Abstract
Low-income, minority women are more likely to be undertreated for breast cancer (BC) treatment-related symptoms than whites. This study assessed the impact of patient–physician communication on symptom resolution. A cross-sectional, California statewide survey was conducted among 921 low-income women with BC. Ethnic/racial differences in BC treatment-related symptoms (pain, nausea/vomiting, depression) reporting and physician’ awareness of these symptoms were assessed by patient report. Multivariate logistic regression models were used to investigate the impact of patient–physician communication on symptom resolution. Depression was the most common symptom reported by patients (66%), yet physicians were the least aware of it (26.3%), especially among less-acculturated Latinas (18.9%) and Asian/Pacific Islanders (14%; P < 0.001). Greater patient-perceived self-efficacy in communication with physicians and greater physician awareness of the symptom positively predicted pain resolution, controlling for sociodemographic variables, comorbidity, and treatment received (AOR = 1.05, P < 0.0001; AOR = 6.12, P < 0.001). Physician awareness was a significant determinant of depression resolution (AOR = 13.46, P < 0.001). Yet patient-perceived self-efficacy played a much more important role than physicians’ awareness in nausea resolution (AOR = 1.04, P = 0.0002). Less-acculturated Latinas tended to achieve less symptom resolution than whites, while this negative impact disappeared or was moderated after patient–physician communication was considered. This study suggests that physicians under-recognized depression, especially among Latinas. The resolution of BC treatment-related symptoms can be addressed by appropriate educational interventions targeted at patient–physician communication. Effective patient–physician communication can moderate disparities in symptom resolution among Latinas, regardless of language acculturation.
Keywords: Breast cancer treatment-related symptoms, Physician–patient communication, Self-efficacy, Medically underserved, Ethnic disparities
Introduction
In the United States, breast carcinoma is the most common malignancy among women; it is estimated that approximately 182,460 new will be diagnosed in 2008 [1]. However, since 1990, early detection and improved treatment have dramatically increased the life-expectancy of women with breast cancer [2-4], leading to an overall 5-year survival rate of 98% for local-stage disease and 81% for regional-stage disease [5]. With these advances in breast cancer care, the goal of breast cancer therapy has changed from simply survival to enhancing patients’ quality of life.
Quality of life is an important endpoint in evaluating the sense of well-being related to disease. Although there is no universal conceptual definition of quality of life, it has usually been defined as a multidimensional measurement of physical, functional, emotional and social well-being [6]. The presence of post breast cancer treatment symptoms indicates perceived disruption in bodily functions [7], and this perception is used as a major measurement of quality of life among breast cancer patients [8].
Pain, nausea, and depression are the three most common treatment-related symptoms for breast cancer patients [9-11]. Reports of these symptoms post treatment by breast cancer patients may vary by treatment received. Chemotherapy and radiation therapy have been associated with nausea and vomiting, as well as fatigue [12, 13]. Moreover, breast cancer patients who have undergone chemotherapy may experience significant depression [14]. Breast cancer surgery, such as mastectomy and lumpectomy, has been found to be associated with a post-surgical pain syndrome [15].
In research among general breast cancer patient populations, patient characteristics, such as age, marital status, income, education, and comorbidity have been documented to be associated with reporting of BC treatment-related symptoms [11, 16-18]. However, very few studies have studied the ethnic/racial differences for BC treatment-related symptoms, and their findings are inconsistent. Some researchers have reported that ethnicity/race was not a predictor of quality of life for breast cancer survivors [19], while others found that white women were more likely to report severe symptoms than minority women [20]. Still another study found that African–American breast cancer survivors reported greater pain than white women and that Latinas reported higher rates of pain and fatigue, as well as depression than whites [13].
Regardless of the variation in symptom reporting among ethnic/racial groups, it is well documented that minority group patients are more likely to be undertreated for cancer treatment-related symptoms than white patients [21]. The implementation of successful symptom management relies on appropriate information exchange between the physician and the patient. Patient self-efficacy in interacting with physicians, that is, their perceived ability in obtaining needed medical information and attention regarding their chief medical concerns from their physicians [22], is a key component for this information transfer process. It has been suggested that vulnerable patient populations might receive less optimal care due to a decreased sense of control over the health care process [23] and less confidence in their ability to get physicians to attend to their health concerns [24]. Thus, interventions to increase patients’ self-efficacy and empower them within the patient–physician encounter might result in a higher rate of resolution of their symptoms. On the physician side, regardless of who initiates information exchange, it is ultimately the physician’s awareness of the symptoms that provides the opportunity to discuss treatment options with the patient, thereby facilitating the possibility of symptom resolution. These points highlight a need to study the potential role of patient–physician communication on symptom resolution post treatment. However, to our knowledge, no study has investigated this issue, especially in low-income and underserved breast cancer patients.
Unequal distribution of the breast cancer burden across socioeconomic groups has been well-documented [25, 26], and low income women may be at particular risk for undertreatment of symptoms and poorer quality of life after BC diagnosis due to their decreased self-efficacy in interacting with physicians [27]. A better understanding of the role of patient–physician communication on treatment-related symptom resolution among low-income women may help to improve quality of life for vulnerable populations of women after a diagnosis of BC.
This study of low-income women with breast cancer in California had two aims: (1) to describe the demographic profile of women’s report of several prevalent post treatment symptoms related to breast cancer (i.e., pain, nausea/vomiting, depression); and (2) to assess the impact of patient self-efficacy in communicating with physicians and physicians’ awareness of these post-treatment symptoms on resolution of these symptoms as a function of ethnicity/race.
Methods
This study was part of a longitudinal survey of low-income women living in California who were aged 18 years and older and approximately 6 months after the diagnosis of breast cancer.
Study sample
Our study population consisted of English-or Spanish-speaking women with newly diagnosed breast cancer (diagnosed 6 months prior to being interviewed), who were enrolled in the Medi-Cal (California’s Medicaid) Breast and Cervical Cancer Treatment Program (BCCTP). Women who were not cognitively able to participate, did not speak English or Spanish, or were receiving treatment for another cancer besides breast cancer were excluded from this study. The BCCTP is a relatively new coverage option legislated by the federal government as part of the Breast and Cervical Cancer Prevention and Treatment Act of 2000 and funded through Medicaid. BCCTP provides coverage to un- and under-insured low-income women (≤200% federal poverty level) diagnosed with breast and cervical cancer who require cancer treatment.
Potential participants were identified through partnership with the California Department of Health Services and were invited to complete a one-hour baseline telephone survey in either English or Spanish. A total of 921 women completed the telephone interview, with a final response rate of 61.1% among 1,509 potential participants. The baseline survey was conducted a mean of 180 days (SD = 20.8) after definitive diagnosis of breast cancer. Non-responders were older than responders by 2 years (mean 52 vs. 50 years, respectively, P < 0.05) and were less likely to be Latina (46 vs. 53%, respectively P < 0.05). Further details of the design and flow of the parent study can be found in a previously published article [28].
Measures
The outcome measurements were (1) occurrence of symptoms and (2) symptom resolution, and were assessed with a series of questions for pain, nausea and depression. Specifically, the questions elicited information about how often the patient experienced these symptoms and how often these symptoms had resolved. For each symptom, both questions had a 0–5 response set, indicating the woman’s experience of the symptom “none of the time,” “a little of the time,” “some of the time,” “most of the time” and “all of the time”. Participants who answered “some of the time,” “most of the time,” and “all of the time” were defined as having or having had the symptom. For symptom resolution, participants who answered “most of the time” or “all of the time” were defined as having or having had significant symptom resolution, while participants who answered “none of the time,” “a little of the time,” and “some of the time” were defined as having less than optimal resolution.
The principal independent variables were measures of: (1) physician’s awareness of the symptoms, and (2) patient-perceived self-efficacy in patient–physician communication. The physician’s awareness of the symptom was measured by participant self report on a 5 point Likertscale question. Answers were classified as indicating “more awareness” if the study participants said that the physicians were aware of their symptoms “most of the time” or “all of the time”, and as indicating “less awareness” if participants answered that the physicians seemed to be aware of their symptoms “none of the time,” “a little of the time” or “some of the time”. Patient-perceived self-efficacy was measured using the previously validated Perceived Efficacy in Patient–Physician Interactions (PEPPI) questionnaire [29]. PEPPI measures participants’ perceived ability to obtain needed medical information and attention to their chief medical concerns from their physicians. Respondents are asked to rate each of 5 items from 0 (not confident at all) to 10 (extremely confident); and the resulting PEPPI sum scale has a range from 0 to 50. Cronbach’s alpha PEPPI for this scale was 0.96 in this sample. PEPPI was categorized into quartiles for analysis since it was skewed.
Other independent variables included age at diagnosis, ethnicity/race (white, African–American, less-acculturated Latina, more-acculturated Latina, Asian/Pacific Islander), married/partnered (yes, no), education (less than high school, high school graduate or more), any major comorbidity (yes, no), and breast cancer treatment received [chemotherapy (yes, no), surgery (lumpectomy, mastectomy, neither)]. Income was not included, as the sample population was by definition uniformly poor. Acculturation to the US culture among Latina women was determined by the five-item Marin Acculturation Scale [30]. This scale is based on language use and preference. The internal consistency reliability was 0.99 for this scale. Latinas were defined as being “more acculturated” if they were equally or more comfortable or conversant with English than Spanish, “less acculturated” otherwise.
Data analysis
Summary statistics, including means and percentages, were calculated to describe participants’ demographic and clinical characteristics and document symptom prevalence. Both unadjusted and adjusted logistic regression analyses were first conducted to identify correlates of the presence of each of the three BC treatment-related symptoms. Similar analyses were then used to investigate relationships among physician awareness, PEPPI and the other independent variables and resolution of these symptoms. In addition, staged logistic regression models were applied to assess the impact of physicians’ awareness and patient’s self-efficacy on moderating the effects of ethnicity/race on symptom resolution.
Multicollinearity was examined and found not to be a problem. The Hosmer–Lemeshow test indicated adequate fit for all of the multiple logistic regression models. All statistical analyses were conducted using SAS version 9.1; P values < 0.05 were considered to be statistically significant.
Results
Descriptive statistics
A total of 921 patients were included in this study. Table 1 shows the sociodemographic and health characteristics of the participants. Their average age was about 51 years and they were mainly Latina (54%) and White (32%). Around half were married or partnered and slightly more than half had graduated from high school. Most of the participants were unemployed (82%) with an annual household income less than $20,000 (87%). Approximately 30% of the participants indicated having been diagnosed with at least one significant comorbidity.
Table 1.
Sociodemographic and health characteristics of the study sample (N = 921)
| Value | |
|---|---|
| Age | |
| Mean (SD) | 50.8(9.5) |
| Range | 25.0–85.0 |
| Ethnicity, N (%) | |
| White | 292(31.7) |
| Latina | 492(53.4) |
| African–American | 54(5.9) |
| Asian/Pacific Islander | 68(7.4) |
| Other | 15(1.6) |
| Education, N (%) | |
| <High school | 377(40.9) |
| ≥High school | 534(59.1) |
| Employment | |
| Not employed | 746(82.1) |
| Employed | 165(17.9) |
| Marital status | |
| Married/partner | 444(48.2) |
| Widowed/divorced/separated | 300(32.6) |
| Single | 177(19.2) |
| Comorbidity, N (%) | |
| None | 646(70.1) |
| Any | 275(29.9) |
The most prevalent self-reported, post BC treatment symptom was depression, with 577 women (63%) reporting some experience of it (Fig. 1). The next most frequent symptom was pain, occurring in 56% of the participants. However, pain was the most recognized symptom by physicians; among patients who experienced pain, over 70% reported their physician(s) being aware of this symptom. Nausea was reported less frequently (42%) than depression and pain in the study sample. However, like pain, nausea also elicited physician recognition about two-thirds of the time. In contrast, physicians were least likely to recognize patients’ depression. Only one-quarter of physicians were aware of depression even though it was the most common symptom reported in this sample. Physicians’ recognition of depression was particularly low among less-acculturated Latinas and Asian/Pacific Islanders. Compared to whites (36%), both minorities were less likely to get physicians’ recognition of depression (less-acculturated Latinas: 19% and Asian/Pacific Islander: 14%, P < 0.001).
Fig. 1.
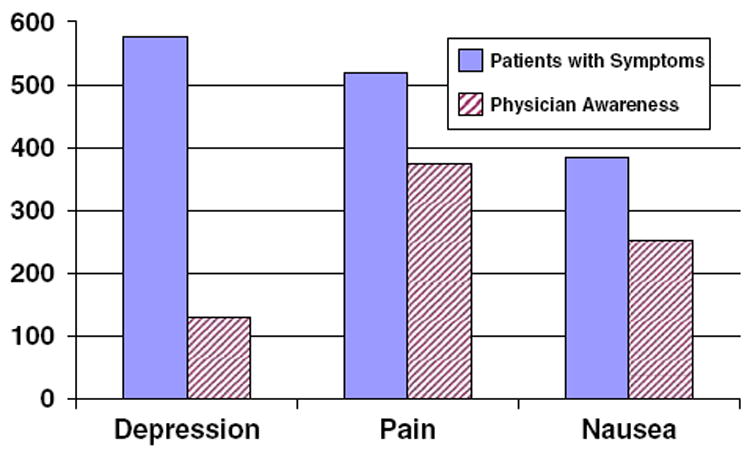
Number of patients and physician awareness of breast cancer treatment-related symptoms of the study sample (N = 921)
Factors associated with the presence of symptoms
Table 2 presents associations between selected factors and the presence of each of the three study symptoms in multivariate analyses. After controlling for patient characteristics and treatment received, nausea was strongly associated with chemotherapy treatment and the presence of one or more co-morbidities, while higher educational background was inversely related to experiencing nausea. Similarly, having one or more co-morbidities was associated with the presence of pain; yet older women were less likely to report pain. In contrast to the positive association with nausea, less-acculturated Latinas were significantly less likely to report pain. The only significant predictor for the presence of depression was age and younger women were more likely to report depression.
Table 2.
Multivariate logistic regression model–factors associated with breast cancer treatment related symptoms
| Depression | Nausea | Pain | |
|---|---|---|---|
| AOR(95%CI) | AOR(95%CI) | AOR(95%CI) | |
| Age | 0.98(0.97–0.99)† | 0.98(0.96–1.00) | 0.98(0.96–0.99)† |
| Race/ethnicity | |||
| White | – | – | – |
| African–American | 0.81(0.45–1.48) | 1.13(0.57–2.25) | 0.67(0.37–1.22) |
| Less-acculturated Latinas | 1.48(0.99–2.19) | 1.79(1.16–2.76)† | 0.62(0.43–0.90)† |
| More-acculturated Latinas | 1.10(0.63–1.92) | 2.09(1.02–4.26)† | 1.23(0.66–2.31) |
| Asian/Pacific Islander | 1.15(0.77–1.70) | 1.11(0.60–1.2.07) | 0.74(0.43–1.27) |
| Education | |||
| <High school | – | – | – |
| ≥High school | 1.15(0.77–1.70) | 0.56(0.36–0.88)† | 0.75(0.51–1.08) |
| Married/partnered | |||
| No | – | – | – |
| Yes | 0.78(0.58–1.05) | 0.94(0.68–1.32) | 0.93(0.70–1.24) |
| Comorbidity | |||
| None | – | – | – |
| Any | 1.04(0.76–1.42) | 1.59(1.11–2.27)† | 1.49(1.10–2.01)† |
| Received treatment | |||
| Chemotherapy | N/A | ||
| No | – | – | |
| Yes | 1.04(0.76–1.42) | 14.58(9.79–21.73)† | |
| Surgery | N/A | ||
| No | – | – | |
| Lumpectomy | 0.92(0.56–1.49) | 1.21(0.77–1.90) | |
| Mastectomy | 0.93(0.68–1.26) | 1.18(0.75–1.85) |
AOR adjusted odds ratio, CI confidence interval, N/A not applicable
P < 0.05
Factors associated with the resolution of symptoms
Unadjusted analyses indicate that symptom resolution varied somewhat by ethnicity/race. In particular, significant differences existed between less-acculturated Latinas and whites for depression (Table 3) and pain (Table 5). Higher educational attainment and greater physician awareness were also positively associated with symptom resolution for depression and pain. Additionally, patients with one or more co-morbidities were more likely to achieve resolution of depression, and patients with higher perceived self-efficacy were more likely to report resolution of their pain. For nausea, only BC treatment with chemotherapy and perceived patient self-efficacy were positively associated with resolution (Table 4).
Table 3.
Multivariate logistic regression model–factors associated with resolution of breast cancer treatment related symptoms (N = 192)
| Depression
|
||
|---|---|---|
| OR (95% CI) | AOR (95%CI) | |
| Age | 1.02(0.99–1.05)† | 1.03(0.99–1.07) |
| Race/ethnicity | ||
| White | – | – |
| African–American | 0.84(0.28–2.49) | 0.81(0.25–2.59) |
| Less-acculturated Latinas | 0.34(0.17–0.68)†† | 0.35(0.14–0.88)† |
| More-acculturated Latinas | 0.77(0.23–2.65) | 0.67(0.18–2.49) |
| Asian/Pacific Islander | 1.08(0.32–3.65) | 1.42(0.38–5.35) |
| Education | ||
| < High school | – | – |
| ≥High school | 1.63(0.79–3.36)† | 0.86(0.31–2.35) |
| Married/partnered | ||
| No | – | – |
| Yes | 1.07(0.88–1.29) | 0.98(0.48–2.02) |
| Comorbidity | ||
| None | – | – |
| Any | 1.88(1.13–3.13)† | 1.20(0.62–2.31) |
| Received treatment | ||
| Chemotherapy | ||
| No | – | – |
| Yes | 0.95(0.50–1.81) | 1.03(0.49–2.18) |
| Surgery | ||
| No | – | – |
| Lumpectomy | 0.85(0.36–2.03) | 0.82(0.29–2.33) |
| Mastectomy | 1.07(0.44–2.61) | 0.81(0.28–2.29) |
| Physician awareness | ||
| Less | – | – |
| More | 2.12(1.10–4.10)† | 2.45(1.19–5.08)† |
| PEPPIa | 1.03(0.99–1.05) | 1.02(0.99–1.05) |
OR odds ratio, AOR adjusted odds ratio, CI confidence interval
P < 0.05;
P < 0.0001
Perceived efficacy in physician–patient interactions
Table 5.
Multivariate logistic regression model–factors associated with resolution of breast cancer treatment related symptoms (N = 407)
| Pain
|
||
|---|---|---|
| OR (95% CI) | AOR (95%CI) | |
| Age | 0.99(0.97–1.02) | 0.99(0.97–1.02) |
| Race/ethnicity | ||
| White | – | – |
| African–American | 0.58(0.24–1.41) | 0.43(0.17–1.08) |
| Less-acculturated Latinas | 0.50(0.32–0.78)†† | 0.63(0.35–1.14) |
| More-acculturated Latinas | 0.87(0.37–2.02) | 0.80(0.33–1.94) |
| Asian/Pacific Islander | 0.80(0.35–1.82) | 0.95(0.40–2.24) |
| Education | ||
| <High School | – | – |
| ≥High School | 1.65(1.07–2.55)† | 1.10(0.60–2.00) |
| Married/partnered | ||
| No | – | – |
| Yes | 1.05(0.93–1.18) | 0.86(0.55–1.32) |
| Comorbidity | ||
| None | – | – |
| Any | 1.00(0.67–1.50) | 0.94(0.60–1.45) |
| Received treatment | ||
| Surgery | ||
| No | – | – |
| Lumpectomy | 0.70(0.37–1.35) | 0.79(0.39–1.60) |
| Mastectomy | 1.05(0.54–2.03) | 1.08(0.53–2.19) |
| Physician awareness | ||
| Less | – | – |
| More | 2.35(1.37–4.03)†† | 2.72(1.53–4.83)†† |
| PEPPIa | 1.04(1.02–1.06)†† | 1.04(1.02–1.07)†† |
OR odds ratio, AOR adjusted odds ratio, CI confidence interval
P < 0.05;
P < 0.0001
Perceived efficacy in physician–patient interactions
Table 4.
Multivariate logistic regression model–factors associated with resolution of breast cancer treatment related symptoms (N = 443)
| Nausea
|
||
|---|---|---|
| OR (95% CI) | AOR (95%CI) | |
| Age | 0.98(0.97–1.00) | 1.00(0.98–1.03) |
| Race/ethnicity | ||
| White | – | – |
| African–American | 1.43(0.59–3.45) | 1.41(0.55–3.65) |
| Less-acculturated Latinas | 0.76(0.49–1.18) | 0.68(0.38–1.20) |
| More-acculturated Latinas | 2.29(0.98–5.36) | 2.14(0.90–5.12) |
| Asian/Pacific Islander | 0.74(0.32–1.70) | 1.01(0.41–2.48) |
| Education | ||
| <High School | – | – |
| ≥High School | 1.01(0.69–1.49) | 0.55(0.32–0.93)† |
| Married/partnered | ||
| No | – | – |
| Yes | 1.03(0.92–1.15) | 0.91(0.60–1.39) |
| Comorbidity | ||
| None | – | – |
| Any | 0.95(0.64–1.41) | 1.00(0.64–1.56) |
| Received treatment | ||
| Chemotherapy | ||
| No | – | – |
| Yes | 3.35(1.44–7.81)†† | 1.04(0.38–2.84) |
| Physician awareness | ||
| Less | – | – |
| More | 1.68(0.94–3.00) | 1.03(0.53–2.00) |
| PEPPIa | 1.04(1.02–1.05)†† | 1.04(1.02–1.06)†† |
OR odds ratio, AOR adjusted odds ratio, CI confidence interval
P < 0.05;
P < 0.0001
Perceived efficacy in physician–patient interactions
Multiple logistic regression models with adjusted OR’s for resolution of each symptom are also shown in Tables 3, 4, and 5. Both physician awareness and patient perceived self-efficacy in communicating with physicians continued to have significant positive associations with resolution of pain (Table 5). Physician awareness remained a significant correlate of resolution of depression when potential confounders were controlled, while self-efficacy became less important (Table 3). In contrast, self-efficacy was positively associated with resolution of nausea, while physician awareness was not important (Table 4).
The ethnic/racial differences in resolution of depression persisted in the adjusted model (Table 3). Less-acculturated Latinas were significantly less likely to have resolution of depression than whites. However, the less-acculturated Latina versus white disparity in pain resolution did not persist in the final model (Table 5). Staged model analysis (data not shown) adding physician awareness and perceived patient self-efficacy to the model as a last step attenuated the differences between less-acculturated Latinas and whites in resolution of pain. Higher educational background was inversely associated with resolution of nausea, while PEPPI was positively associated with resolution of symptoms in both unadjusted and adjusted models.
Discussion
As breast cancer survival rates continue to improve, there is an increased interest in studying BC treatment-related quality of life. While most previous studies have focused on investigating the impact of patients’ ethnicity/race, sociodemographic and clinical factors on disparities in prevalence and/or resolution of breast cancer-related symptoms [11, 16-18], this study added a further dimension of patient–physician communication. This study aimed to increase understanding of these disparities by investigating whether they resulted at least in part, from differences in physician–patient communication. To our knowledge, this is the first study that has attempted to quantify both physician and patient aspects of the patient–physician interaction and examined their independent impact on resolution of breast cancer-related symptoms. Further, the study was conducted in a large population of low-income, medically underserved women with substantial ethnic/racial minority representation and at risk for undertreatment, with its attendant quality of life and survivorship implications.
The diagnosis and treatment of breast cancer is a relatively complicated and intense process and is commonly associated with depressive symptoms [31]. Therefore, it is not surprising that depression was the most prevalent symptom in this study sample. Although Latina and Asian/Pacific Islander ethnicity, higher educational attainment, BC treatment with chemotherapy, and being without a partner have all been shown to be risk factors for depression in previous studies [13, 14], the only significant predictor for depression in our sample was age; older women were less likely to report depression. In general, older women have been found to experience depression less frequently than younger women [32].
Less than 30% of the participants who reported depression were recognized as such by physicians in this study. This finding is consistent with previous studies showing that under-treatment of depression is common among cancer patients [33, 34]. Not surprisingly, the findings revealed that under-awareness of depression by physicians, as reported by patients, was a major factor in non-resolution of depressive symptoms. Often, physicians tend to focus on cancer treatment details during patient visits and may overlook psychiatric symptoms [22]. In addition, physicians may feel less well-equipped to deal with psychiatric issues than with medical problems [35].
In this sample, less-acculturated Latinas were particularly unlikely to achieve resolution of their depression. Patients may be reluctant to report their depressive symptoms because of culturally based negative attitudes about depression [36], embarrassment about discussing emotional problems with physicians, 25 fear of being stigmatized as having a psychological problem [37], or worry that emotional complaints may distract the physician from curative efforts [38]. The fact that patient self-efficacy was associated with symptom resolution and that physician awareness was a strong predictor further underscores the need for physician initiative with regard to diagnosing this condition as one means of overcoming social stigma. And, lack of physician awareness of depression may, in fact, result in patient hesitancy to report emotional problems. Thus, physicians should not rely on patient reports/complaints, but should more actively question patients to be able to discern and diagnose psychological problems.
Pain is another common symptom among breast cancer patients. Similar to other researchers’ findings [11, 12], younger age and co-morbidity were inversely associated with presence of pain. In addition, less-acculturated Latinas were less likely to report pain than whites. This finding contrasts with previous studies, in which Latinas reported more pain than whites [13, 39]. However, other studies have shown that ethnic minorities, including Latinas, report fewer severe symptoms during cancer treatment [18]. These mixed findings raise an important issue about whether less-acculturated Latinas actually experience less pain or whether they are more reluctant to report the symptom due to cultural factors and to beliefs and attitudes about pain.
It is also well documented that ethnic minority patients are undertreated for cancer-related pain [40, 41]. Our results are consistent with this contention in that less-acculturated Latinas were less likely to get pain resolution than whites when physician–patient communication was not controlled. However, this disparity was no longer apparent when physician–patient communication was considered and the remaining ethnic minority groups did not have significantly lower odds of pain resolution than whites. Physician–patient communication may play an especially important role in resolving pain for low income, less-acculturated Latinas and may overcome language barriers in the group.
Lack of information on pain management has been identified as a significant barrier for pain control among minorities [40]. Latinas have also been reported to be reluctant to take pain medications due to worries about their side effects [36]. In one study, very few Latinos reported receiving information on possible side effects from pain medications or how to manage them in advance; further, one-third of those who did receive information stated that they had to initially bring up concerns about pain control to their physicians [36]. In that study, Latinos also tended to not adhere to their prescribed regimens because of concerns about possible addiction to pain medications. In addition, it appears that Latinos may hold certain beliefs that constitute barriers to timely pain resolution, such as one should be strong enough to not rely on pain medications and one should avoid taking medications until one has severe pain [42].
Another significant barrier to pain control among Latinas may be underestimated pain severity by physicians [43]. Language and cultural barriers may make it difficult for minority individuals to disclose symptoms. Reluctance to report pain might lead to less physician awareness of the symptom and inadequate pain assessment. In one study, over two-thirds of Latinas reported no quantified measurement of their pain and 35% of them stated they received inadequate treatment for their pain [35]. However, in our study, aspects of patient–physician communication appeal to overcome language and cultural barriers on resolution of pain symptoms.
In contrast to depression and pain symptoms, there were no ethnic/racial disparities in the presence and resolution of nausea. Women with higher educational levels were less likely to experience nausea than those with lower educational levels. One possible explanation is that more educated women might have had more resources to obtain information on preventing and treating nausea. Nausea was found to be strongly associated with chemotherapy and the presence of one or more co-morbitidies, consistent with findings of previous studies [9, 10]. However, patients who undergo chemotherapy usually receive antiemetic treatment at the same time as part of a routine protocol, which has been demonstrated to be effective in improving the control of nausea [44-46]. This protocol may be one of the reasons patient-perceived self-efficacy was significantly associated with resolution of nausea, while physicians’ awareness was not. Since antiemetic treatment has been widely adopted as standard care for patients who undergo chemotherapy, physicians may pay less attention to patients’ nausea due to automatic reliance on the effectiveness of antiemetic protocols. As a result, patients experiencing nausea may need to be more proactive in their communication with physicians to address their unresolved symptoms. Surprisingly, women with higher educational attainment were less likely to obtain resolution of their nausea, controlling for other factors. It is possible that patients with higher educational levels might have had greater expectations of treatment outcomes, which resulted in less satisfaction with symptom relief that may have biased reports of resolution.
Few studies have examined the effect of patient–physician communication on breast cancer treatment-related symptom resolution. The three symptoms focused on in this study are the most common treatment related symptoms among breast cancer patients. A better understanding of factors related to these symptoms and to their control could have important implications for improving the quality of life for women with breast cancer. Another strength of this research is that it included a large, low-income sample, with significant ethnic/racial minority group representation, allowing for a detailed examination of factors associated with potentially quality of life-enhancing provider responses among at-risk populations. In particular, this study had a large representation of Latina BC patients, the largest and fastest growing racial/ethnic minority population in the US [47].
There are several limitations to this study. First, we did not use validated objective measures to assess the presence and the resolution of breast cancer treatment-related symptoms; subjective bias and recall error should be considered. Also, physician awareness of the symptoms was measured by patient report. Second, because the study was conducted in a sample of low-income, medically underserved women in a specific Medicaid BC treatment coverage program in California, external generalizability of the findings to other low-income populations and to women in the general population may be limited. Lastly, the cross-sectional study design prevents an assumption of causality between independent predictors and dependent variables.
Taken together, the study results suggest that resolution of breast cancer treatment-related symptoms can be improved at the patient–physician communication level. Both perceived patient self-efficacy in communicating with physicians and physician awareness help to enhance resolution of nausea. However, perceived patient self-efficacy was more powerful in enhancing the resolution of pain, while physician awareness played a more important role in symptom resolution for depression. In addition, patient–physician communication moderated Latinas’ disparity in pain resolution, regardless of language acculturation. Resolution of breast cancer treatment-related symptoms among low-income women can be ameliorated by appropriate educational interventions targeting patient–physician communication. Comprehensive strategies aimed at both patients and physicians to improve patient–physician communication with regard to BC treatment-related symptoms, including increasing cultural sensitivity, can enhance quality of life among women during and after treatment for breast cancer.
Acknowledgments
This study was funded by the American Cancer Society (# TURSG-02-081) and the California Breast Cancer Research Program (# 7PB-0070).
Contributor Information
Rose C. Maly, Department of Family Medicine, David Geffen School of Medicine at UCLA, 10880 Wilshire Blvd, Ste 1800, Los Angeles, CA 90024, USA, rmaly@mednet.ucla.edu
Yihang Liu, Department of Family Medicine, David Geffen School of Medicine at UCLA, 10880 Wilshire Blvd, Ste 1800, Los Angeles, CA 90024, USA.
Barbara Leake, Department of Family Medicine, David Geffen School of Medicine, and School of Nursing at UCLA, Los Angeles, CA, USA.
Amardeep Thind, Department of Family Medicine, Department of Epidemiology & Biostatistics, University of Western Ontario, London, ON, Canada.
Allison L. Diamant, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
References
- 1.National Cancer Institute Breast Cancer. [29 May 2008];2008 Available from URL: http://www.cancer.gov/cancertopic/types/breast.
- 2.Soerjomataram I, Louwman MWJ, Bibot JG, Roukema JA, Coebergh JWW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. 2008;107:309–330. doi: 10.1007/s10549-007-9556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor R, Davis P, Boyages J. Long-term survival of women with breast cancer in New South Wales. Eur J Cancer. 2003;39:215–222. doi: 10.1016/S0959-8049(02)00486-0. [DOI] [PubMed] [Google Scholar]
- 4.Peto R, Boreham J, Clarke M, et al. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet. 2000;355:1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Institute SEER Cancer Statistics Review. [29 May 2008];1975–2002 Available from URL: http://seer.cancer.org/csr/1975_2002/
- 6.Cella D. Quality of life: concepts and definition. J Pain Symptom Manage. 1994;9:186–192. doi: 10.1016/0885-3924(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 7.Powe BD, Hamilton J, Hancock N, et al. Quality of life of African American cancer survivors: a review of the literature. Cancer. 2007;109:435–445. doi: 10.1002/cncr.22358. [DOI] [PubMed] [Google Scholar]
- 8.Smith KW, Avis NE, Assmann SF. Distinguish between quality of life and health status in quality of life research: a meta-analysis. Qual Life Res. 1999;8(5):447–459. doi: 10.1023/A:1008928518577. [DOI] [PubMed] [Google Scholar]
- 9.Mith W, Bourne D, Squair J, Phililips D, Chambers W. A retrospective cohort study of post mastectomy pain syndrome. Pain. 1999;83:91–95. doi: 10.1016/S0304-3959(99)00076-7. [DOI] [PubMed] [Google Scholar]
- 10.Booth CM, Clemons M, Dranitsaris G, et al. Chemotherapy-induced nausea and vomiting in breast cancer patients: a prospective observational study. J Support Oncol. 2007;5(8):374–380. [PubMed] [Google Scholar]
- 11.Badger TA, Braden CJ, Mishel MH. Depression brudent, self-help interveetions, and side effect experience in women receiving treatment for breast cancer. Oncol Nurs Forum. 2001;28:567–574. [PubMed] [Google Scholar]
- 12.Byar KL, Berger AM, Bakken SL. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum. 2006;33:E18–E26. doi: 10.1188/06.ONF.E18-E26. [DOI] [PubMed] [Google Scholar]
- 13.Booth CM, Clemons M, Dranitsaris B, et al. Chemotherapy-induced nausea and vomiting in breast cancer patients: a prospective observational study. J Support Oncol. 2007;5:374–380. [PubMed] [Google Scholar]
- 14.Walker LG, Heys SD, Walker MB, et al. Psychological factors can predict the response to primary chemotherapy in patients with locally advanced breast cancer. Eur J Cancer. 1999;35(13):1783–1788. doi: 10.1016/S0959-8049(99)00169-0. [DOI] [PubMed] [Google Scholar]
- 15.Stevens P, Dibble SL, Miakowski C. Prevalence, characteristics and impact of post-mastectomy pain syndrome. Pain. 1995;83:91–95. [Google Scholar]
- 16.Eversley R, Estrin D, Dibble S, et al. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250–256. doi: 10.1188/05.ONF.250-256. [DOI] [PubMed] [Google Scholar]
- 17.Janz NK, Mujahid M, Chung LK, et al. Symptom experience and quality of life of women following breast cancer treatment. J Womens Health. 2007;16:1348–1361. doi: 10.1089/jwh.2006.0255. [DOI] [PubMed] [Google Scholar]
- 18.Yoon J, Marlin JL, Tao ML, et al. Symptoms after breast cancer treatment: are they influenced by patient characteristics? Breast Cancer Res Treat. 2008;108(1):69–77. doi: 10.1007/s10549-007-9580-1. [DOI] [PubMed] [Google Scholar]
- 19.Ashing-Giwa K, Ganz PA, Petersen L. Quality of life of African–Americans and white long term breast carcinoma survivors. Cancer. 1999;85:418–426. doi: 10.1002/(SICI)1097-0142(19990115)85:2<418∷AID-CNCR20>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97(6):448–456. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 21.Castel LD, Saville BR, Depuy V, et al. Racial differences in pain during 1 year among women with metastatic breast cancer: a hazards analysis of interval-censored data. Cancer. 2008;112:162–170. doi: 10.1002/cncr.23133. [DOI] [PubMed] [Google Scholar]
- 22.Maly RC, Umezawa Y, Leake B, Silliman RA. Determinants of participation in treatment decision-making by older breast cancer patients. Breast Cancer Res Treat. 2004;85:201–209. doi: 10.1023/B:BREA.0000025408.46234.66. [DOI] [PubMed] [Google Scholar]
- 23.Woodward NJ, Wallston BS. Age and health care beliefs: self-efficacy as mediator of low desire for control. Psychol Aging. 1987;2:3–8. doi: 10.1037/0882-7974.2.1.3. [DOI] [PubMed] [Google Scholar]
- 24.Greene MG, Adelman R, Charon R, Hoffman S. Ageism in the medical encounter: an exploratory study of the doctor–elderly patient relationship. Lang Commun. 1986;6:113–124. doi: 10.1016/0271-5309(86)90010-8. [DOI] [PubMed] [Google Scholar]
- 25.Breen N, Kessler LG, Brown ML. Breast cancer control among the underserved—an overview. Breast Cancer Res Treat. 1996;40:105–115. doi: 10.1007/BF01806006. [DOI] [PubMed] [Google Scholar]
- 26.Newman LA, Martin IK. Disparities in breast cancer. Curr Probl Cancer. 2007;31(3):134–156. doi: 10.1016/j.currproblcancer.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Pendleton DA, Bochner S. The communication of medical information in general practice consultations as a function of patients’ social class. Soc Sci Med. 1980;14A:669–673. doi: 10.1016/0160-7979(80)90072-7. [DOI] [PubMed] [Google Scholar]
- 28.Chen JY, Diamant AL, Thind A, Maly RC. Determinants of breast cancer knowledge among newly diagnosed, low-income, medically underserved women with breast cancer. Cancer. 2008;112(5):1153–1161. doi: 10.1002/cncr.23262. [DOI] [PubMed] [Google Scholar]
- 29.Maly RC, Frank JC, Marshall GN, DiMatteo MR, Reuben DB. Perceived efficacy in patient–physician interactions (PEPPI): validation of an instrument in older persons. J Am Geriatr Soc. 1998;46(7):889–894. doi: 10.1111/j.1532-5415.1998.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 30.Marin G, Sabogal F, Marin B, Otero-Sabogal R, Perez-Stable E. Development of a short acculturation scale for Hispanics. Hisp J Behav Sci. 1987;9:183–205. doi: 10.1177/07399863870092005. [DOI] [Google Scholar]
- 31.Badger TA, Braden CJ, Mishel MH. Depression brudent, self-help interventions, and side effect experience in women receiving treatment for breast cancer. Oncol Nurs Forum. 2001;28:567–574. [PubMed] [Google Scholar]
- 32.Compas BE, Stoll MF, Thomsen AH, et al. Adjustment to breast cancer: age-related differences in coping and emotional distress. Breast Cancer Res Treat. 1999;54:195–203. doi: 10.1023/A:1006164928474. [DOI] [PubMed] [Google Scholar]
- 33.Sharpe M, Strong V, Allen K, et al. Major depression in outpatients attending a regional caner center: screening and unmet treatment needs. Br J Cancer. 2004;90:314–320. doi: 10.1038/sj.bjc.6601578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 35.Valente SM, Saunders JM, Cohen MZ. Evaluating depression among patients with cancer. Cancer Pract. 1994;2:65–71. [PubMed] [Google Scholar]
- 36.Interian A, Martinez IE, Guarnaccia PJ, et al. A qualitative analysis of the perception of stigma among Latinos receiving antidepressants. Psychiatr Serv. 2007;58:1591–1594. doi: 10.1176/appi.ps.58.12.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maguire P. Improving the detection of psychiatric problems in cancer patients. Soc Sci Med. 1985;20:819–823. doi: 10.1016/0277-9536(85)90336-3. [DOI] [PubMed] [Google Scholar]
- 38.Passik SD, Dugan W, McDonald MV, et al. Oncologists’ recognition of depression in their patients with cancer. J Clin Oncol. 1998;16:1594–1600. doi: 10.1200/JCO.1998.16.4.1594. [DOI] [PubMed] [Google Scholar]
- 39.Payne R, Medina E, Hampton JW. Quality of life concerns in patients with breast cancer: evidence for disparity of outcomes and experiences in pain management and palliative care among African–American women. Cancer. 2003;97:311–317. doi: 10.1002/cncr.11017. [DOI] [PubMed] [Google Scholar]
- 40.Cleeland CS, Gonin R, Baez L, et al. Pain and treatment of pain in minority patients with cancer. The Eastern cooperative oncology group minority outpatient pain study. Ann Intern Med. 1997;127(9):813–816. doi: 10.7326/0003-4819-127-9-199711010-00006. [DOI] [PubMed] [Google Scholar]
- 41.Anderson KO, Richman SP, Hurley J. Cancer pain management among underserved minority outpatients. Cancer. 2001;94(8):2295–2304. doi: 10.1002/cncr.10414. [DOI] [PubMed] [Google Scholar]
- 42.Juarez G, Ferrell B, Borneman T. Cultural considerations in education for cancer pain management. J Cancer Educ. 1999;14:168–173. doi: 10.1080/08858199909528610. [DOI] [PubMed] [Google Scholar]
- 43.Anderson KO, Mendoza TR, Valero V, et al. Minority cancer patients and their providers-pain management attitudes and practice. Cancer. 2000;88:1929–1938. doi: 10.1002/(SICI)1097-0142(20000415)88:8<1929∷AID-CNCR23>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 44.Kris MG, Hesketh PJ, Somerfield MR, et al. American society of clinical oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol. 2006;24:2932–2947. doi: 10.1200/JCO.2006.06.9591. [DOI] [PubMed] [Google Scholar]
- 45.Roila F, Hesketh PJ, Herrstedt J, et al. Antiemetic subcommittee of the multinational association of supportive care in cancer. Prevention of chemotherapy-and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol. 2006;17:20–28. doi: 10.1093/annonc/mdj936. [DOI] [PubMed] [Google Scholar]
- 46.National Comprehensive Cancer Network NCCN. Clinical Practice Guidelines in Oncology: Antiemesis, V.1. 2007. [3 Aug 2007]; Available from URL: http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf.
- 47.Bernstein R. US Census Bureau News, US Department of Commerce; Washington, D.C.: [9 Sept 2005]. Hispanic Population Passes 40 million: Census Bureau Reports. Released June 9, 2005. Available from URL: http://www.census.gov/population/www/cen2000/briefs.html. [Google Scholar]


