco E, Parati G. Zofenopril plus hydrochlorothiazide fixed combination in the treatment of hypertension and associated clinical conditions. Cardiovasc Ther. 2009;27(4):275–288. doi: 10.1111/j.1755-5922.2009.00102.x. [DOI] [PubMed] [Google Scholar]
- 2.Borghi C, Bacchelli S, Degli Esposti D, Ambrosioni E. A review of the angiotensin-converting enzyme inhibitor, zofenopril, in the treatment of cardiovascular diseases. Expert Opin Pharmacother. 2004;5(9):1965–1977. doi: 10.1517/14656566.5.9.1965. [DOI] [PubMed] [Google Scholar]
- 3.Zanchetti A, Parati G, Malacco E. Zofenopril plus hydrochlorothiazide: combination therapy for the treatment of mild to moderate hypertension. Drugs. 2006;66(8):1107–1115. doi: 10.2165/00003495-200666080-00006. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosioni E. Defining the role of zofenopril in the management of hypertension and ischemic heart disorders. Am J Cardiovasc Drugs. 2007;7(1):17–24. doi: 10.2165/00129784-200707010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ambrosioni E, Borghi C, Magnani B Survival of Myocardial Infarction Long-Term Evaluation (SMILE) Study Investigators. The effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. N Engl J Med. 1995;332(2):80–85. doi: 10.1056/NEJM199501123320203. [DOI] [PubMed] [Google Scholar]
- 6.Borghi C, Ambrosioni E Survival of Myocardial Infarction Longterm Evaluation-2 Working Party. Double-blind comparison between zofenopril and lisinopril in patients with acute myocardial infarction: results of the Survival of Myocardial Infarction Long-term Evaluation-2 (SMILE-2) study. Am Heart J. 2003;145(1):80–87. doi: 10.1067/mhj.2003.24. [DOI] [PubMed] [Google Scholar]
- 7.Borghi C, Ambrosioni E Survival of Myocardial Infarction Long-term Evaluation Study Group. Effects of zofenopril on myocardial ischemia in post-myocardial infarction patients with preserved left ventricular function: the Survival of Myocardial Infarction Long-term Evaluation (SMILE)-ISCHEMIA study. Am Heart J. 2007;153(3):445. e7–e14. doi: 10.1016/j.ahj.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Binkley PF, Haas GJ, Starling RC, et al. Sustained augmentation of parasympathetic tone with angiotensin-converting enzyme inhibition in patients with congestive heart failure. J Am Coll Cardiol. 1993;21(3):655–661. doi: 10.1016/0735-1097(93)90098-l. [DOI] [PubMed] [Google Scholar]
- 9.Kelbaek H, Agner E, Wroblewski H, Vasehus Madsen P, Marving J. Angiotensin converting enzyme inhibition at rest and during exercise in congestive heart failure. Eur Heart J. 1993;14(5):692–695. doi: 10.1093/eurheartj/14.5.692. [DOI] [PubMed] [Google Scholar]
- 10.Zofenopril calcium + hydrochlorothiazide [Investigator’s brochure]. Version 5.0. Release Date: April 18, 2008. [Google Scholar]
- 11.Parati G, Omboni S, Malacco E. Antihypertensive efficacy of zofenopril and hydrochlorothiazide combination on ambulatory blood pressure. Blood Press. 2006;15(Suppl 1):7–17. [Google Scholar]
- 12.Nilsson P. Antihypertensive efficacy of zofenopril compared with atenolol in patients with mild to moderate hypertension. Blood Press. 2007;16(Suppl 2):25–30. doi: 10.1080/08038020701561745. [DOI] [PubMed] [Google Scholar]
- 13.Narkiewicz K. Comparison of home and office blood pressure in hypertensive patients treated with zofenopril or losartan. Blood Press. 2007;16(Suppl 2):7–12. doi: 10.1080/08038020701561687. [DOI] [PubMed] [Google Scholar]
- 14.Mallion JM. An evaluation of the initial and long-term antihypertensive efficacy of zofenopril compared with enalapril in mild to moderate hypertension. Blood Press. 2007;16(Suppl 2):13–18. doi: 10.1080/08038020701561703. [DOI] [PubMed] [Google Scholar]
- 15.Farsang C. Blood pressure control and response rates with zofenopril compared with amlodipine in hypertensive patients. Blood Press. 2007;16(Suppl 2):19–24. doi: 10.1080/08038020701561737. [DOI] [PubMed] [Google Scholar]
- 16.Leonetti G, Rappelli A, Omboni S. A similar 24-h blood pressure control is obtained by zofenopril and candesartan in primary hypertensive patients. Blood Press. 2006;15(Suppl 1):18–26. [Google Scholar]
- 17.Malacco E, Piazza S, Omboni S on behalf of the Zofenopril Study Group. Zofenopril versus lisinopril in the treatment of essential hypertension in elderly patients: a randomised, double-blind, multicentre study. Clin Drug Investig. 2005;25(3):175–182. doi: 10.2165/00044011-200525030-00003. [DOI] [PubMed] [Google Scholar]
- 18.Cushman DW, Wang FL, Fung WC, Harvey CM, DeForrest JM. Differentiation of angiotensin-converting enzyme (ACE) inhibitors by their selective inhibition of ACE in physiologically important target organs. Am J Hypertens. 1989;2(4):294–306. doi: 10.1093/ajh/2.4.294. [DOI] [PubMed] [Google Scholar]
- 19.Subissi A, Evangelista S, Giachetti A. Preclinical profile of zofenopril: an angiotensin converting enzyme inhibitor with peculiar cardioprotective properties. Cardiovasc Drug Rev. 1999;17(2):115–133. [Google Scholar]
- 20.Sun Y, Mendelsohn FA. Angiotensin converting enzyme inhibition in heart, kidney, and serum studied ex vivo after administration of zofenopril, captopril, and lisinopril. J Cardiovasc Pharmacol. 1991;18(4):478–486. doi: 10.1097/00005344-199110000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Noun L. Drug-induced respiratory disorders: incidence, prevention and management. Drug Saf. 2000;23(2):143–164. doi: 10.2165/00002018-200023020-00005. [DOI] [PubMed] [Google Scholar]
- 22.Havelka J, Vetter H, Studer A, et al. Acute and chronic effects of the angiotensin-converting enzyme inhibitor captopril in severe hypertension. Am J Cardiol. 1982;49(6):1467–1474. doi: 10.1016/0002-9149(82)90362-9. [DOI] [PubMed] [Google Scholar]
- 23.Sesoko S, Kaneko Y. Cough associated with the use of captopril. Arch Intern Med. 1985;145(8):1524. [PubMed] [Google Scholar]
- 24.Coulter DM, Edwards IR. Cough associated with captopril and enalapril. Br Med J (Clin Res Ed) 1987;294(6586):1521–1523. doi: 10.1136/bmj.294.6586.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med. 1992;117(3):234–242. doi: 10.7326/0003-4819-117-3-234. [DOI] [PubMed] [Google Scholar]
- 26.Verme-Gibboney C. Oral angiotensin-converting-enzyme inhibitors. Am J Health Syst Pharm. 1997;54(23):2689–2703. doi: 10.1093/ajhp/54.23.2689. [DOI] [PubMed] [Google Scholar]
- 27.Izzo JL, Jr, Weir MR. Angiotensin-converting enzyme inhibitors. J Clin Hypertens (Greenwich) 2011;13(9):667–675. doi: 10.1111/j.1751-7176.2011.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piepho RW. Overview of the angiotensin-converting-enzyme inhibitors. Am J Health Syst Pharm. 2000;57(Suppl 1):S3–7. doi: 10.1093/ajhp/57.suppl_1.S3. [DOI] [PubMed] [Google Scholar]
- 29.Stoller JK, Elghazawi A, Mehta AC, Vidt DG. Captopril-induced cough. Chest. 1988;93(3):659–661. doi: 10.1378/chest.93.3.659. [DOI] [PubMed] [Google Scholar]
- 30.Lacourcière Y, Brunner H, Irwin R, et al. Losartan Cough Study Group. Effects of modulators of the renin-angiotensin-aldosterone system on cough. J Hypertens. 1994;12(12):1387–1393. [PubMed] [Google Scholar]
- 31.Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(Suppl 1):169S–173S. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- 32.Overlack A. ACE inhibitor-induced cough and bronchospasm. Incidence, mechanisms and management. Drug Saf. 1996;15(1):72–78. doi: 10.2165/00002018-199615010-00006. [DOI] [PubMed] [Google Scholar]
- 33.Karlberg BE. Cough and inhibition of the renin-angiotensin system. J Hypertens Suppl. 1993;11(3):S49–52. [PubMed] [Google Scholar]
- 34.Just PM. The positive association of cough with angiotensin-converting enzyme inhibitors. Pharmacotherapy. 1989;9(2):82–87. doi: 10.1002/j.1875-9114.1989.tb04107.x. [DOI] [PubMed] [Google Scholar]
- 35.Fletcher AE, Palmer AJ, Bulpitt CJ. Cough with angiotensin converting enzyme inhibitors: how much of a problem? J Hypertens Suppl. 1994;12(2):S43–47. [PubMed] [Google Scholar]
- 36.Lee YJ, Chiang YF, Tsai JC. Severe nonproductive cough and cough-induced stress urinary incontinence in diabetic postmenopausal women treated with ACE inhibitor. Diabetes Care. 2000;23(3):427–428. doi: 10.2337/diacare.23.3.427. [DOI] [PubMed] [Google Scholar]
- 37.Gandhi TK, Burstin HR, Cook EF, et al. Drug complications in outpatients. J Gen Intern Med. 2000;15(3):149–154. doi: 10.1046/j.1525-1497.2000.04199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obergassel L, Carlsson J, Tebbe U. ACE inhibitor-associated interstitial lung infiltrates. Dtsch Med Wochenschr. 1995;120(38):1273–1277. doi: 10.1055/s-2008-1055475. German. [DOI] [PubMed] [Google Scholar]
- 39.Packard KA, Wurdeman RL, Arouni AJ. ACE inhibitor-induced bronchial reactivity in patients with respiratory dysfunction. Ann Pharmacother. 2002;36(6):1058–1067. doi: 10.1345/aph.1A332. [DOI] [PubMed] [Google Scholar]
- 40.Wood R. Bronchospasm and cough as adverse reactions to the ACE inhibitors captopril, enalapril and lisinopril. A controlled retrospective cohort study. Br J Clin Pharmacol. 1995;39(3):265–270. doi: 10.1111/j.1365-2125.1995.tb04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terajima T, Yamagata S, Satoh N, Ueda S. Meta-analysis: effect of ACE-inhibitors on outcomes in patients with renal insufficiency. P T. 2003;28(2):98–112. [Google Scholar]
- 42.Matchar DB, McCrory DC, Orlando LA, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008;148(1):16–29. doi: 10.7326/0003-4819-148-1-200801010-00189. [DOI] [PubMed] [Google Scholar]
- 43.Chalmers D, Dombey SL, Lawson DH. Post marketing surveillance of captopril (for hypertension): a preliminary report. Br J Clin Pharmacol. 1987;24(3):343–349. doi: 10.1111/j.1365-2125.1987.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeşil S, Yeşil M, Bayata S, Postaci N. ACE inhibitors and cough. Angiology. 1994;45(9):805–808. doi: 10.1177/000331979404500908. [DOI] [PubMed] [Google Scholar]
- 45.Amir M, Khan B, Tahir M. Incidence of angiotensin converting enzyme inhibitor induced cough. Professional Med J. 2005;12(4):435–439. [Google Scholar]
- 46.De Smedt RH, Denig P, Haaijer-Ruskamp FM, Jaarsma T. Perceived medication adverse effects and coping strategies reported by chronic heart failure patients. Int J Clin Pract. 2009;63(2):233–242. doi: 10.1111/j.1742-1241.2008.01954.x. [DOI] [PubMed] [Google Scholar]
- 47.Mackay FJ, Pearce GL, Mann RD. Cough and angiotensin II receptor antagonists: cause or confounding? Br J Clin Pharmacol. 1999;47(1):111–114. doi: 10.1046/j.1365-2125.1999.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cameron HA, Higgins TJ. Clinical experience with lisinopril. Observations on safety and tolerability. J Hum Hypertens. 1989;3(Suppl 1):177–186. [PubMed] [Google Scholar]
- 49.Rosenthal JR, Osowski U. Tolerability and efficacy of antihypertensive treatment with cilazapril in general practice. Cardiology. 1996;87(1):54–59. doi: 10.1159/000177060. [DOI] [PubMed] [Google Scholar]
- 50.Todd PA, Benfield P. Ramipril. A review of its pharmacological properties and therapeutic efficacy in cardiovascular disorders. Drugs. 1990;39(1):110–135. doi: 10.2165/00003495-199039010-00009. [DOI] [PubMed] [Google Scholar]
- 51.Kaplan NM CARE Investigators. The CARE Study: a post-marketing evaluation of ramipril in 11,100 patients. Clin Ther. 1996;18(4):658–670. doi: 10.1016/s0149-2918(96)80216-5. [DOI] [PubMed] [Google Scholar]
- 52.Speirs C, Wagniart F, Poggi L. Perindopril postmarketing surveillance: a 12 month study in 47,351 hypertensive patients. Br J Clin Pharmacol. 1998;46(1):63–70. doi: 10.1046/j.1365-2125.1998.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edwards C, Blowers DA, Pover GM. Fosinopril national survey: a postmarketing surveillance study of fosinopril (Staril) in general practice in the UK. Int J Clin Pract. 1997;51(6):394–398. [PubMed] [Google Scholar]
- 54.Haziza HM, Francillon A, Mottier D, Heintzmann F, Serrurier D. Antihypertensive action and predictive factors of efficacy of benazepril in mild-to-moderate hypertension: clinical trial in general medical practice on 16,987 patients. Ann Cardiol Angeiol (Paris) 1998;47(1):33–41. French. [PubMed] [Google Scholar]
- 55.Karpati P, Alberici M, Tocci G, Musumeci MB, Cosentino F, Volpe M. Long-term tolerability and efficacy of the fixed combination of manidipine and delapril in patients with essential hypertension. High Blood Press Cardiovasc Prev. 2003;10(2):81–86. [Google Scholar]
- 56.Yeo WW, Ramsay LE. Persistent dry cough with enalapril: incidence depends on method used. J Hum Hypertens. 1990;4(5):517–520. [PubMed] [Google Scholar]
- 57.Ravid D, Lishner M, Lang R, Ravid M. Angiotensin-converting enzyme inhibitors and cough: a prospective evaluation in hypertension and in congestive heart failure. J Clin Pharmacol. 1994;34(11):1116–1120. doi: 10.1002/j.1552-4604.1994.tb01989.x. [DOI] [PubMed] [Google Scholar]
- 58.Bangalore S, Kumar S, Messerli FH. Angiotensin-converting enzyme inhibitor associated cough: deceptive information from the Physicians’ Desk Reference. Am J Med. 2010;123(11):1016–1030. doi: 10.1016/j.amjmed.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 59.Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990;141(3):640–647. doi: 10.1164/ajrccm/141.3.640. [DOI] [PubMed] [Google Scholar]
- 60.Smyrnios NA, Irwin RS, Curley FJ. Chronic cough with a history of excessive sputum production. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Chest. 1995;108(4):991–997. doi: 10.1378/chest.108.4.991. [DOI] [PubMed] [Google Scholar]
- 61.Prabhu M, Palaian S, Malhotra A, et al. Therapeutic dimensions of ACE inhibitors – a review of literature and clinical trials. Kathmandu Univ Med J (KUMJ) 2005;3(3):296–304. [PubMed] [Google Scholar]
- 62.Szmidt M, Minc P. Cough, bronchoconstriction and bronchial hyper-reactivity in relation to treatment with angiotensin-converting enzyme. Pol Merkur Lekarski. 1999;6(35):281–285. Polish. [PubMed] [Google Scholar]
- 63.Fuller RW. Cough associated with angiotensin-converting enzyme inhibitors. J Hum Hypertens. 1989;3(Suppl 1):159–161. [PubMed] [Google Scholar]
- 64.Agustí A, Bonet S, Arnau JM, Vidal X, Laporte JR. Adverse effects of ACE inhibitors in patients with chronic heart failure and/or ventricular dysfunction: meta-analysis of randomised clinical trials. Drug Saf. 2003;26(12):895–908. doi: 10.2165/00002018-200326120-00004. [DOI] [PubMed] [Google Scholar]
- 65.Os I, Bratland B, Dahløf B, Gisholt K, Syvertsen JO, Tretli S. Lisinopril or nifedipine in essential hypertension? A Norwegian multicenter study on efficacy, tolerability and quality of life in 828 patients. J Hypertens. 1991;9(12):1097–1104. [PubMed] [Google Scholar]
- 66.Strocchi E, Valtancoli G, Ricci C, Malini PL, Bassein L, Ambrosioni E. Post-marketing studies of subjective side effects; a case for strict methodological criteria and careful analysis of data. Pharmacol Res. 1992;25(Suppl 1):79–80. doi: 10.1016/1043-6618(92)90549-q. [DOI] [PubMed] [Google Scholar]
- 67.Gibson GR. Enalapril-induced cough. Arch Intern Med. 1989;149(12):2701–2703. [PubMed] [Google Scholar]
- 68.Tseng DS, Kwong J, Rezvani F, Coates AO. Angiotensin-converting enzyme-related cough among Chinese-Americans. Am J Med. 2010;123(2):183. e11–e15. doi: 10.1016/j.amjmed.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 69.McDowell SE, Coleman JJ, Ferner RE. Systematic review and metaanalysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ. 2006;332(7551):1177–1181. doi: 10.1136/bmj.38803.528113.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morimoto T, Gandhi TK, Fiskio JM, et al. Development and validation of a clinical prediction rule for angiotensin-converting enzyme inhibitor-induced cough. J Gen Intern Med. 2004;19(6):684–691. doi: 10.1111/j.1525-1497.2004.30016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dykewicz MS. Cough and angioedema from angiotensin-converting enzyme inhibitors: new insights into mechanisms and management. Curr Opin Allergy Clin Immunol. 2004;4(4):267–270. doi: 10.1097/01.all.0000136759.43571.7f. [DOI] [PubMed] [Google Scholar]
- 72.Semple PF. Putative mechanisms of cough after treatment with angiotensin converting enzyme inhibitors. J Hypertens Suppl. 1995;13(3):S17–21. doi: 10.1097/00004872-199509003-00004. [DOI] [PubMed] [Google Scholar]
- 73.Morice AH, Brown MJ, Higenbottam T. Cough associated with angiotensin converting enzyme inhibition. J Cardiovasc Pharmacol. 1989;13(Suppl 3):S59–S62. doi: 10.1097/00005344-198900133-00015. [DOI] [PubMed] [Google Scholar]
- 74.Trifilieff A, Da Silva A, Gies JP. Kinins and respiratory tract diseases. Eur Respir J. 1993;6(4):576–587. [PubMed] [Google Scholar]
- 75.Fox AJ, Lalloo UG, Belvisi MG, Bernareggi M, Chung KF, Barnes PJ. Bradykinin-evoked sensitization of airway sensory nerves: a mechanism for ACE-inhibitor cough. Nat Med. 1996;2(7):814–817. doi: 10.1038/nm0796-814. [DOI] [PubMed] [Google Scholar]
- 76.Lalloo UG, Barnes PJ, Chung KF. Pathophysiology and clinical presentations of cough. J Allergy Clin Immunol. 1996;98(5 Pt 2):S91–S96. discussion S96–97. [PubMed] [Google Scholar]
- 77.Yeo WW, Chadwick IG, Kraskiewicz M, Jackson PR, Ramsay LE. Resolution of ACE inhibitor cough: changes in subjective cough and responses to inhaled capsaicin, intradermal bradykinin and substance-P. Br J Clin Pharmacol. 1995;40(5):423–429. [PMC free article] [PubMed] [Google Scholar]
- 78.Mas S, Gassò P, Alvarez S, et al. Pharmacogenetic predictors of angiotensin-converting enzyme inhibitor-induced cough: the role of ACE, ABO, and BDKRB2 genes. Pharmacogenet Genomics. 2011;21(9):531–538. doi: 10.1097/FPC.0b013e328348c6db. [DOI] [PubMed] [Google Scholar]
- 79.Mukae S, Aoki S, Itoh S, Iwata T, Ueda H, Katagiri T, Bradykinin B. (2) receptor gene polymorphism is associated with angiotensin-converting enzyme inhibitor-related cough. Hypertension. 2000;36(1):127–131. doi: 10.1161/01.hyp.36.1.127. [DOI] [PubMed] [Google Scholar]
- 80.O’Connell F, Thomas VE, Pride NB, Fuller RW. Capsaicin cough sensitivity decreases with successful treatment of chronic cough. Am J Respir Crit Care Med. 1994;150(2):374–380. doi: 10.1164/ajrccm.150.2.8049818. [DOI] [PubMed] [Google Scholar]
- 81.Nikpoor B, Duan QL, Rouleau GA. Acute adverse reactions associated with angiotensin-converting enzyme inhibitors: genetic factors and therapeutic implications. Expert Opin Pharmacother. 2005;6(11):1851–1856. doi: 10.1517/14656566.6.11.1851. [DOI] [PubMed] [Google Scholar]
- 82.Allen TL, Gora-Harper ML. Cromolyn sodium for ACE inhibitor-induced cough. Ann Pharmacother. 1997;31(6):773–775. [PubMed] [Google Scholar]
- 83.Dicpinigaitis PV. Use of baclofen to suppress cough induced by angiotensin-converting enzyme inhibitors. Ann Pharmacother. 1996;30(11):1242–1245. doi: 10.1177/106002809603001106. [DOI] [PubMed] [Google Scholar]
- 84.Cazzola M, Matera MG, Liccardi G, De Prisco F, D’Amato G, Rossi F. Theophylline in the inhibition of angiotensin-converting enzyme inhibitor-induced cough. Respiration. 1993;60(4):212–215. doi: 10.1159/000196201. [DOI] [PubMed] [Google Scholar]
- 85.Gilchrist NL, Richards AM, March R, Nicholls MG. Effect of sulindac on angiotensin converting enzyme inhibitor-induced cough: randomised placebo-controlled double-blind cross-over study. J Hum Hypertens. 1989;3(6):451–455. [PubMed] [Google Scholar]
- 86.Luque CA, Vazquez Ortiz M. Treatment of ACE inhibitor-induced cough. Pharmacotherapy. 1999;19(7):804–810. doi: 10.1592/phco.19.10.804.31563. [DOI] [PubMed] [Google Scholar]
- 87.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326(7404):1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mancia G, De Backer G, Dominiczak A, et al. Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. 2007 guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25(6):1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 89.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 90.Andersen RS, Christensen S. Angiotensin II antagonists versus ACE inhibitors in the treatment of raised blood pressure. Ugeskr Laeger. 2001;163(50):7036–7039. Danish. [PubMed] [Google Scholar]
- 91.Lacourcière Y, Lefebvre J. Modulation of the renin-angiotensinaldosterone system and cough. Can J Cardiol. 1995;11(Suppl F):33F–39F. [PubMed] [Google Scholar]
- 92.Yusuf S, Teo KK, Pogue J, et al. ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 93.Lakhdar R, Al-Mallah MH, Lanfear DE. Safety and tolerability of angiotensin-converting enzyme inhibitor versus the combination of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker in patients with left ventricular dysfunction: a systematic review and meta-analysis of randomized controlled trials. J Card Fail. 2008;14(3):181–188. doi: 10.1016/j.cardfail.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 94.Evangelista S, Manzini S. Antioxidant and cardioprotective properties of the sulphydryl angiotensin-converting enzyme inhibitor zofenopril. J Int Med Res. 2005;33(1):42–54. doi: 10.1177/147323000503300103. [DOI] [PubMed] [Google Scholar]
- 95.Przyklenk K, Kloner RA. Angiotensin converting enzyme inhibitors improve contractile function of stunned myocardium by different mechanisms of action. Am Heart J. 1991;121(5):1319–1330. doi: 10.1016/0002-8703(91)90134-4. [DOI] [PubMed] [Google Scholar]
- 96.Cialdai C, Giuliani S, Valenti C, Tramontana M, Maggi CA. Differences between zofenopril and ramipril, two ACE inhibitors, on cough induced by citric acid in guinea pigs: role of bradykinin and PGE2. Naunyn Schmiedebergs Arch Pharmacol. 2010;382(5–6):455–461. doi: 10.1007/s00210-010-0563-0. [DOI] [PubMed] [Google Scholar]
- 97.Mutolo D, Bongianni F, Evangelista S, Cinelli E, Pantaleo T. Effects of zofenopril and ramipril on cough reflex responses in anesthetized and awake rabbits. J Cardiovasc Pharmacol Ther. 2010;15(4):384–392. doi: 10.1177/1074248410379413. [DOI] [PubMed] [Google Scholar]
- 98.Kohlstedt K, Busse R, Fleming I. Signaling via the angiotensin-converting enzyme enhances the expression of cyclooxygenase-2 in endothelial cells. Hypertension. 2005;45(1):126–132. doi: 10.1161/01.HYP.0000150159.48992.11. [DOI] [PubMed] [Google Scholar]
- 99.Swartz SL, Williams GH. Angiotensin-converting enzyme inhibition and prostaglandins. Am J Cardiol. 1982;49(6):1405–1409. doi: 10.1016/0002-9149(82)90351-4. [DOI] [PubMed] [Google Scholar]