Abstract
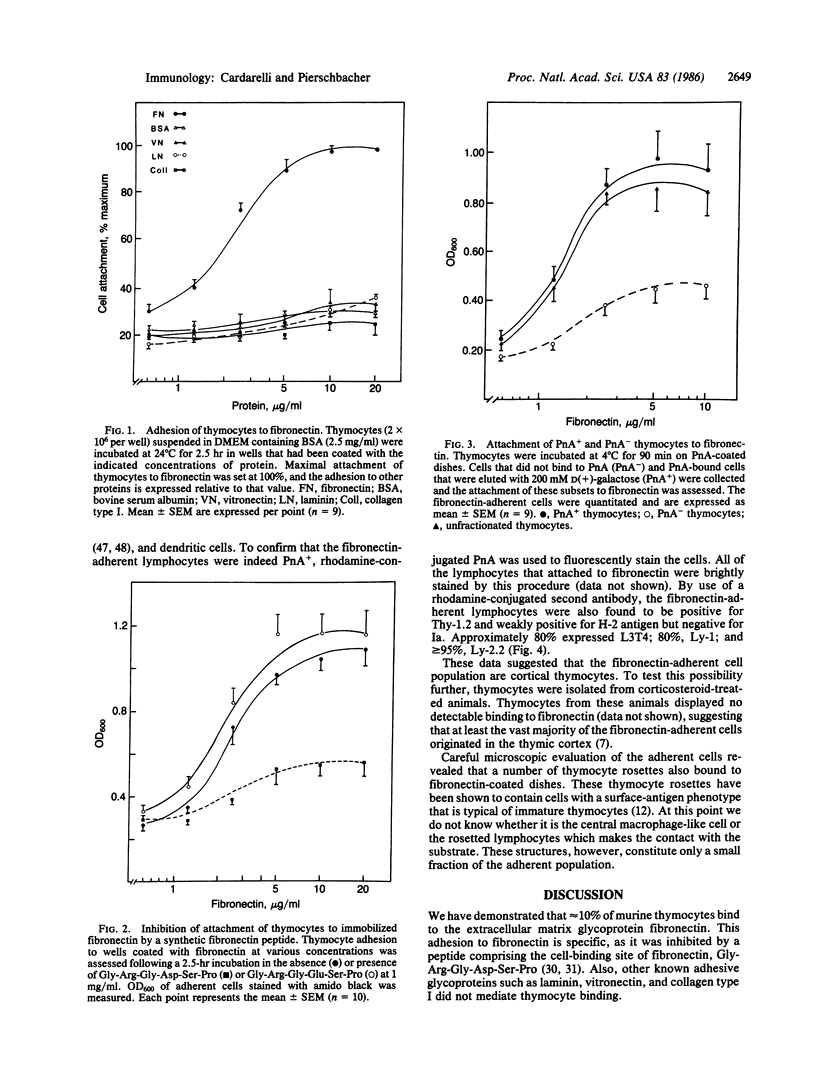
A population of murine thymocytes adheres specifically to fibronectin but not to vitronectin, laminin, or collagen type I. The interaction of these thymocytes with fibronectin could be inhibited by the synthetic peptide Gly-Arg-Gly-Asp-Ser-Pro, which comprises the previously identified cell-attachment determinant of the molecule, suggesting that the cell attachment site on fibronectin is recognized by these cells. A similar peptide, in which the aspartate residue had been replaced with glutamate, had no effect on this adhesion. The fibronectin-adherent thymocytes were found to be cortisone-sensitive; to bind peanut agglutinin; to have a Thy-1.2+, Ia- surface phenotype; and to express H-2 antigen only weakly on their surface. In addition, approximately 80% of the fibronectin-adherent cells expressed L3T4 and 80% expressed Ly-1 on their surface, whereas greater than 95% were positive for Ly-2. The data suggest that these cells, which constitute 10% of all thymic lymphocytes, are cortical thymocytes. We propose that their adhesion to fibronectin may be important for their differentiation. The binding to fibronectin provides a means to selectively isolate these cells for study.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P., Shortman K., Scollay R., Potworowski E. F., Kruisbeek A. M., Goldstein G., Trainin N., Bach J. F. Thymus hormones do not induce proliferative ability or cytolytic function in PNA+ cortical thymocytes. Cell Immunol. 1985 Apr 1;91(2):455–466. doi: 10.1016/0008-8749(85)90243-6. [DOI] [PubMed] [Google Scholar]
- Barnes D. W., Silnutzer J. Isolation of human serum spreading factor. J Biol Chem. 1983 Oct 25;258(20):12548–12552. [PubMed] [Google Scholar]
- Barnes D., Wolfe R., Serrero G., McClure D., Sato G. Effects of a serum spreading factor on growth and morphology of cells in serum-free medium. J Supramol Struct. 1980;14(1):47–63. doi: 10.1002/jss.400140106. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Boucaut J. C., Darribère T., Poole T. J., Aoyama H., Yamada K. M., Thiery J. P. Biologically active synthetic peptides as probes of embryonic development: a competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest cell migration in avian embryos. J Cell Biol. 1984 Nov;99(5):1822–1830. doi: 10.1083/jcb.99.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant B. J. Renewal and fate in the mammalian thymus: mechanisms and inferences of thymocytokinetics. Eur J Immunol. 1972 Feb;2(1):38–45. doi: 10.1002/eji.1830020109. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Lymphocyte adherence to high endothelial venules: characterization of a modified in vitro assay, and examination of the binding of syngeneic and allogeneic lymphocyte populations. J Immunol. 1979 Nov;123(5):1996–2003. [PubMed] [Google Scholar]
- Cardarelli P. M., Blumenstock F. A., Saba T. M., Rourke F. J. Fibronectin-enhanced attachment of gelatin-coated erythrocytes to isolated hepatic Kupffer cells. J Leukoc Biol. 1984 Oct;36(4):477–492. doi: 10.1002/jlb.36.4.477. [DOI] [PubMed] [Google Scholar]
- Carlsson R., Engvall E., Freeman A., Ruoslahti E. Laminin and fibronectin in cell adhesion: enhanced adhesion of cells from regenerating liver to laminin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2403–2406. doi: 10.1073/pnas.78.4.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman J. R., Hök M., Rees D. A., Timpl R. Adhesion, growth, and matrix production by fibroblasts on laminin substrates. J Cell Biol. 1983 Jan;96(1):177–183. doi: 10.1083/jcb.96.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Doran T. I., Vidrich A., Sun T. T. Intrinsic and extrinsic regulation of the differentiation of skin, corneal and esophageal epithelial cells. Cell. 1980 Nov;22(1 Pt 1):17–25. doi: 10.1016/0092-8674(80)90150-6. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Ezine S., Weissman I. L., Rouse R. V. Bone marrow cells give rise to distinct cell clones within the thymus. Nature. 1984 Jun 14;309(5969):629–631. doi: 10.1038/309629a0. [DOI] [PubMed] [Google Scholar]
- Fink P. J., Gallatin W. M., Reichert R. A., Butcher E. C., Weissman I. L. Homing receptor-bearing thymocytes, an immunocompetent cortical subpopulation. Nature. 1985 Jan 17;313(5999):233–235. doi: 10.1038/313233a0. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Scheid M., Hammerling U., Schlesinger D. H., Niall H. D., Boyse E. A. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):11–15. doi: 10.1073/pnas.72.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Grinnell F., Hays D. G., Minter D. Cell adhesion and spreading factor. Partial purification and properties. Exp Cell Res. 1977 Nov;110(1):175–190. doi: 10.1016/0014-4827(77)90284-1. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Engvall E., A'Hearn E., Barnes D., Pierschbacher M., Ruoslahti E. Cell attachment on replicas of SDS polyacrylamide gels reveals two adhesive plasma proteins. J Cell Biol. 1982 Oct;95(1):20–23. doi: 10.1083/jcb.95.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman E. G., Pierschbacher M. D., Ohgren Y., Ruoslahti E. Serum spreading factor (vitronectin) is present at the cell surface and in tissues. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4003–4007. doi: 10.1073/pnas.80.13.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman E. G., Pierschbacher M. D., Ruoslahti E. Detachment of cells from culture substrate by soluble fibronectin peptides. J Cell Biol. 1985 Jun;100(6):1948–1954. doi: 10.1083/jcb.100.6.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F. The human thymic microenvironment. Adv Immunol. 1984;36:87–142. doi: 10.1016/s0065-2776(08)60900-1. [DOI] [PubMed] [Google Scholar]
- Holmes R. Preparation from human serum of an alpha-one protein which induces the immediate growth of unadapted cells in vitro. J Cell Biol. 1967 Feb;32(2):297–308. doi: 10.1083/jcb.32.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Thomas J. A., Bollum F. J., Granger S., Pizzolo G., Bradstock K. F., Wong L., McMichael A., Ganeshaguru K., Hoffbrand A. V. The human thymic microenvironment: an immunohistologic study. J Immunol. 1980 Jul;125(1):202–212. [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebe R. J. Isolation of a collagen-dependent cell attachment factor. Nature. 1974 Jul 19;250(463):248–251. doi: 10.1038/250248a0. [DOI] [PubMed] [Google Scholar]
- Kyewski B. A., Rouse R. V., Kaplan H. S. Thymocyte rosettes: multicellular complexes of lymphocytes and bone marrow-derived stromal cells in the mouse thymus. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5646–5650. doi: 10.1073/pnas.79.18.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- McKenzie I. F., Potter T. Murine lymphocyte surface antigens. Adv Immunol. 1979;27:179–338. doi: 10.1016/s0065-2776(08)60263-1. [DOI] [PubMed] [Google Scholar]
- Owen J. J., Jenkinson E. J. Embryology of the lymphoid system. Prog Allergy. 1981;29:1–34. [PubMed] [Google Scholar]
- Ozato K., Mayer N. M., Sachs D. H. Monoclonal antibodies to mouse major histocompatibility complex antigens. Transplantation. 1982 Sep;34(3):113–120. doi: 10.1097/00007890-198209000-00001. [DOI] [PubMed] [Google Scholar]
- Pearlstein E. Plasma membrane glycoprotein which mediates adhesion of fibroblasts to collagen. Nature. 1976 Aug 5;262(5568):497–500. doi: 10.1038/262497a0. [DOI] [PubMed] [Google Scholar]
- Pennypacker J. P., Hassell J. R., Yamada K. M., Pratt R. M. The influence of an adhesive cell surface protein on chondrogenic expression in vitro. Exp Cell Res. 1979 Jul;121(2):411–415. doi: 10.1016/0014-4827(79)90022-3. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Hayman E. G., Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981 Oct;26(2 Pt 2):259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M., Hayman E. G., Ruoslahti E. Synthetic peptide with cell attachment activity of fibronectin. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1224–1227. doi: 10.1073/pnas.80.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Reichert R. A., Gallatin W. M., Butcher E. C., Weissman I. L. A homing receptor-bearing cortical thymocyte subset: implications for thymus cell migration and the nature of cortisone-resistant thymocytes. Cell. 1984 Aug;38(1):89–99. doi: 10.1016/0092-8674(84)90529-4. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Linker-Israeli M., Sharon N. Separation of mouse thymocytes into two subpopulations by the use of peanut agglutinin. Cell Immunol. 1976 Jul;25(1):129–134. doi: 10.1016/0008-8749(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Rojkind M., Gatmaitan Z., Mackensen S., Giambrone M. A., Ponce P., Reid L. M. Connective tissue biomatrix: its isolation and utilization for long-term cultures of normal rat hepatocytes. J Cell Biol. 1980 Oct;87(1):255–263. doi: 10.1083/jcb.87.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. D., Singer M. S., Yednock T. A., Stoolman L. M. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science. 1985 May 24;228(4702):1005–1007. doi: 10.1126/science.4001928. [DOI] [PubMed] [Google Scholar]
- Rothenberg E. A specific biosynthetic marker for immature thymic lymphoblasts. Active synthesis of thymus-leukemia antigen restricted to proliferating cells. J Exp Med. 1982 Jan 1;155(1):140–154. doi: 10.1084/jem.155.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin K., Johansson S., Pettersson I., Ocklind C., Obrink B., Hök M. Attachment of rat hepatocytes to collagen and fibronectin; a study using antibodies directed against cell surface components. Biochem Biophys Res Commun. 1979 Nov 14;91(1):86–94. doi: 10.1016/0006-291x(79)90586-2. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin in cell adhesion and invasion. Cancer Metastasis Rev. 1984;3(1):43–51. doi: 10.1007/BF00047692. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G. Two active sites with different characteristics in fibronectin. FEBS Lett. 1979 Jan 15;97(2):221–224. doi: 10.1016/0014-5793(79)80088-5. [DOI] [PubMed] [Google Scholar]
- SAINTE-MARIE G., LEBLOND C. P. Origin and fate of cells in the medulla of rat thymus. Proc Soc Exp Biol Med. 1958 Aug-Sep;98(4):909–915. doi: 10.3181/00379727-98-24226. [DOI] [PubMed] [Google Scholar]
- Scheid M. P., Hoffmann M. K., Komuro K., Hämmerling U., Abbott J., Boyse E. A., Cohen G. H., Hooper J. A., Schulof R. S., Goldstein A. L. Differentiation of T cells induced by preparations from thymus and by nonthymic agents. J Exp Med. 1973 Oct 1;138(4):1027–1032. doi: 10.1084/jem.138.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollay R., Bartlett P., Shortman K. T cell development in the adult murine thymus: changes in the expression of the surface antigens Ly2, L3T4 and B2A2 during development from early precursor cells to emigrants. Immunol Rev. 1984 Dec;82:79–103. doi: 10.1111/j.1600-065x.1984.tb01118.x. [DOI] [PubMed] [Google Scholar]
- Scollay R., Shortman K. Identification of early stages of T lymphocyte development in the thymus cortex and medulla. J Immunol. 1985 Jun;134(6):3632–3642. [PubMed] [Google Scholar]
- Scollay R., Wilson A., Shortman K. Thymus cell migration: analysis of thymus emigrants with markers that distinguish medullary thymocytes from peripheral T cells. J Immunol. 1984 Mar;132(3):1089–1094. [PubMed] [Google Scholar]
- Stutman O. Intrathymic and extrathymic T cell maturation. Immunol Rev. 1978;42:138–184. doi: 10.1111/j.1600-065x.1978.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Sundqvist K. G., Wanger L. Anchorage and lymphocyte function II. Contact with non-cellular surfaces, cell density and T-cell activation. Immunology. 1981 Jul;43(3):573–580. [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Oldberg A., Hayman E. G., Pierschbacher M. D., Ruoslahti E. Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. EMBO J. 1985 Oct;4(10):2519–2524. doi: 10.1002/j.1460-2075.1985.tb03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Pierschbacher M. D., Hayman E. G., Nguyen K., Ohgren Y., Ruoslahti E. Domain structure of vitronectin. Alignment of active sites. J Biol Chem. 1984 Dec 25;259(24):15307–15314. [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Weissman I. L., McGrath M. S., Pillemer E., Hollander N., Rouse R. V., Jerabek L., Stevens S. K., Scollay R. G., Butcher E. C. Normal and neoplastic lymphocyte maturation. J Supramol Struct Cell Biochem. 1981;15(3):303–314. doi: 10.1002/jsscb.1981.380150307. [DOI] [PubMed] [Google Scholar]
- Woodruff J. J., Rasmussen R. A. In vitro adherence of lymphocytes to unfixed and fixed high endothelial cells of lymph nodes. J Immunol. 1979 Nov;123(5):2369–2372. [PubMed] [Google Scholar]




