Abstract
Over the past decade, interest has increased in the use of exogenous stem cells to optimize lung repair and serve as carriers of a therapeutic gene for genetic airway disease such as cystic fibrosis. We investigated the survival and the engraftment of exogenous stem cells after intratracheal injection, in a murine model of acute epithelial airway injury already used in gene therapy experiments on cystic fibrosis. Embryonic stem cells and mesenchymal stem cells were intratracheally injected 24hr after 2% polidocanol administration, when epithelial airway injury was maximal. Stem cells were transfected with reporter genes immediately prior to administration. Reporter gene expression was analyzed in trachea-lungs and bronchoalveolar lavages using non-fluorescent, quantitative and sensitive methods. ELISA quantitative results showed that 0.4 to 5.5% stem cells survived in the injured airway. Importantly, no stem cells survived in healthy airway or in the epithelial lining fluid. Using X-Gal staining, transduced mesenchymal stem cells were detected in injured trachea and bronchi lumen. When the epithelium was spontaneously regenerated, the in vivo amount of engrafted mesenchymal stem cells from cell line decreased dramatically. No stem cells from primary culture were located within lungs at 7 days. This study demonstrated the feasibility of the intratracheal cell delivery for airway diseases with acute epithelial injury.
Keywords: Analysis of Variance, Animals, Bronchoalveolar Lavage, DNA Primers, genetics, Embryonic Stem Cells, transplantation, Enzyme-Linked Immunosorbent Assay, Galactosides, Genetic Engineering, methods, Genetic Vectors, Indoles, Male, Mesenchymal Stem Cell Transplantation, methods, Mice, Respiratory Tract Diseases, therapy, Tissue Therapy, methods, Trachea, cytology, Transfection, beta-Galactosidase
Introduction
Respiratory diseases remain one of the main causes of morbidity and mortality in the world. Interest has increased as to the possibility of optimizing the repair of the lung with the use of stem cells (SCs). In particular, combining SC ability to engraft into damaged lungs and their ability to serve as carriers of a therapeutic gene has a great potential for pulmonary fibrosis (Loebinger et al., 2008) and for genetic airway disease such as cystic fibrosis. This developing therapeutic approach has been stimulated by early reports demonstrating that both embryonic stem cells and SCs derived from adult bone marrow, including mesenchymal stem cells, can differentiate into respiratory cells in vitro, thus acquiring phenotypic and functional markers of airway and alveolar epithelial cells (Coraux et al., 2005; Wang et al., 2005; Rippon et al., 2006). Systemic administration of adult SCs from bone marrow in mice after total-body irradiation (Pereira et al., 1995; Loebinger et al., 2008; Sueblinvong et al., 2008) and/or pollutant-reagent treatment have been reported recently (Beckett et al., 2005; MacPherson et al., 2006; Mei et al., 2007; Serikov et al., 2007; Xu et al., 2007; Loebinger et al., 2008) and showed that the administered SCs were mainly engrafted in alveolar spaces (Pereira et al., 1995; Beckett et al., 2005; Mei et al., 2007; Serikov et al., 2007; Xu et al., 2007; Loebinger et al., 2008; Sueblinvong et al., 2008) and sometimes in conducting airway (Pereira et al., 1995; Krause et al., 2001; MacPherson et al., 2005; Loi et al., 2006; MacPherson et al., 2006; Serikov et al., 2007). The SC differentiation as pneumocytes (I or II) or airway epithelial cells was mostly evaluated using different fluorescence techniques. Reported engraftment rates of adult bone marrow SC-derived cells ranged from 0% (Wagers et al., 2002) up to 20% (Krause et al., 2001) in the lungs, and from 0.025% (Loi et al., 2006) up to 4% (Krause et al., 2001) in conducting airway. These discrepancies have been partly attributed to the different fluorescence techniques used to detect the donor-derived epithelial cells, leading to significant artifacts (Loebinger et al., 2008). Importantly, lung SC engraftment is currently estimated to be between 0.01% and 0.1% (Krause 2005; Bruscia et al., 2006). Despite this low engraftment level, there is evidence that transplanted SC post-injury have some therapeutic effects (Loebinger et al., 2008).
In most of these studies, systemic administration of SCs required total-body irradiation of the recipient to promote SC bone marrow engraftment, a difficult condition to apply in the clinical setting, especially in patients suffering from airway disease associated with chronic infections such as cystic fibrosis. Alternatively, considering the advantages of the intratracheal route to target the airway and the respiratory epithelium, more recent studies reported the intratracheal administration of adult SCs in reagent-injured lungs (Gupta et al., 2007; Wong et al., 2007). Using fluorescent techniques SC engraftment was enhanced with the intratracheal route as compared to the intravenous route (Wong et al., 2007; Wong et al., 2009) but remained 5–10% (Gupta et al., 2007; Wong et al., 2007; Wong et al., 2009). The intratracheal route was also used for the administration of differentiated cells and showed both positive (Serrano-Mollar et al., 2007) and negative results (Kuang et al., 2005; Gupta et al., 2007). SC or differentiated cell engraftment levels in these studies were mostly assessed using fluorescence techniques, which could explain these conflicting results.
The aims of our study were to evaluate the survival and the engraftment of different types of exogenous SCs and differentiated cells after intratracheal injection. We used a murine model presenting acute epithelial airway injury without total-body irradiation. This model was developed for gene therapy experiments on cystic fibrosis and consisted in intranasal injection of polidocanol detergent (Parsons et al., 1998). Quantification of cell survival rates and cell location within lungs were performed using ELISA, biochemical assay and PCR.
Materials and methods
Animal model and intratracheal administration
Male, 8- to 10-week old Swiss mice were obtained from Janvier Laboratories (Le Genest St Isle, France). Animal experiments were performed under the approval of the Guide for The Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996). Mice were anesthetized with an intraperitoneal injection of xylazine (15mg/kg) and ketamine (70mg/kg), and placed supine. Briefly, intratracheal administration was performed using a 22-gauge catheter placed within an intubation canula (Harvard Apparatus) that was removed immediately before injection. Airway injury was induced after anaesthesia by intratracheal administration of 25μl of 2% polidocanol (PDOC: polyoxyethylene 9-lauryl ether, Sigma, (Borthwick et al., 2001; MacPherson et al., 2005)) in phosphate-buffered saline (PBS) through the 22-gauge catheter. Cells (106cells/25μl) or PBS were intratracheally injected 24hr after PDOC administration using the same protocol. Mice were sacrificed by an intraperitoneal lethal injection of nesdonal 24hr or 7 days after intratracheal administration.
Bronchoalveolar lavages
Bronchoalveolar lavages (BALs) were performed 24hr after CAT-transfected cell administration in healthy or injured airway. Mice were anesthetized and thoracic cavity was opened by careful dissection. The trachea was exposed, and a small transverse incision performed just below the level of the larynx. BAL was then performed using one dose of 1 ml of PBS, ensuring that both lungs inflated during the lavage process and that there was no leakage of lavage fluid from the trachea. BAL fluid was centrifuged at 400 g for 5 min. The supernatant was removed and stored at −80°C until ELISA. Cell pellet was suspended in lysis buffer and stored at −80°C until ELISA.
Cell cultures
Undifferentiated mouse embryonic stem cells (ESCs, R1 cell line, (Nagy et al., 1993)) were cultured in dishes coated with 0.1% gelatine (Sigma). The culture medium was composed of Dulbecco minimum essential medium with 4.5g/l glucose (DMEM, Invitrogen) supplemented with 15% FCS (Invitrogen), 0.1% non essential amino acids (Invitrogen), 1mM sodium pyruvate (Invitrogen), 2mM L-Glutamine (Invitrogen), 10−7M βmercaptoethanol (Sigma), penicillin/streptomycin (100U/ml and 100μg/ml respectively, Invitrogen) and 2000U/ml of LIF (Sigma).
Undifferentiated murine mesenchymal stem cells (BMC9s, (Dennis and Caplan 1996)) were cultured as previously described (Chateauvieux et al., 2007) in alpha MEM medium with nucleosides (Invitrogen) supplemented with 10% FCS (Invitrogen), 2mM L-Glutamine (Invitrogen), penicillin/streptomycin (100U/ml and 100μg/ml respectively, Invitrogen).
A human hepatocellular carcinoma cell line (Hep 3B2.1-7, ATCC number HB-8064), transformed African green monkey kidney fibroblast cell line (COS-7, ATCC Number CRL-1651) and murine osteosarcoma cell line (mOS-J, (Joliat et al., 2002)) were used for control experiments. Cells were cultured in DMEM medium with 4.5 g/l glucose supplemented with 15% FCS, 2 mM L-Glutamine, penicillin/streptomycin (100U/ml and 100μg/ml respectively).
Primary culture of adult mesenchymal stem cells
Total bone marrow was obtained from wild type adult male Swiss mice and male Rosa26 lacZ-mice (background C57Bl/6J x 129S2, kindly provided by Dr M.F. Gardahaut, Nantes, France) by flushing femurs and tibias with culture medium. Cells were plated at a density of 500 000 cells/cm2 in medium composed of alpha MEM with nucleosides supplemented with 10% FCS, 2 mM L-Glutamine, penicillin/streptomycin (100U/ml and 100μg/ml respectively) and 2ng/ml of human FGF2 (AbCys). The culture medium was changed at day 3 to remove non-adherent cells and was subsequently replaced weekly. The cells were grown for 2–3 weeks until confluence. Adherent cells were then detached by 0.5% trypsin-EDTA and plated at a density of 10 000 cells/cm2. Subsequent passages and seeding of the cells were performed at a density of 5 000 cells/cm2. From passage 8, MSC cultures were characterized by FACS analysis (FACS Calibur Instrument, BD Biosciences, CellQuestPro Software) after incubation with anti-CD45-PE, anti-CD90-PE, anti-CD29-CyChrome, anti-Sca1-PE, anti CD106-CyChrome (BD Biosciences) (Dominici et al., 2006).
In vitro cell transfection with CAT or GFP reporter genes and evaluation of gene transfer system efficacy
ESCs, BMC9s and differentiated cells were transfected with pCIK-CAT (4.7 Kb) and pEGFP-C1 (4.7 Kb) plasmids encoding for Chloramphenicol AcetylTransferase (CAT) protein and GFP, using synthetic vectors just prior to intratracheal administration (Pitard et al., 2001). DNA plasmids were complexed with ICAfectin® 441 according to the manufacturer’s instruction (In-Cell-Art, Nantes, France). After 2hr, transfection complexes were removed by changing growth medium and cells were lysed for dosages or kept in culture for 24hr, 7 days, or injected intratracheally as indicated above.
MSCs from Swiss mice were transfected with pCIK-CAT and pEGFP-C1 plasmids using nucleofection (Nucleofector Solution, Amaxa Biosystem) just prior to intratracheal administration (Aluigi et al., 2006). After 2hr, growth medium was changed and cells were lysed for dosages or kept in culture for 24hr, 7 days, or injected intratracheally as indicated above. The percentage of GFP-expressing cells was evaluated 24hr after GFP nucleofection using FACS analysis.
The efficacy of gene transfer systems was evaluated using cytometry for GFP-expressing cells and ELISA for CAT-expressing cells. The percentage of GFP-expressing cells was evaluated 24hr after GFP transfection using FACS analysis (FACS Calibur Instrument, BD Biosciences, CellQuestPro Software). CAT protein quantity per stem cell at 24hr was then calculated using in vitro data: CAT protein quantity in cell lysis buffer was divided by the cell number and by the percentage of GFP-expressing cells (obtained by FACS analysis). Finally, this value was used to determine the minimum number of CAT-expressing stem cells required to be detected by ELISA in vitro or in vivo considering that CAT ELISA detection threshold was 50pg CAT protein (see below).
In vitro stem cell transduction with nls-lacZ reporter gene
BMC9s were transduced with an amphotropic recombinant murine retrovirus vector containing the nls-LacZ reporter gene (LacZ gene encoding β galactosidase (βgal) coupled to a nuclear localization signal (nls), kindly provided by Dr. N. Ferry, Nantes, France). This retrovirus vector was obtained from the human producer cell line TELCeB6AF7 derived from the Te671 cell line as described in (Aubert et al., 2002). 24hr recombinant retroviral supernatant was harvested from the confluent producer cell line and filtered through a 0.45 μm membrane. The titer of the βgal supernatant, determined by endpoint dilution using Te671 target cells, was 2 × 108 transducing particles/ml. After BMC9 transduction, retrovirus vector was removed by changing growth medium and cells were lysed for dosages or kept in culture or injected intratracheally as indicated above.
To evaluate the percentage of βgal-transduced BMC9s, cells were fixed in 4% paraformaldehyde, washed with PBS and incubated in 5mM K4Fe(CN)6, 5mM K3Fe3(CN)6 and 2mM MgCl2 in PBS containing 0.5mg/ml X-Gal ([5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside] dissolved in N,N-dimethylformamide at 20mg/ml before dilution into the reaction mixture, Sigma) for 2hr, at 37°C. Cells were identified as positive for βgal activity by blue nuclear staining after X-Gal reaction. The percentage of βgal expressing cells in vitro was obtained by counting the number of nuclear-blue cells in a total of 200 cells.
MSCs from Swiss mice were transduced with a lentivirus vector containing the nls-lacZ reporter gene. The vector was derived from pHR’ LacZ (Zufferey et al., 1997) in which a nls from the SV40 T Ag was cloned in frame 5’ to the LacZ cDNA. The lentivirus vector was produced on human embryonic kidney 293T cells as previously described (Selig et al., 1999). After transduction with 50 MOI (Multiplicity Of Infection), lentivirus vector was removed by changing growth medium and cells were lysed for dosages or kept in culture or injected intratracheally as indicated above. The percentage of βgal-transduced MSCs was evaluated by in vitro X-Gal staining and counting as described above.
Reporter gene assay and estimation of survival rate
CAT-transfected cells, BAL cells and BAL fluid, or whole frozen trachea-lungs were homogenized in reporter lysis buffer (Roche Diagnostics) supplemented with protease inhibitors (Roche Diagnostics). After centrifugation at 10 000 rpm for 5min, CAT quantity was measured in supernatant with a VICTOR2 multilabel counter (PerkinElmer), using a CAT enzyme-linked immunosorbent assay (ELISA) kit according to the instructions of the supplier (Roche Diagnostics). Each sample was analyzed in duplicate. CAT detection threshold was 50pg. Protein content was measured with a bicinchoninic acid (BCA) protein assay kit (Pierce).
To estimate cell survival rate 24hr after intratracheal administration, we first calculated CAT protein quantity per CAT-expressing cell, using in vitro data: CAT quantity in cell lysis buffer was divided by the cell number and by the percentage of GFP-expressing cells (obtained by FACS analysis). Finally, the total in vivo CAT protein quantity per animal was divided by this in vitro value, in order to estimate the number of CAT-expressing cells per animal and subsequently the cell survival rate.
BMC9s and Rosa26 MSCs or whole frozen murine trachea-lungs were homogenized in 1ml reporter lysis buffer (Roche Diagnostics) supplemented with a protease inhibitor cocktail (Roche Diagnostics). After centrifugation at 10 000 rpm for 5min, βgal activity was determined by enzymatic fluorimetric assay using 4MUG (4-methylumbelliferyl-β-D-galactoside, Sigma) as a fluorescent substrate. Each cell and trachea-lung samples were analyzed in duplicate. βgal activity detection threshold was 230pg. Protein content was measured with a BCA protein assay kit. Additional experiments were done with lysates of βgal-transduced BMC9s or Rosa26 MSCs after three cycles of freezing and thawing. Viability was ascertained by trypan blue exclusion.
Detection of β galactosidase activity
Tissues were fixed in 4% paraformaldehyde (at room temperature, 20min) and rinsed 3 times with PBS. Immediately after fixation, trachea-lungs were stained by intratracheal infusion (though a 19-gauge needle, total volume = 5ml) and immersion in 5mM K4Fe(CN)6, 5mM K3Fe3(CN)6 and 2mM MgCl2 in PBS containing 0.5mg/ml X-Gal (as indicated above) for 6hr, at 30°C to avoid the detection of endogenous βgal activity (Weiss et al., 1997). Tissues were again washed with PBS and immediately embedded in paraffin. Tissues were identified as positive for β galactosidase activity by nuclear blue staining after X-Gal reaction. Sections (4μm thick) were stained with nuclear fast red (Sigma).
Detection of nls-lacZ reporter gene
Total DNA from whole murine trachea-lungs was isolated for PCR analysis. Tissues were digested overnight with lysis buffer containing proteinase K (10mg/ml, Sigma). Total DNA was extracted with phenol/chloroform/isoamyl alcohol (Sigma) method and then precipitated in ethanol. Total DNA from 106 transduced MSCs was also extracted for PCR experiments. PCR were performed using primers that specifically bind the nls sequence and the lacZ gene encoded in the nls-lacZ lentivirus vector: 5′-GTA ACA ACT CCG CCC CAT T -3′ and 5′-GAC AGT ATC GGC CTC AGG AA -3′.
Statistical analysis
Data are expressed as means ± S.E.M. Statistical significance was evaluated using the analysis of variance (ANOVA) method to compare control and treatment groups of 3 or more. Tukey’s test was subsequently used for pair-wise comparisons. A non parametric Mann and Whitney test was performed to compare two groups. Statistical significance was set at p<0.05.
Results
Transient epithelial airway injury induced by polidocanol intratracheal administration
Intratracheal administration of 2% PDOC induced macroscopic lung injury at 24hr with haemorrhage (Figs. 1b,k,n). The major histological findings at 24hr were acute injury of the murine airway epithelium with focal shedding areas observed in the epithelial surface, with only a remaining layer of basal cells or a total denudation of the basement membrane (Figs. 1e,h). However, in some regions, the luminal layer of epithelial cells was just disrupted or sloughed whereas the basal cell layer and the basement membrane remained intact (not shown), as already described after intratracheal or intranasal administration (Driscoll et al., 2000 and Southam et al., 2002, respectively). Inflammation was present in pulmonary parenchyma (Figs. 1k,n, arrow heads). Trachea-lung sections at 7 days after PDOC administration demonstrated significant improvement of haemorrhage (Figs. 1c,l) and of airway injury with epithelial surface again covered by cilia (Figs. 1f,i). PDOC effect did not alter significantly animal survival (Table S1 in supplementary data).
Figure 1.
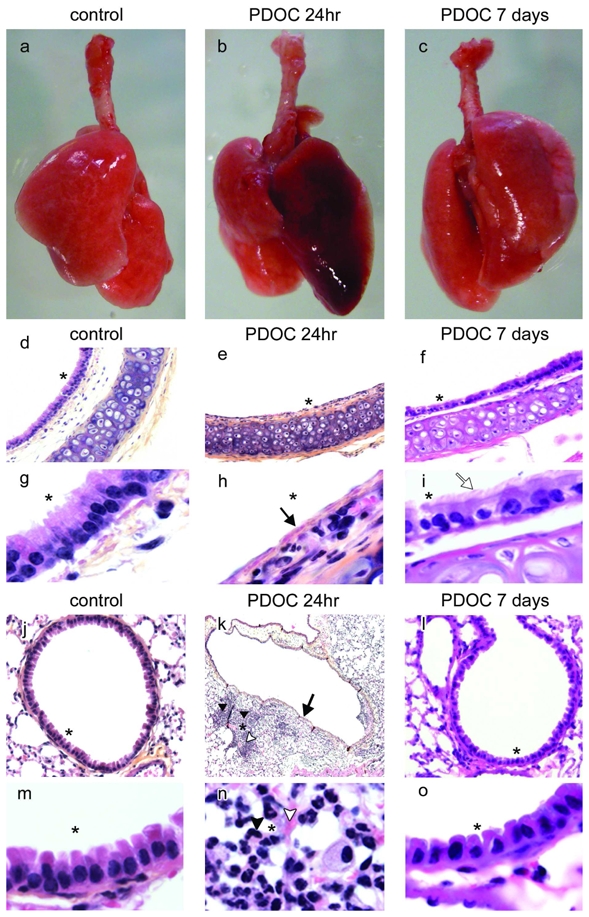
Epithelial damage induced by intratracheal administration of polidocanol (PDOC). (a–c) Trachea-lungs from (a) control animal, (b) animals injected with PDOC at 24hr and (c) 7 days after PDOC administration. (d–i) tracheal and (j–o) bronchial sections from (d,g,j,m) control animal, (e,h,k,n) animals injected with PDOC at 24hr and (f,i,l,o) at 7 days. (k) Magnification 4x. (d–f) Magnification 10x. (j,l) Magnification 20x. (g–i,m–o) Magnification 100x. Haematoxylin counterstaining, airway epithelial damage (arrows), ciliated cells (white arrow), inflammation areas (arrow heads), haemorrhagic areas (white arrow heads). Areas marked by asterisks were magnified in the adjacent pictures.
ESC and BMC9 survival in murine airway with acute epithelial airway injury
In a first set of experiments, embryonic stem cells (ESCs) and adult mesenchymal stem cells (BMC9s) from well-characterized cell lines were intratracheally injected. ESCs and BMC9s were injected 24hr after PDOC administration, when epithelial airway injury induced by PDOC was acute (MacPherson et al., 2005). Stem cell survival was evaluated 24hr after stem cell administration. To calculate the survival rate, we used a quantitative method based on in vivo expression of reporter genes by transplanted cells. Stem cells were transfected in vitro with plasmids encoding CAT or GFP reporter genes 2hr prior to intratracheal injection. CAT-transfected stem cells were then intratracheally injected, at a time where they did not yet express the foreign genes (see below). We hypothesized that only surviving stem cells would express in vivo CAT protein in trachea-lungs 24hr after intratracheal cell injection.
Two hours after the beginning of cell incubation with both plasmids and synthetic vectors, transfection complexes were removed and reporter gene expression was evaluated. Neither CAT protein nor cell fluorescence was detected in stem cells using ELISA (Fig. 2b) and microscopy (not shown), demonstrating that stem cells did not express CAT nor GFP protein at the time of in vivo injection. In vitro reporter gene expression was also evaluated 24hr and 7 days after incubation with plasmids and synthetic vectors. As evaluated by FACS analysis, 19.4 ± 0.2% ESCs (n = 6) and 37.0 ± 4.4% BMC9s (n = 6) expressed GFP in vitro at 24hr (Fig. 2a). However, CAT and GFP were not detected at 7 days after transfection (Fig. 2b), suggesting that transgene expression was transient. Using CAT ELISA quantitative results and GFP-expressing cell rates, we calculated that CAT-expressing ESCs and CAT-expressing BMC9s contained at 24hr 0.03 ± 0.01pgCAT/cell (n = 10) and 0.08 ± 0.02pgCAT/cell (n = 6), respectively. Cell values were further used to evaluate the number of in vivo surviving cells after intratracheal injection.
Figure 2.
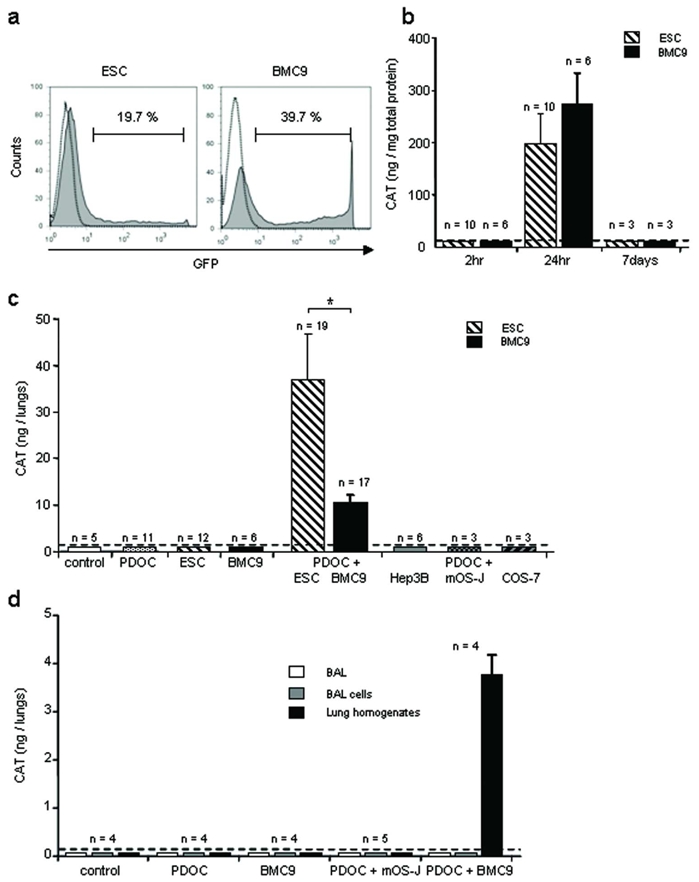
In vitro cell transfection with reporter genes and in vivo CAT gene expression into injured airway 24hr after intratracheal stem cell administration. To follow stem cell survival in vivo, murine embryonic stem cells (ESCs) or adult mesenchymal stem cells (BMC9s) were transfected in vitro with plasmids encoding CAT or GFP reporter genes just prior to intratracheal administration. (a) Representative histograms of FACS analysis of GFP-expressing ESCs or BMC9s 24hr after in vitro transfection. (b) CAT protein quantification in ESC and BMC9 lysates using ELISA, 2hr, 24hr or 7 days after incubation with plasmids and synthetic vectors. (c) CAT protein quantification in whole trachea-lung homogenates using ELISA, 24hr after ESC or BMC9 intratracheal injection. Evaluated groups included animals that were intratracheally injected with the followings: no injection (control), PDOC only (PDOC), ESCs or BMC9s only (ESC, BMC9), PDOC and ESCs or BMC9s 24hr later (PDOC + ESC, BMC9), PDOC and differentiated cells 24hr later (PDOC + Hep3B, mOS-J, COS-7). (d) CAT protein quantification in bronchoalveolar lavages and whole trachea-lung homogenates using ELISA, 24hr after BMC9 or murine differentiated cells (mOS-J) intratracheal injection. Evaluated groups included animals that were intratracheally injected with the followings: no injection (control), PDOC only (PDOC), BMC9s only (BMC9), PDOC and mOS-Js 24hr later (PDOC + mOS-J), PDOC and BMC9s 24hr later (PDOC + BMC9). Dotted line depicts control threshold. * p<0.05.
For intratracheal administration, CAT-transfected ESCs and CAT-transfected BMC9s were harvested 2hr after the beginning of transfection and injected intratracheally. Mice were sacrificed 24hr after intratracheal administration; trachea-lungs were used in toto to quantify CAT protein by ELISA (Fig. 2c). No CAT protein was detected in control animals or animals injected with PDOC only. Importantly, CAT protein was detected in 61% (19/31) animals intratracheally injected with PDOC and ESCs and in 81% (17/21) animals intratracheally injected with PDOC and BMC9s, suggesting that ESCs and BMC9s survived within lungs and expressed reporter genes 24hr after intratracheal injection. To estimate the number of surviving cells within the lungs at 24hr, total CAT protein quantity per animal was divided by CAT quantity per cell in vitro. The survival rate of 106 injected stem cells was 3.69 ± 0.86% ESCs (n = 19), whereas this survival rate was significantly less with BMC9s (0.43 ± 0.12%, n = 17, Tukey’s test, p = 0.02). CAT cell quantities were also used to calculate the in vivo cell detection threshold. A minimum of 10 ± 1.2×103 ESCs and 4.3 ± 1.1×103 BMC9s could be detected in one animal in vivo, corresponding to 0.4–1% injected cells. These results suggest that even if the percentage of cells expressing reporter gene in vitro was low, this was still a sensitive method for in vivo detection of a small cell number.
Influence of differentiation state and airway environment on ESC and BMC9 survival
To determine whether cell survival ability in murine injured airway was specific to stem cells, differentiated cells from various origins (Hep3B, mOS-J and COS-7 cells) were transfected in vitro with CAT plasmids in conditions and results similar to that of ESCs and BMC9s (not shown). CAT-transfected differentiated cells were then intratracheally injected 2hr after the beginning of incubation with CAT plasmid. No CAT protein was detected in any animal injected with PDOC and differentiated cells at 24hr (Fig. 2c), suggesting that only stem cells survived after intratracheal injection in injured airway. CAT-transfected ESCs and BMC9s were injected in control healthy animals. No CAT protein was detected in any animal with healthy airway 24hr after stem cell injection (Fig. 2c), suggesting that stem cell survival was favoured by airway injury.
Cell survival in the epithelial lining fluid
To evaluate whether cells expressing CAT reporter protein were present within the epithelial lining fluid or after phagocytosis by alveolar macrophages, bronchoalveolar lavages (BALs) were performed at 24hr in in vivo conditions when phagocytosis was expected maximal, e.g. when CAT ELISA results were negative: mesenchymal stem cells (BMC9s) in healthy airway and murine differentiated cells (mOS-J) in injured airway. As further controls, BALs were also performed in control and PDOC animals and in animals intratracheally injected with PDOC and BMC9s. No CAT protein was detected in any BAL fluid nor BAL cell sample from any animal (Fig. 2d), suggesting that if stem cells or differentiated cells were phagocytosed by alveolar macrophages, such phagocytosis did not alter CAT protein measurement nor cell survival detection. Importantly, in animals intratracheally injected with PDOC and BMC9s, high levels of CAT protein were measured in trachea and lung homogenates whereas no CAT protein was detected in BAL fluid nor BAL cell samples, suggesting that CAT-expressing cells were mostly present within the lungs and did not survive in the epithelial lining fluid (Fig. 2d).
BMC9 location at 24hr and 7 days
Next, we investigated BMC9 location at 7 days when the airway epithelium was known to be spontaneously regenerating (MacPherson et al., 2005). As CAT expression using synthetic vectors was transient, we used integrative virus vectors allowing long-term foreign gene expression. BMC9s were transduced with a retrovirus vector encoding nls-lacZ gene. As evaluated by X-Gal staining, 57.2 ± 0.1% BMC9s expressed nuclear β galactosidase (βgal) when they were injected (Fig. 3a, n = 6). βgal activity in transduced BMC9s was significantly increased just prior to injection as compared to endogenous βgal activity in BMC9s (Fig. 3b, Mann and Whitney test, p = 0.02) and remained stable at 7 days (not shown). Animals were assessed for βgal activity 24hr and 7 days after intratracheal administration, using the 4 MUG method (Fig. 3c). In contrast to previous experiments, βgal-transduced BMC9s expressed nlslacZ gene at the time of intratracheal injections. Therefore, as a further control, we injected lysates of βgal-transduced BMC9s, and no significant increase in βgal activity was observed as compared to endogenous βgal activity (Tukey’s test, p = 0.98). In animals intratracheally injected with PDOC and βgal-transduced BMC9s, 24hr after injection βgal activity significantly increased as compared to that of control animals (Tukey’s test, p = 0.003) and to animals injected with PDOC and lysate of βgal-transduced BMC9s (Tukey’s test, p = 0.004), confirming results with CAT gene transfer. However, 7 days after stem cell injection βgal activity was similar to that of control animals (Tukey’s test, p = 0.98) and animals injected with PDOC and lysate of βgal-transduced BMC9s (Tukey’s test, p = 0.94), suggesting that the number of surviving BMC9s in the airway epithelium decreased significantly between 24hr and 7 days.
Figure 3.
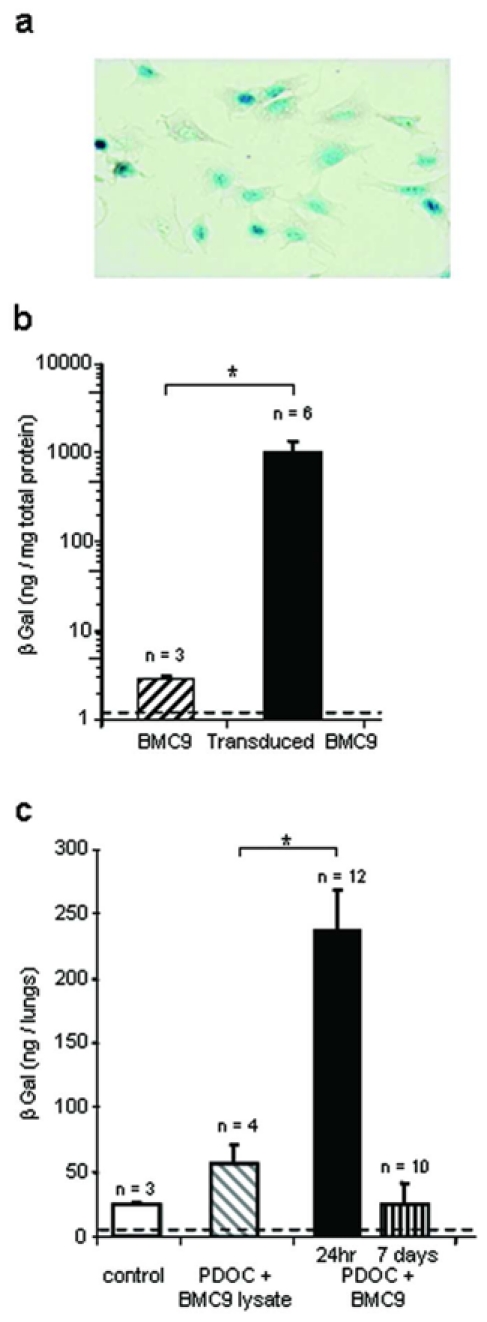
In vitro and in vivo detection of βgal activity 24hr or 7 days after βgal-transduced BMC9 intratracheal administration. BMC9s were transduced in vitro with a retrovirus vector encoding nls-lacZ gene prior to in vivo administration. (a) Representative example of βgal-transduced BMC9s just prior to injection, after X-Gal staining. Magnification 20x. (b) In vitro βgal activity in BMC9s and βgal-transduced BMC9s just prior to injection. (c) βgal activity in whole trachea-lung homogenates, 24hr or 7 days after BMC9 intratracheal injection. Evaluated groups included animals that were intratracheally injected with the followings: no injection (control), PDOC and lysate of βgal-transduced BMC9s (PDOC + BMC9 lysate), PDOC and βgal-transduced BMC9s (PDOC + BMC9). Dotted line depicts control threshold. * p<0.05.
To locate BMC9s after intratracheal injection into injured airway, we performed X-Gal staining of in toto trachea-lungs at 24hr and 7 days (Fig. 4). No blue staining was observed in trachea-lungs (Fig. 4a) and histological sections (Figs. 4b-d) from any control animal (Fig. 4a, n = 3), animal injected with PDOC (Fig. 4b, n = 3), or animal intratracheally injected with βgal-transduced BMC9s only (Fig. 4c, n = 5). No cell with nuclear blue staining was observed in any animal injected with PDOC and lysate of βgal-transduced BMC9s (Fig. 4d, n = 5), suggesting that cells with nuclear blue staining expressed the nls-lacZ gene de novo. In contrast, macroscopic and microscopic strong nuclear blue staining was observed in trachea and pulmonary lobes at 24hr (Figs. 4e-m, n = 16) and 7 days (Figs. 4n-p, n = 7) in each animal intratracheally injected with PDOC and βgal-transduced BMC9s. At 24hr, macroscopic analyses showed blue spots (<5 spots) in trachea from 8/16 animals (Figs. 4e,f) and blue spots in one (15/16, Figs. 4g,h) or two pulmonary lobes (1/16, Figs. 4i,j). Blue spots were also observed in large bronchi from 5/16 animals (Figs. 4i,j). Histological analyses confirmed the macroscopic result for each sample, showing β-gal positive cells on histological sections where blue spots had been observed. Clusters of cells with blue nuclei were observed in the lumen of injured trachea (Fig. 4k) and injured bronchi or bronchioles (Figs. 4l,m), but not in lung parenchyma (Fig. 4l). At 7 days, blue spots were observed in 7/7 animals, in the lumen of bronchioles (Figs. 4n-p). No blue cell was observed in trachea or in pulmonary parenchyma (not shown). Clusters of blue cells were sometimes located in polyp-like structures, located in bronchioles and large bronchi. Polyp-like structures were not observed in any animal receiving PDOC only. Further quantitative and statistical analyses demonstrated that the development of polyp-like structures at 7 days was not due to stem cell administration but rather to a second intratracheal administration (PBS or BMC9) 24hr after PDOC injection (Table S2 in supplementary data). Histological analyses at high magnification showed that βgal-expressing cells did not have a respiratory phenotype, with no cilia at 24hr or 7 days (not shown).
Figure 4.
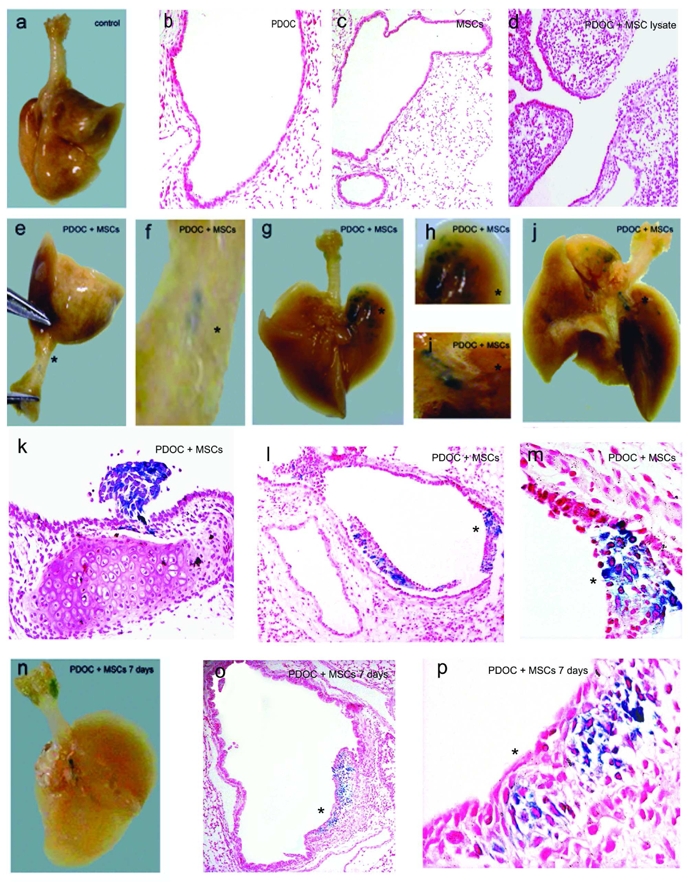
BMC9 location 24hr and 7 days after intratracheal injection, using X-Gal staining. (a–d) Trachea-lungs or histological sections at 24hr after X-Gal staining from (a) control animal, (b) animal intratracheally injected with PDOC or (c) βgal-transduced BMC9s or (d) with PDOC and 24hr later βgal-transduced BMC9 lysate. No blue staining was observed in any control animals. (e–j) Trachea-lungs, (k) tracheal and (l,m) bronchial sections from animals intratracheally injected with PDOC and 24hr later βgal-transduced BMC9s. Animals were sacrificed 24hr after BMC9 injection and stained using X-Gal. Blue spots were observed (e,f) in trachea and (g–j) in pulmonary lobes. (f,h,i) Higher power views of the area marked by asterisks in Figs. 4e,g,j, respectively. (m) Higher power view of the area marked by asterisk in Fig. 4l. (n–p) Trachea-lungs and histological sections from animal intratracheally injected with PDOC and βgal-transduced BMC9s, sacrificed 7 days after BMC9 injection and stained using X Gal. Blue spots were observed in the lumen of bronchioles. (p) Higher power view of the area marked by asterisk in Fig. 4o. (c,d,o) Magnification 4x. (b,l) Magnification 10x. (k) Magnification 20x. (m,p) Magnification 40x. Nuclear fast red counterstaining.
MSC survival in murine airway with acute epithelial airway injury
In a second set of experiments, to avoid in vivo cell survival linked to cell line transformation, we repeated previous experiments with the same experimental design, using murine MSCs from primary cultures. MSC cultures were characterised according to (Dominici et al., 2006) and (Chateauvieux et al., 2007). FACS analysis demonstrated that MSCs were CD45− (Fig. 5a, 0.4 ± 0.1%, n = 4), CD90 − (0.43 ± 0.2 %, n = 6) and CD29 +, Sca-1+ (Fig. 5a, 96.9 ± 1.3% and 98.1 ± 0.7% respectively, n = 4) and CD106+ (96 ± 0.8 %, n = 4).
Figure 5.
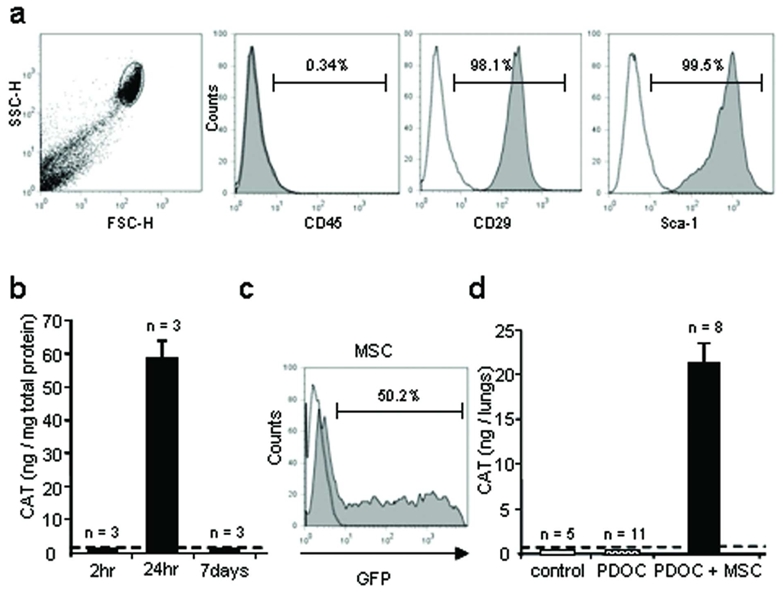
In vitro cell transfection with reporter genes and in vivo CAT gene expression 24hr after intratracheal administration into injured airway of murine adult mesenchymal stem cells (MSCs) from primary cultures. (a) MSC morphology and representative histograms of CD45, CD29 and Sca-1 labeling, using FACS analysis just prior to intratracheal injection. (b–d) To follow MSC survival in vivo, MSCs were transfected in vitro with plasmids encoding CAT or GFP reporter genes using nucleofection, just prior to intratracheal injection. (b) CAT protein quantification in MSCs using ELISA, 2hr, 24hr or 7 days after incubation with plasmid and nucleofection solution. (c) FACS analysis of GFP-expressing MSCs 24hr after incubation with GFP plasmid and nucleofection solution. (d) CAT protein quantification in whole trachea-lung homogenates using ELISA, 24hr after CAT-transduced MSC intratracheal injection. Evaluated groups included animals that were intratracheally injected with the followings: no injection (control), PDOC only (PDOC), PDOC and 24hr later CAT-transduced MSCs (PDOC + MSC). Dotted line depicts control threshold.
MSCs were transiently transfected in vitro with plasmids encoding CAT or GFP reporter genes. In contrast to ESCs and BMC9s, MSC transfection with synthetic vectors was ineffective (not shown), therefore nucleofection was used to obtain significant gene transfer rate. As evaluated by FACS analysis, 56.8 ± 2.9% MSCs expressed GFP in vitro at 24hr (Fig. 5c, n = 3), but CAT and GFP were not detected 7 days after nucleofection (Fig. 5b), suggesting that foreign gene expression was again transient and that nucleofection could not be used for 7 days in vivo experiments. CAT-transfected MSCs contained 0.35 ± 0.02pg CAT/cell at 24hr (n = 6). CAT-transfected MSCs were then intratracheally injected 2hr after the beginning of incubation with CAT plasmid. In control mice and mice injected with PDOC only, no CAT protein was detected at 24hr (Fig. 5d). Importantly, CAT protein was detected in each animal injected with PDOC and MSCs (Fig. 5d, n = 8, Tukey’s test, p<0.001), suggesting that MSCs from primary cultures survived in injured airway and expressed CAT protein after intratracheal administration. MSC survival rate was 5.52 ± 1.9% (n = 8), a minimum of 710 ± 45 CAT-expressing MSCs could be detected in one animal in vivo, corresponding to 0.07% injected cells, confirming CAT ELISA sensitivity for in vivo experiments.
MSC location at 24hr and 7 days
To investigate whether MSCs could be located into the airway epithelium 7 days after cell injection, we first used MSCs in primary cultures from βgal+ transgenic (Rosa26) mice, thus expressing continuously cytoplasmic βgal (Fig. 6a). Using the 4 MUG method (Fig. 6c), in vitro βgal activity was significantly increased in Rosa26 MSCs as compared to MSCs from Swiss mice (Figure 6c, Mann and Whitney test, p = 0.03). Mice were assessed for βgal activity 24hr and 7 days after intratracheal administration of Rosa26 MSCs or of a lysate of Rosa26 MSCs (Fig. 6d). As observed in previous experiments with βgal-transduced BMC9s, βgal activity was similar in murine airway from control animals and animals intratracheally injected with lysates of Rosa26 MSCs (Tukey’s test, p = 0.68). In animals intratracheally injected with PDOC and Rosa26 MSCs, βgal activity was significantly increased as compared to that of both control groups (Tukey’s test, p = 0.01). However, in contrast to previous experiments with βgal-transduced BMC9s, no blue staining was observed in trachea-lungs from animals injected intratracheally with PDOC and Rosa26 MSCs at 24hr, and Rosa26 MSCs could not be located on histological slides (not shown). Finally, βgal activity in murine injured airway returned to baseline at 7 days after Rosa26 MSC intratracheal injection (Tukey’s test, p = 0.85).
Figure 6.
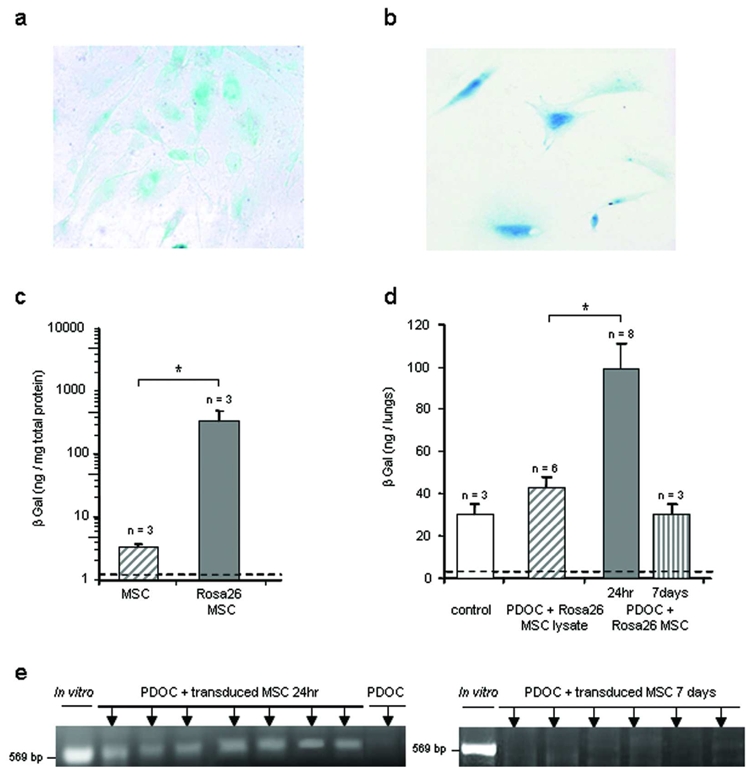
In vitro and in vivo detection of Rosa26 MSCs and βgal-transduced MSCs 24hr or 7 days after administration. MSCs from wild type mice were transduced with a lentivirus vector encoding nls-lacZ gene. (a,b) Representative example of (a) Rosa26 MSCs or (b) βgal-transduced MSCs after in vitro X-Gal staining just prior to intratracheal injection. Magnification 20x. (c) In vitro βgal activity in MSCs and Rosa26 MSCs just prior to intratracheal injection. (d) βgal activity in whole trachea-lungs homogenates, 24hr or 7 days after Rosa26 MSC intratracheal administration. Evaluated groups included animals that were intratracheally injected with the followings: no injection (control), PDOC and lysate of Rosa26 MSCs (PDOC + Rosa26 MSC lysate), PDOC and Rosa26 MSCs (PDOC + Rosa26 MSC). (e) In vivo detection of the nls-lacZ gene by PCR 24hr and 7 days after intratracheal injection of βgal-transduced MSCs. Dotted line depicts control threshold. * p<0.05.
In a last set of experiments, as previously performed with BMC9s we overexpressed nls-lacZ gene in MSCs from wild-type Swiss mice, to locate them in vivo at 24hr and 7 days. MSC transduction with the βgal retrovirus vector remained ineffective. Therefore, MSCs from Swiss mice were transduced prior to intratracheal injection with a lentivirus vector encoding the nls-lacZ gene (Conrad et al., 2007). After lentiviral transduction, 20.1 ± 3.0% MSCs expressed βgal (Fig. 6b, n = 4). Nevertheless, no blue staining was observed in trachea-lungs from animals injected intratracheally with PDOC and βgal-transduced MSCs at 7 days (not shown). Since rapid down regulation of foreign gene expression under cytomegalovirus promoter has been described in lungs (Pringle et al., 2005), we concurrently used PCR to detect the presence of nls-lacZ gene even without gene expression. PCR experiments using primers specific to nls-lacZ gene were performed 24hr and 7 days after βgal-transduced MSC injection (Fig. 6e). At 24hr, PCR was positive in trachea-lungs from each animal injected with PDOC and βgal-transduced MSCs (Fig. 6e, n = 7), confirming that βgal-transduced MSCs were present 24hr after injection in injured airway. However, no nls-lacZ gene was detected in animals at 7 days after βgal-transduced MSC injection, suggesting that no βgal-transduced MSCs from primary culture survived at this time once the epithelium is regenerated (Fig. 6e, n = 6).
Discussion
Using different and independent methods based on reporter gene transfer we demonstrated the short term survival of different types of exogenous stem cells, including embryonic and adult mesenchymal stem cells, after intratracheal injection in murine airway presenting acute epithelial airway injury and without total-body irradiation. In contrast to differentiated cells, 0.43 to 5.5 % stem cells were capable of surviving within the injured lungs at 24hr. Importantly no SCs survived in healthy airway or in the epithelial lining fluid. Biochemical staining showed that transduced MSCs were located in the lumen of conducting airway at 24hr and at 7 days. However, the in vivo amount of engrafted MSCs decreased dramatically with time. No primary MSCs were located within lungs at 7 days.
In our study, several gene transfer systems were used, according to our aims and to cell types. Using careful timing, non-viral vectors were useful to allow expression of the foreign gene only after intratracheal cell injection. Gene delivery with viral vectors was used for in vivo studies at 7 days, but we had to use different viral vectors for transducing MSCs from cell lines and primary MSCs. Depending on the gene transfer system and the cell type, gene transfer efficacy was variable in vitro. Studies with retrovirus vectors showed high efficacy and stable MSC transduction (up to 97%, (Sales et al., 2007)) whereas studies with lentivirus vectors showed variable efficacy between murine MSCs (up to 50%, (Ricks et al., 2008; Santoni de Sio et al., 2008; Xu et al., 2008)) and human MSCs (up to 93%, (Chan et al., 2005)). These data confirmed that primary murine MSCs are more difficult to transduce than human MSCs.
Engraftment of exogenous stem cells into the lungs has been reported in several in vivo studies, but cell engraftment rate remains controversial (Loebinger et al., 2008). Our results highlight the main advantage in using ELISA and biochemical methods to detect and quantify a small number of administered cells in the whole organ. Although lacZ gene is widely used to locate stem cells, some reports have shown that standard protocols for X-Gal staining can lead, especially in the lungs, both to false positive results as well as to failure to adequately detect βgal expressing cells (Weiss et al., 1997). In our study, while βgal activity significantly increased after intratracheal injection of MSCs from Rosa26 mice, surviving Rosa26 MSCs could not be located in vivo on histological slides, as already described in another report (MacPherson et al., 2005). The lacZ gene coupled with a nuclear localization signal had already been used in several studies from our group (Lemarchand et al., 1994; Chapelier et al., 1996) and others (Mastrangeli et al., 1993) and was shown to allow easy detection of endogenous from exogenous βgal activity. Using nls-LacZ gene, we were able to detect and locate nuclear βgal-expressing cells, even when βgal activity in the whole lungs was not significantly different from baseline.
We investigated whether stem cells would be capable of locating specifically on the epithelial side of airway after intratracheal administration. BAL experiments showed that stem cells expressing reporter protein did not survive in the epithelial lining fluid. X-Gal staining showed that stem cells were localised into conducting airway in vivo. By injecting stem cells in healthy airway, we also confirmed the major role of injured environment on the engraftment (MacPherson et al., 2005). Stem cells may not engraft into healthy airway because of mucociliary clearance mechanism that could impede access to airway epithelium, as described for in vivo gene transfer to the lungs (Weiss 2002). We speculated that mucociliary clearance would be disrupted in polidocanol (PDOC)-treated airway and that the freshly denuded basement membrane may also promote specifically stem cell adherence and engraftment in the airway epithelium (Engelhardt et al., 1992; Nikolova et al., 2006). In the first set of experiments, murine ESCs were intratracheally injected and a significant survival cell rate was observed, although in vitro ESC transfection rate was low. This confirms the great potential of ESC pluripotency for regenerative medicine and in particular airway injury. Nevertheless, ESCs for cell-based therapy have met with ethical, moral and political challenges, and with inherent risks associated with immune rejection (Chidgey et al., 2008). In further experiments, we focused our attention on MSCs because they are already used in the clinical setting (Uccelli et al., 2008). Our ELISA data indicated that MSC survival rate varied according to immortalized or primary cultures, probably because of the different gene transfer systems we used. Survival rates (from 0.4% to 5.5%) 24hr after intratracheal administration were higher than those described after intravenous administration (from 0.01% to 0.1%) and in agreement with those usually described in stem cell therapy protocols, including cardiology (Robey et al., 2008) or diabetology (Bottino et al., 2003) protocols, and lung injury models (Gupta et al., 2007; Wong et al., 2007; Wong et al., 2009).
We hypothesized that MSC incorporation in the lungs at 7 days could be masked by a down regulation of transgene expression into the lungs because of the common cytomegalovirus enhancer/promoter element in the virus vector constructs (Alton et al., 1999; Pringle et al., 2005). The negative results of PCR experiments on nls-lacZ gene at 7 days demonstrated that this was not the case. Allogeneic rejection seems unlikely to be responsible for the lack of survival of differentiated cells at 24hr since ESCs, although known to be targeted for rejection by the immune system (Chidgey et al., 2008), survived at 24hr. Nevertheless, allogeneic MSC rejection cannot be ruled out at 7 days.
Another hypothesis to explain MSC negative results at 7 days is the spontaneous regeneration of the airway epithelium that may have hampered MSC incorporation into epithelial airway. At 24hr, e.g. when there is a desquamation of the epithelial surface and a denuded basement membrane, the PDOC murine model is a good model to evaluate stem cell survival and presence in injured airway but stem cell differentiation into airway epithelial cells is unlikely at this time. At 7 days after PDOC administration, the epithelium was spontaneously regenerated, demonstrating that PDOC did not alter endogenous lung capability to regenerate. Therefore, the PDOC model may not be an adequate model to evaluate stem cell capability in regenerating lung epithelium, since the endogenous repair mechanisms may impede MSC contribution to achieve structural lung regeneration. This limitation was described in (Wong et al., 2007) study in which the naphthalene-injured airway epithelium was still undergoing rapid cell turnover and regeneration and the number of administered stem cells also decreased with time. A more appropriate animal model to study stem cell contribution to structural lung regeneration would be a model with chronic epithelial airway injury and a recurrent loss of the epithelial surface, due to a lack or an exhaustion of endogenous progenitor cells. This hypothesis was evaluated in animal models with permanent retinal epithelial degeneration such as the RCS rat model (D’Cruz et al., 2000) and the rhodopsin knock-out mouse (Humphries et al., 1997). MSC engraftment and epithelial regeneration involving MSCs were observed in these animal models (Arnhold et al., 2007; Inoue et al., 2007). For lung tissue, a recent murine model overexpressing the beta-subunit of epithelial Na+ channel (ENaC+/+) and showing epithelial degeneration in newborn animals (Mall et al., 2008) may be more appropriate to evaluate MSC contribution to lung regeneration.
Finally, an important limitation of our study was also the lack of phenotypic characterisation of surviving cells. This characterisation of stem cell-derived epithelial cells has been hampered by methodological problems, in the lungs as in many other organs such as the heart (Murry et al., 2004) or the brain (Castro et al., 2002), and remains difficult and controversial. In this regard, additional studies using complex transgenic models with reporter genes under the control of lung-specific promoters in vivo will be essential.
Supplementary Material
Acknowledgments
We thank Nicolas Ferry for providing the nls-lacZ retrovirus vector (Nantes, France) and Marie-France Gardahaut for providing Rosa26 mice (Nantes, France). We are grateful to Beatrice Delasalle for statistical analyses. This work was supported in part by a grant from “Vaincre la Mucoviscidose” (Paris, France).
Footnotes
No competing financial interests exist.
References
- Alton EW, Stern M, Farley R, Jaffe A, Chadwick SL, Phillips J, Davies J, Smith SN, Browning J, Davies MG, Hodson ME, Durham SR, Li D, Jeffery PK, Scallan M, Balfour R, Eastman SJ, Cheng SH, Smith AE, Meeker D, Geddes DM. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet. 1999;353(9157):947–54. doi: 10.1016/s0140-6736(98)06532-5. [DOI] [PubMed] [Google Scholar]
- Aluigi M, Fogli M, Curti A, Isidori A, Gruppioni E, Chiodoni C, Colombo MP, Versura P, D’Errico-Grigioni A, Ferri E, Baccarani M, Lemoli RM. Nucleofection is an efficient nonviral transfection technique for human bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24(2):454–61. doi: 10.1634/stemcells.2005-0198. [DOI] [PubMed] [Google Scholar]
- Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol. 2007;245(3):414–22. doi: 10.1007/s00417-006-0382-7. [DOI] [PubMed] [Google Scholar]
- Aubert D, Menoret S, Chiari E, Pichard V, Durand S, Tesson L, Moullier P, Anegon I, Ferry N. Cytotoxic immune response blunts long-term transgene expression after efficient retroviral-mediated hepatic gene transfer in rat. Mol Ther. 2002;5(4):388–96. doi: 10.1006/mthe.2002.0561. [DOI] [PubMed] [Google Scholar]
- Beckett T, Loi R, Prenovitz R, Poynter M, Goncz KK, Suratt BT, Weiss DJ. Acute lung injury with endotoxin or NO2 does not enhance development of airway epithelium from bone marrow. Mol Ther. 2005;12(4):680–6. doi: 10.1016/j.ymthe.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24(6):662–70. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- Bottino R, Lemarchand P, Trucco M, Giannoukakis N. Gene- and cell-based therapeutics for type I diabetes mellitus. Gene Ther. 2003;10(10):875–89. doi: 10.1038/sj.gt.3302015. [DOI] [PubMed] [Google Scholar]
- Bruscia EM, Ziegler EC, Price JE, Weiner S, Egan ME, Krause DS. Engraftment of donor-derived epithelial cells in multiple organs following bone marrow transplantation into newborn mice. Stem Cells. 2006;24(10):2299–308. doi: 10.1634/stemcells.2006-0166. [DOI] [PubMed] [Google Scholar]
- Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297(5585):1299. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- Chan J, O’Donoghue K, de la Fuente J, Roberts IA, Kumar S, Morgan JE, Fisk NM. Human fetal mesenchymal stem cells as vehicles for gene delivery. Stem Cells. 2005;23(1):93–102. doi: 10.1634/stemcells.2004-0138. [DOI] [PubMed] [Google Scholar]
- Chapelier A, Danel C, Mazmanian M, Bacha EA, Sellak H, Gilbert MA, Herve P, Lemarchand P. Gene therapy in lung transplantation: feasibility of ex vivo adenovirus-mediated gene transfer to the graft. Hum Gene Ther. 1996;7(15):1837–45. doi: 10.1089/hum.1996.7.15-1837. [DOI] [PubMed] [Google Scholar]
- Chateauvieux S, Ichante JL, Delorme B, Frouin V, Pietu G, Langonne A, Gallay N, Sensebe L, Martin MT, Moore KA, Charbord P. Molecular profile of mouse stromal mesenchymal stem cells. Physiol Genomics. 2007;29(2):128–38. doi: 10.1152/physiolgenomics.00197.2006. [DOI] [PubMed] [Google Scholar]
- Chidgey AP, Layton D, Trounson A, Boyd RL. Tolerance strategies for stem-cell-based therapies. Nature. 2008;453(7193):330–7. doi: 10.1038/nature07041. [DOI] [PubMed] [Google Scholar]
- Conrad C, Gupta R, Mohan H, Niess H, Bruns CJ, Kopp R, von Luettichau I, Guba M, Heeschen C, Jauch KW, Huss R, Nelson PJ. Genetically engineered stem cells for therapeutic gene delivery. Curr Gene Ther. 2007;7(4):249–60. doi: 10.2174/156652307781369119. [DOI] [PubMed] [Google Scholar]
- Coraux C, Nawrocki-Raby B, Hinnrasky J, Kileztky C, Gaillard D, Dani C, Puchelle E. Embryonic stem cells generate airway epithelial tissue. Am J Respir Cell Mol Biol. 2005;32(2):87–92. doi: 10.1165/rcmb.2004-0079RC. [DOI] [PubMed] [Google Scholar]
- D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9(4):645–51. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Caplan AI. Differentiation potential of conditionally immortalized mesenchymal progenitor cells from adult marrow of a H-2Kb-tsA58 transgenic mouse. J Cell Physiol. 1996;167(3):523–38. doi: 10.1002/(SICI)1097-4652(199606)167:3<523::AID-JCP16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Driscoll KE, Costa DL, Hatch G, Henderson R, Oberdorster G, Salem H, Schlesinger RB. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: uses and limitations. Toxicol Sci. 2000;55(1):24–35. doi: 10.1093/toxsci/55.1.24. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Yankaskas JR, Wilson JM. In vivo retroviral gene transfer into human bronchial epithelia of xenografts. J Clin Invest. 1992;90(6):2598–607. doi: 10.1172/JCI116155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179(3):1855–63. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15(2):216–9. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Iriyama A, Ueno S, Takahashi H, Kondo M, Tamaki Y, Araie M, Yanagi Y. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res. 2007;85(2):234–41. doi: 10.1016/j.exer.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Joliat MJ, Umeda S, Lyons BL, Lynes MA, Shultz LD. Establishment and characterization of a new osteogenic cell line (MOS-J) from a spontaneous C57BL/6J mouse osteosarcoma. In Vivo. 2002;16(4):223–8. [PubMed] [Google Scholar]
- Krause DS. Engraftment of bone marrow-derived epithelial cells. Ann N Y Acad Sci. 2005;1044:117–24. doi: 10.1196/annals.1349.015. [DOI] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105(3):369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Kuang PP, Lucey E, Rishikof DC, Humphries DE, Bronsnick D, Goldstein RH. Engraftment of neonatal lung fibroblasts into the normal and elastase-injured lung. Am J Respir Cell Mol Biol. 2005;33(4):371–7. doi: 10.1165/rcmb.2004-0319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarchand P, Jones M, Danel C, Yamada I, Mastrangeli A, Crystal RG. In vivo adenovirus-mediated gene transfer to lungs via pulmonary artery. J Appl Physiol. 1994;76(6):2840–5. doi: 10.1152/jappl.1994.76.6.2840. [DOI] [PubMed] [Google Scholar]
- Loebinger MR, Aguilar S, Janes SM. Therapeutic potential of stem cells in lung disease: progress and pitfalls. Clin Sci (Lond) 2008;114(2):99–108. doi: 10.1042/CS20070073. [DOI] [PubMed] [Google Scholar]
- Loebinger MR, Sage EK, Janes SM. Mesenchymal stem cells as vectors for lung disease. Proc Am Thorac Soc. 2008;5(6):711–6. doi: 10.1513/pats.200801-009AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173(2):171–9. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson H, Keir P, Webb S, Samuel K, Boyle S, Bickmore W, Forrester L, Dorin J. Bone marrow-derived SP cells can contribute to the respiratory tract of mice in vivo. J Cell Sci. 2005;118(Pt 11):2441–50. doi: 10.1242/jcs.02375. [DOI] [PubMed] [Google Scholar]
- MacPherson H, Keir PA, Edwards CJ, Webb S, Dorin JR. Following damage, the majority of bone marrow-derived airway cells express an epithelial marker. Respir Res. 2006;7:145. doi: 10.1186/1465-9921-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall MA, Harkema JR, Trojanek JB, Treis D, Livraghi A, Schubert S, Zhou Z, Kreda SM, Tilley SL, Hudson EJ, O’Neal WK, Boucher RC. Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. Am J Respir Crit Care Med. 2008;177(7):730–42. doi: 10.1164/rccm.200708-1233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangeli A, Danel C, Rosenfeld MA, Stratford-Perricaudet L, Perricaudet M, Pavirani A, Lecocq JP, Crystal RG. Diversity of airway epithelial cell targets for in vivo recombinant adenovirus-mediated gene transfer. J Clin Invest. 1993;91(1):225–34. doi: 10.1172/JCI116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4(9):e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90(18):8424–8. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10(3):397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Parsons DW, Grubb BR, Johnson LG, Boucher RC. Enhanced in vivo airway gene transfer via transient modification of host barrier properties with a surface-active agent. Hum Gene Ther. 1998;9(18):2661–72. doi: 10.1089/hum.1998.9.18-2661. [DOI] [PubMed] [Google Scholar]
- Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92(11):4857–61. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitard B, Oudrhiri N, Lambert O, Vivien E, Masson C, Wetzer B, Hauchecorne M, Scherman D, Rigaud JL, Vigneron JP, Lehn JM, Lehn P. Sterically stabilized BGTC-based lipoplexes: structural features and gene transfection into the mouse airways in vivo. J Gene Med. 2001;3(5):478–87. doi: 10.1002/jgm.211. [DOI] [PubMed] [Google Scholar]
- Pringle IA, Raman S, Sharp WW, Cheng SH, Hyde SC, Gill DR. Detection of plasmid DNA vectors following gene transfer to the murine airways. Gene Ther. 2005;12(15):1206–14. doi: 10.1038/sj.gt.3302518. [DOI] [PubMed] [Google Scholar]
- Ricks DM, Kutner R, Zhang XY, Welsh DA, Reiser J. Optimized lentiviral transduction of mouse bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2008;17(3):441–50. doi: 10.1089/scd.2007.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon HJ, Polak JM, Qin M, Bishop AE. Derivation of distal lung epithelial progenitors from murine embryonic stem cells using a novel three-step differentiation protocol. Stem Cells. 2006;24(5):1389–98. doi: 10.1634/stemcells.2005-0465. [DOI] [PubMed] [Google Scholar]
- Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45(4):567–81. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales VL, Mettler BA, Lopez-Ilasaca M, Johnson JA, Jr, Mayer JE., Jr Endothelial progenitor and mesenchymal stem cell-derived cells persist in tissue-engineered patch in vivo: application of green and red fluorescent protein-expressing retroviral vector. Tissue Eng. 2007;13(3):525–35. doi: 10.1089/ten.2006.0128. [DOI] [PubMed] [Google Scholar]
- Santoni de Sio FR, Gritti A, Cascio P, Neri M, Sampaolesi M, Galli C, Luban J, Naldini L. Lentiviral vector gene transfer is limited by the proteasome at postentry steps in various types of stem cells. Stem Cells. 2008;26(8):2142–52. doi: 10.1634/stemcells.2007-0705. [DOI] [PubMed] [Google Scholar]
- Selig L, Pages JC, Tanchou V, Preveral S, Berlioz-Torrent C, Liu LX, Erdtmann L, Darlix J, Benarous R, Benichou S. Interaction with the p6 domain of the gag precursor mediates incorporation into virions of Vpr and Vpx proteins from primate lentiviruses. J Virol. 1999;73(1):592–600. doi: 10.1128/jvi.73.1.592-600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serikov VB, Popov B, Mikhailov VM, Gupta N, Matthay MA. Evidence of temporary airway epithelial repopulation and rare clonal formation by BM-derived cells following naphthalene injury in mice. Anat Rec (Hoboken) 2007;290(9):1033–45. doi: 10.1002/ar.20574. [DOI] [PubMed] [Google Scholar]
- Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A, Bulbena O. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2007;176(12):1261–8. doi: 10.1164/rccm.200610-1491OC. [DOI] [PubMed] [Google Scholar]
- Southam DS, Dolovich M, O’Byrne PM, Inman MD. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L833–9. doi: 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177(7):701–11. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297(5590):2256–9. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA, Jr, Spees JL, Bertucci D, Peister A, Weiss DJ, Valentine VG, Prockop DJ, Kolls JK. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci U S A. 2005;102(1):186–91. doi: 10.1073/pnas.0406266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DJ. Delivery of gene transfer vectors to lung: obstacles and the role of adjunct techniques for airway administration. Mol Ther. 2002;6(2):148–52. doi: 10.1006/mthe.2002.0662. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Liggitt D, Clark JG. In situ histochemical detection of beta-galactosidase activity in lung: assessment of X-Gal reagent in distinguishing lacZ gene expression and endogenous beta-galactosidase activity. Hum Gene Ther. 1997;8(13):1545–54. doi: 10.1089/hum.1997.8.13-1545. [DOI] [PubMed] [Google Scholar]
- Wong AP, Dutly AE, Sacher A, Lee H, Hwang DM, Liu M, Keshavjee S, Hu J, Waddell TK. Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L740–52. doi: 10.1152/ajplung.00050.2007. [DOI] [PubMed] [Google Scholar]
- Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A, Hu J, Waddell TK. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest. 2009;119(2):336–48. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Qu J, Cao L, Sai Y, Chen C, He L, Yu L. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214(4):472–81. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L131–41. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15(9):871–5. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


