Abstract
Molecular data have provided many insights into cetacean evolution but some unsettled issues still remain. We estimated the topology and timing of cetacean evolutionary relationships using Bayesian and maximum likelihood analyses of complete mitochondrial genomes. In order to clarify the phylogenetic placement of Sotalia and Steno within the Delphinidae, we sequenced three new delphinid mitogenomes. Our analyses support three delphinid clades: one joining Steno and Sotalia (supporting the revised subfamily Stenoninae); another placing Sousa within the Delphininae; and a third, the Globicephalinae, which includes Globicephala, Feresa, Pseudorca, Peponocephala and Grampus. We also conclude that Orcinus does not belong in the Globicephalinae, but Orcaella may be part of that subfamily. Divergence dates were estimated using the relaxed molecular clock calibrated with fossil data. We hypothesise that the timing of separation of the marine and Amazonian Sotalia species (2.3 Ma) coincided with the establishment of the modern Amazon River basin.
Introduction
The phylogeny of cetaceans has been intensively investigated over the last decade using molecular data. Classical arrangements have been drastically modified, such as the positioning of the clade within the artiodactyls [1], [2], [3], [4], [5], [6], [7], [8] and the monophyletic status of several genera and higher taxonomic groups [9], [10], [11], [12], [13], [14]. Modern Cetacea consists of two evolutionary lineages supported by morphological [15], [16] and molecular data [11], [17]: the Mysticeti (baleen whales) and the Odontoceti (toothed whales). Odontocetes are of particular evolutionary interest as they include several species that have adapted to riverine environments. Furthermore, the rapid diversification of the Delphinidae makes the phylogenetic inference of their evolutionary history challenging.
Within the Delphinidae, the systematics of the genus Sotalia has been the focus of several recent studies. After the recognition that the genus comprises two species, S. fluviatilis and S. guianensis, the former became the only known exclusively freshwater delphinid in the world [18]. However, the phylogenetic placement of Sotalia within the family is still unresolved [5], [10], [19], [20], [21], [22]. Moreover, different studies have estimated different timings for the separation between the two Sotalia species [20], [21], [23], [24]. Although it is generally believed that the changes in the Amazon during the Plio-Pleistocene drove the diversification of Sotalia species [18], [23], a clearer evolutionary scenario can only be depicted in light of reliable estimates of the phylogenetic position and the chronology of Sotalia speciation.
In order to better assess such historical information on Sotalia evolution and to establish its phylogenetic position within the Delphinidae, we sequenced the complete mitochondrial genomes of S. fluviatilis, S. guianensis and Steno bredanensis. Besides providing a more precise estimate of the timing of separation of Sotalia species, our analyses also shed light on delphinid phylogeny and increased the evidence in favour of Steno being a sister taxon to Sotalia.
Results
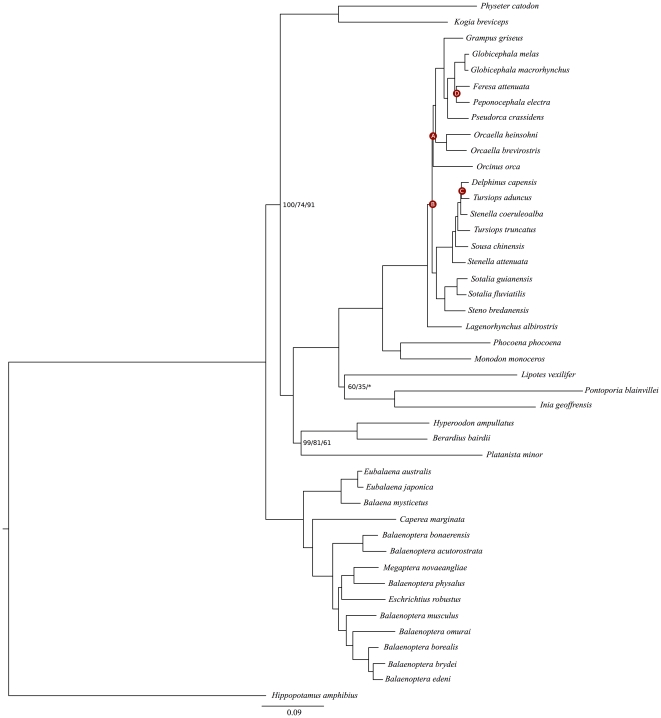
Both Bayesian and maximum likelihood trees were topologically congruent and presented a similar pattern of statistical support distribution for the nodes (Figure 1). Except for delphinid relationships, our phylogeny is largely in agreement with recent mitogenomic studies [11], [25], [26], [27], [28].
Figure 1. Phylogeny of Cetacea.
Support values represent PP/aLRT/BS. Nodes without information were supported by 100/100/100. (A) 61/52/*, (B) 100/80/*, (C) 55/89/*, (D) 100/91/73. (*) Indicates that RAxML BS is <50.
Within the Delphinidae, two major lineages were statistically supported (100% PP, aLRT and BS); the Delphininae (Tursiops+Stenella+Delphinus+Sousa) and the Stenoninae (Sotalia+Steno) clades. Those two clades are closely related (100% PP and aLRT, 99% BS). A third clade, the Globicephalinae (Globicephala+Pseudorca+Grampus+Peponocephala+Feresa), may include Orcaella, and is only supported with the exclusion of Orca. Alternatively, subfamily Orcaellinae could be a sister taxon to Globicephalinae. There is no support for subfamily Orcininae (Orcinus+Orcaella). The position of Orcinus within the family is unclear, and the white-beaked dolphin, Lagenorhynchus albirostris, was inferred as a sister to the remaining delphinids (100% PP, aLRT and BS).
Contrary to recent works of Caballero et al. [19] and McGowen et al. [21], [22], our analysis of complete dolphin mitochondrial genomes show that Steno bredanensis is phylogenetically more related to Sotalia dolphins than to the Globicephalinae. We have investigated whether the arrangement proposed by the former papers [19], [21], [22] was statistically superior to the one supported by our tree via the Kishino-Hasegawa test [29]. The topology presented in this study (Figure 1) significantly increases the likelihood of our data (ΔlnL = 339.0, p≅0), rejecting the null hypothesis that the likelihoods of both trees are equal.
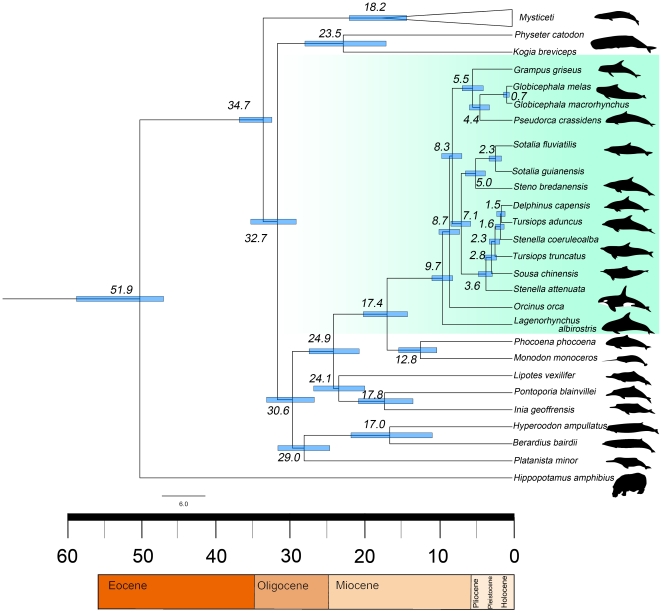
Beginning in the late Miocene (9.5±1.4 Ma, Mega annum) the Delphinidae experienced a rapid diversification (Figure 2). The clades that presented significant statistical support within the Delphinidae diversified around the Pliocene (5.5 – 3.5 Ma). The separation between the marine and riverine species of Sotalia was estimated at approximately 2.3 Ma (1.3–3.4 Ma). Species with difficult taxonomic assignment at the generic level, such as Tursiops spp. and Stenella spp., were all inferred to have diversified in the late Pliocene and Pleistocene.
Figure 2. Timescale of Odontoceti evolution.
Discussion
Mitogenomic analyses have the potential to disclose delphinid evolutionary relationships because the group has undergone rapid and recent diversification. The effective population size of mitochondrial lineages is smaller than that of nuclear gene lineages. Thus, the probability of reaching reciprocal monophyly in small time intervals is higher in mitochondrial genomes [30]. As more cetacean mitogenomes are sequenced, phylogenetic reconstructions become more capable of settling long standing issues. The inclusion of three new dolphin mitogenomes in the analyses have contributed to the resolution of uncertain delphinid evolutionary affinities, especially concerning the phylogenetic placement of Sotalia and Steno dolphins.
Delphinidae
The grouping of Sotalia and Steno was strongly supported, as well as the positioning of Sousa within the Delphininae. These results indicate that Steno is the sister group of Sotalia, thus supporting the revised subfamily Stenoninae as proposed by LeDuc et al. [10]. Although those authors could not reach a definitive conclusion regarding the sister group relationship between Steno and Sotalia based on their cytochrome b data, they decided to maintain both genera in Stenoninae as indicated by earlier morphological studies. However, they suggested that the revised Stenoninae does not include Sousa, a genus also traditionally assigned to that subfamily based on morphology. Instead, they placed Sousa in Delphininae. Our phylogeny reinforces the view that Sousa belongs in the Delphininae, as formerly indicated by other studies [5], [19], [20], [21], [25], [28].
The close affinity between Steno and Sotalia and the placement of Sousa in the Delphininae were previously suggested using cytochrome b data [5], [31] and a supermatrix of nine nuclear and six mitochondrial genes [20]. Three studies disputed this conclusion. Caballero et al. [19], and McGowen et al. [21], [22] proposed placing both Sotalia and Sousa in the Delphininae, and grouping Steno with Globicephalinae+Grampus+Orcaella. In those studies, conclusions were based on a combined nuclear and mitochondrial dataset. In the case of Caballero et al. [19] and McGowen et al. [21], [22], the combined phylogeny seemed to be driven mainly by their nuclear data, since their mitochondrial phylogeny did not support such groupings. Interestingly, however, Steeman et al. [20] also combined nuclear and mitochondrial data, and recovered the same topology observed here and in the studies mentioned above. In a recent mitogenomic phylogeny of the Delphinidae Steno was also placed outside the Globicephalinae [28].
The new conformation of Stenoninae implies that the phylogenetic placement of the fossil genus Astadelphis requires revision. Astadelphis has been assigned to this subfamily, and is only recorded from Pliocene deposits (3.1–3.8 Ma) of Italy. It has been considered by different authors as phylogenetically close to Steno and Sotalia [32] or to Sousa [33]. If that latter view is correct, Astadelphis does not belong in Stenoninae.
Our phylogeny confirms the phylogenetic position of Grampus within the Globicephalinae, as first proposed by LeDuc et al. [10] and contrary to the traditional view, based on morphology, which included Grampus in the subfamily Delphininae [34]. Previous studies based on the cytochrome b [5], mitochondrial genomes [28], [35] and combined mitochondrial and nuclear genes [19], [20], [21], [22], [36] also recovered the placement of Grampus in the Globicephalinae.
On the other hand, Orcinus, traditionally placed in the Globicephalinae [34], usually figures in molecular phylogenies either as “incertae sedis” [10], [20], [21], [36], or pooled with Orcaella [5]. Recently, however, a mitogenomic study proposed the inclusion of both Orcinus and Orcaella in the Globicephalinae [28], in agreement with early views based on morphology. Our phylogeny strongly supports the exclusion of Orcinus from Globicephalinae. We also refute the existence of the subfamily Orcininae (Orcinus+Orcaella), as proposed by LeDuc et al. [10] and Agnarsson and May-Collado [5] based on cytochrome b data. Instead, Orcaella may be part of the Globicephalinae, or warrant a separate subfamily, as proposed by Perrin [34]. The relationship of Orcinus to the other delphinid subfamilies remains unresolved.
Lagenorhynchus albirostris occupies the most basal position within the delphinids. Unfortunately, since our phylogeny lacks mitogenomes from the genera Lissodelphis, Cephalorhynchus and the other Lagenorhynchus species, it is not yet possible to ascertain the evolutionary relationships of all Delphinidae subfamilies, nor the monophyly of Lissodelphininae, which has been questioned [10], [37].
Unsurprisingly, our analyses were unable to shed light on the Stenella-Delphinus-Tursiops complex, due to the current lack of mitochondrial genomes from many species. Our phylogeny only reinforced previous findings concerning the para- or polyphyly of Stenella and Tursiops [5], [10], [20], [21]. The recent speciation (<4 Ma) of these lineages poses many difficulties in phylogenetic inference (such as low number of informative characters, incomplete lineage sorting and the possible existence of fertile hybrids), and understanding their evolution may require the aid of phylogeographical approaches.
Timing of Sotalia speciation
Dating the divergence between riverine and marine Sotalia is crucial to understand the phylogeography of S. fluviatilis, as it indicates when this species became genetically isolated after colonising the Amazon basin. To date, all divergence estimates have been based either on the mitochondrial control region [23], cytochrome b [20], [21] or on both markers [18], [24]. Thus, our mitogenomic phylogeny provides the best opportunity so far to date more precisely the divergence between S. guianensis and S. fluviatilis.
Four previous studies attempted to estimate the timing of Sotalia speciation. The genetic divergence (p distance) between the Sotalia species observed by Cunha et al. [18] for both the control region and the cytochrome b was 2.5%. Taking into consideration the evolutionary rates of these markers in cetaceans – control region: 0.5% to 1% per million years [38]; cytochrome b: 1%/Ma, [39] – the speciation event that separated both lineages would have happened between 2.5 and 1.25 Ma, during the early Pleistocene [24]. A somewhat similar estimate was obtained using a relaxed molecular clock and cytochrome b data, 1.99 Ma (0.63–3.67) [21]. The dates proposed by Cunha et al. [24] and McGowen et al. [21] overlap with our estimate – 2.3 Ma (1.3–3.4). A different timing of the speciation was proposed by Caballero et al. [23], who calibrated a molecular clock for the control region using the estimated divergence between Sotalia and Phocoena phocoena based on the fossil record (10 to 11 Ma). Therefore, they arrived at a faster substitution rate, and dated the divergence between S. fluviatilis and S. guianensis much later, at 1.0 to 1.2 Ma. Finally, the oldest time estimate for the separation of Sotalia species (3.5 Ma) was obtained by Steeman et al. [20]. It is noteworthy that, in spite of being a supermatrix analysis (of 15 mitochondrial and nuclear markers) using seven fossil calibration points and relaxed clock models, the divergence between the Sotalia species in that study was based exclusively on the cytochrome b gene (the only marker analysed from S. guianensis by the authors).
Besides being the most precise estimate available, our dating coincides remarkably well with the establishment of the modern Amazon River basin. Until recently, authors accepted that the modern Amazon basin was already established in the Miocene, but that view has changed based on new geological data. Sediment analyses showed that the Amazon River only attained its present conformation by the beginning of the Pleistocene, approximately 2.5 Ma [40], [41]. At the same time, there was a major lowering of sea level from 3 to 2 Ma [42], which could have been partly responsible for changing the river's course eastwards, coupled with Andean tectonics [40], [41]. Irrespective of the environmental conditions prevailing at the time, Sotalia dolphins that colonised the Amazon basin certainly had an Atlantic origin, because the connection with the Caribbean via the Paleo-Orinoco river and the Paleo-Maracaibo had been closed since the rising of the northern Andes cordillera, 8 Ma [43], [44].
Methods
DNA extraction, amplification and sequencing
Total DNA was isolated from skin samples of Steno bredanensis, Sotalia guianensis and S. fluviatilis using the standard phenol-chloroform procedure [45]. Two long fragments (about 9 Mb and 7 Mb), comprising the entire mitochondrial genome, were PCR-amplified using the primers described by Sasaki et al. [46]. Amplifications were carried out in 50 µL reactions using the Qiagen LongRange PCR Kit. Reagent concentrations and cycling profile followed the manufacturer's instructions.
Long-PCR products were then used as templates for amplification of smaller fragments, using the primers described in Xiong et al. [25] and others developed in this study (Table S1). PCR reactions (30 µL) contained 1.5 U Taq, 200 µM dNTP, 2.5 mM MgCl2, 15 µg BSA and 0.5 µM of each primer. Amplification thermal conditions were as follows: 3 min at 93°C, 30 cycles of 1 min at 92°C, 1 min at 50°C or 55°C, 1 min at 72°C, and 5 min of final extension at 72°C. PCR products were purified using ExoSap (GE) and both strands were sequenced in an ABI3130 using BigDye chemistry.
Sequences were edited in SeqMan 7 (DNAStar), using the complete mitochondrial genome of Sousa chinensis [GenBank EU557091] as template to build the contigs. The complete mitogenomes were deposited in GenBank (JF681038, JF681039 and JF681040).
Alignment and evolutionary analyses
All cetacean mitochondrial genomes available in GenBank as of March 2011 were included in this study (Table S2). Alignments were conducted for each gene individually in ClustalW [47] and manually checked. A supermatrix of 15,873 bp was used in phylogeny estimation and divergence time inference. It consisted of the concatenation of all 13 protein coding genes, tRNA and rRNA genes and D-loop. Protein coding genes were further separated into three partitions containing only first, second and third codon positions. This was done to maximise rate variation among partitions [48]. Phylogeny estimation was performed in MrBayes 3 [49], PhyML 3 [50] and RAxML 7.0.4 [51]. In MrBayes and RAxML, each partition was allowed to evolve independently under the GTR+G+I model.
For the Bayesian inference, the Markov chain Monte Carlo (MCMC) settings used were as follows. Two independent runs, with three Markov chains each, were sampled every 100th generation during 107 generations, resulting in 100,000 trees in each run, of which 25% were discarded as burn-in. In RAxML, maximum likelihood (ML) topology estimation was conducted independently from 200 different starting trees. Then, 1,000 bootstrap pseudoreplicates were run to obtain the statistical support for the nodes of the tree with the highest log-likelihood. ML tree search in PhyML was performed by the SPR algorithm and the aLRT statistic [52] was used to evaluate node confidence.
Divergence time inference was conducted in BEAST 1.6.1 [53] using the same data partitioning, substitution model and MCMC settings described above. Substitution rate evolution was modelled by the uncorrelated lognormal distribution. Tree topology prior followed the Yule process.
Calibration information used as priors
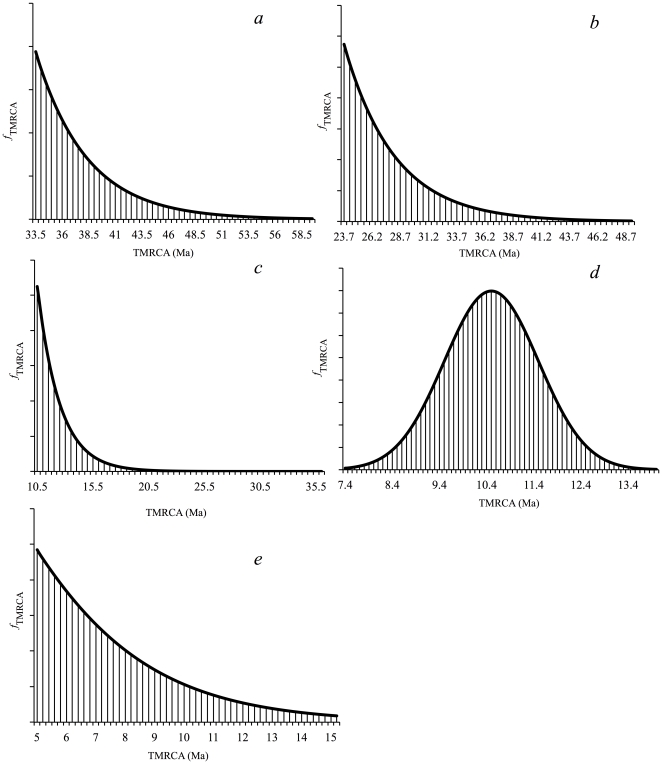
The ages of five nodes were constrained by calibration information based on the fossil record of cetaceans (Figure 3):
Figure 3. Calibration information used as priors in Bayesian dating analyses.
(a) TMRCA of modern Cetacea; (b) TMRCA of Odontoceti; (c) Age of the Monodon/Phocoena split; (d) Age of the Delphinidae diversification; (e) Age of the Iniidae/Pontoporidae divergence.
The time since the most recent common ancestor (TMRCA) of Cetacea was calibrated by a gamma prior with shape = 1.0, scale = 4.8 and offset = 33.5 Ma. This is based on the early Mysticeti fossils at the Eocene/Oligocene boundary [54], [55]. The gamma prior was adjusted so that the tail of the distribution would include the Archaeoceti fossils, which are supposedly the stem cetacean lineage.
The earliest members of the Odontoceti [56] were found in the late Oligocene [57]. By around 23.7 Ma, odontocetes had already diversified, because this is the age of Ferecetotherium, which presents autapomorphies of the Physeteridae [16]. We have used a gamma prior with shape = 1, scale = 4.5 and offset = 23 Ma. The tail of the gamma distribution was extended to the Eocene, to safely incorporate stem lineages of the mysticetes and odontocetes.
The age of the Monodon/Phocoena split was constrained by a gamma prior with shape = 1, scale = 2 and offset = 10.5 Ma. Prior information was based on the oldest fossil Phocoenidae, Salumiphocaena stocktoni, from the late Miocene of North America [58]. The shape of the distribution was set in order to accommodate the Miocene epoch.
The diversification of Delphinidae was constrained by a normal prior with mean = 10.5 and standard deviation = 1.0 Ma, which resulted in a 95% interval from 8.5 to 12.5. This prior was based on the record of early delphinids from the late Miocene [59].
The divergence between Iniidae and Pontoporiidae had already taken place in the early Pliocene. We used a gamma prior with shape = 1.7, scale = 2.5 and offset = 5 Ma to estimate the TMRCA of Inia/Pontoporia. Xiong et al. [25] used the fossil Brachydelphis to constrain the Inia/Pontoporia divergence. However, the extensive morphological revision by Geisler and Sanders [16] places Brachydelphis as stem lineage of “Platanistoidea”, which includes extant Lipotes and Platanista as well as Inia and Pontoporia. Thus, we chose not to consider this fossil as a stem Pontoporiidae and established an offset for the gamma prior at 5 Ma, which safely includes late Miocene South American fossils of iniids and pontoporiids [60].
Supporting Information
Additional primers designed to enable complete sequencing of the delphinid mitochondrial genome.
(DOC)
Accession numbers of the species used in this study.
(DOC)
Acknowledgments
Skin samples were collected under permits 022-01/CMA/IBAMA and IBAMA 02001.0002344/96-11, issued by the Brazilian Environmental Agency (IBAMA). The authors would like to thank David Marjanovic and an anonymous referee for comments that improved the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was financially supported by grants 103.136/2008, 110.838/2010 and 110.028/2011 from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and 308147/2009-0 from Conselho Nacional de Desenvolvimento Científico e Tecnológico to CS. HC is supported by a post-doctoral scholarship from FAPERJ (E26/100.209/2008). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Graur D, Higgins DG. Molecular Evidence for the Inclusion of Cetaceans within the Order Artiodactyla. Molecular Biology and Evolution. 1994;11:357–364. doi: 10.1093/oxfordjournals.molbev.a040118. [DOI] [PubMed] [Google Scholar]
- 2.Gatesy J, Hayashi C, Cronin MA, Arctander P. Evidence from milk casein genes that cetaceans are close relatives of hippopotamid artiodactyls. Molecular Biology and Evolution. 1996;13:954–963. doi: 10.1093/oxfordjournals.molbev.a025663. [DOI] [PubMed] [Google Scholar]
- 3.Nikaido M, Rooney AP, Okada N. Phylogenetic relationships among cetartiodactyls based on insertions of short and long interpersed elements: Hippopotamuses are the closest extant relatives of whales. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10261–10266. doi: 10.1073/pnas.96.18.10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatesy J, O'Leary MA. Deciphering whale origins with molecules and fossils. Trends in Ecology & Evolution. 2001;16:562–570. [Google Scholar]
- 5.Agnarsson I, May-Collado LJ. The phylogeny of Cetartiodactyla: The importance of dense taxon sampling, missing data, and the remarkable promise of cytochrome b to provide reliable species-level phylogenies. Molecular Phylogenetics and Evolution. 2008;48:964–985. doi: 10.1016/j.ympev.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 6.Geisler JH, Theodor JM. Hippopotamus and whale phylogeny. Nature. 2009;458:E1–E4. doi: 10.1038/nature07776. [DOI] [PubMed] [Google Scholar]
- 7.Spaulding M, O'Leary MA, Gatesy J. Relationships of Cetacea (Artiodactyla) Among Mammals: Increased Taxon Sampling Alters Interpretations of Key Fossils and Character Evolution. Plos One. 2009;4 doi: 10.1371/journal.pone.0007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy WJ, Eizirik E, Johnson WE, Zhang YP, Ryder OA, et al. Molecular phylogenetics and the origins of placental mammals. Nature. 2001;409:614–618. doi: 10.1038/35054550. [DOI] [PubMed] [Google Scholar]
- 9.Arnason U, Gullberg A. Cytochrome b nucleotide sequences and the identification of five primary lineages of extant Cetaceans. Molecular Biology and Evolution. 1996;13:407–417. doi: 10.1093/oxfordjournals.molbev.a025599. [DOI] [PubMed] [Google Scholar]
- 10.LeDuc RG, Perrin WF, Dizon AE. Phylogenetic relationships among the delphinid cetaceans based on full cytochrome B sequences. Marine Mammal Science. 1999;15:619–648. [Google Scholar]
- 11.Arnason U, Gullberg A, Janke A. Mitogenomic analyses provide new insights into cetacean origin and evolution. Gene. 2004;333:27–34. doi: 10.1016/j.gene.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Yan H, Zhou KY, Yang G. Molecular phylogenetics of ‘river dolphins’ and the baiji mitochondrial genome. Molecular Phylogenetics and Evolution. 2005;37:743–750. doi: 10.1016/j.ympev.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 13.May-Collado L, Agnarsson I. Cytochrome b and Bayesian inference of whale phylogeny. Molecular Phylogenetics and Evolution. 2006;38:344–354. doi: 10.1016/j.ympev.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Cassens I, Vicario S, Waddell VG, Balchowsky H, Van Belle D, et al. Independent adaptation to riverine habitats allowed survival of ancient cetacean lineages. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11343–11347. doi: 10.1073/pnas.97.21.11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messenger SL, McGuire JA. Morphology, molecules, and the phylogenetics of cetaceans. Systematic Biology. 1998;47:90–124. doi: 10.1080/106351598261058. [DOI] [PubMed] [Google Scholar]
- 16.Geisler JH, Sanders AE. Morphological Evidence for the Phylogeny of Cetacea. Journal of Mammalian Evolution. 2003;10:106. [Google Scholar]
- 17.Nikaido M, Matsuno F, Hamilton H, Brownell RL, Cao Y, et al. Retroposon analysis of major cetacean lineages: The monophyly of toothed whales and the paraphyly of river dolphins. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7384–7389. doi: 10.1073/pnas.121139198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunha HA, da Silva VMF, Lailson-Brito J, Santos MCO, Flores PAC, et al. Riverine and marine ecotypes of Sotalia dolphins are different species. Marine Biology. 2005;148:449–457. [Google Scholar]
- 19.Caballero S, Jackson J, Mignucci-Giannoni AA, Barrios-Garrido H, Beltran-Pedreros S, et al. Molecular systematics of South American dolphins Sotalia: Sister taxa determination and phylogenetic relationships, with insights into a multi-locus phylogeny of the Delphinidae. Molecular Phylogenetics and Evolution. 2008;46:252–268. doi: 10.1016/j.ympev.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Steeman ME, Hebsgaard MB, Fordyce RE, Ho SYW, Rabosky DL, et al. Radiation of Extant Cetaceans Driven by Restructuring of the Oceans. Systematic Biology. 2009;58:573–585. doi: 10.1093/sysbio/syp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGowen MR, Spaulding M, Gatesy J. Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Molecular Phylogenetics and Evolution. 2009;53:891–906. doi: 10.1016/j.ympev.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 22.McGowen MR. Toward the resolution of an explosive radiation-A multilocus phylogeny of oceanic dolphins (Delphinidae). Molecular Phylogenetics and Evolution. 2011;60:345–357. doi: 10.1016/j.ympev.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Caballero S, Trujillo F, Vianna JA, Barrios-Garrido H, Montiel MG, et al. Taxonomic status of the genus Sotalia: Species level ranking for “tucuxi” (Sotalia fluviatilis) and “costero” (Sotalia guianensis) dolphins. Marine Mammal Science. 2007;23:358–386. [Google Scholar]
- 24.Cunha HC, da Silva VMF, Solé-Cava AM. Molecular Ecology and Systematics of Sotalia dolphins. In: Ruiz-Garcia M, Shostell J, editors. Biology, Evolution and Conservation of River Dolphins within South America and Asia. New York: Nova Science; 2010. pp. 261–283. [Google Scholar]
- 25.Xiong Y, Brandley MC, Xu SX, Zhou KY, Yang G. Seven new dolphin mitochondrial genomes and a time-calibrated phylogeny of whales. Bmc Evolutionary Biology. 2009;9 doi: 10.1186/1471-2148-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang XG. Bayesian inference of cetacean phylogeny based on mitochondrial genomes. Biologia. 2009;64:811–818. [Google Scholar]
- 27.Ho SYW, Lanfear R. Improved characterisation of among-lineage rate variation in cetacean mitogenomes using codon-partitioned relaxed clocks. Mitochondrial DNA. 2010;21:138–146. doi: 10.3109/19401736.2010.494727. [DOI] [PubMed] [Google Scholar]
- 28.Vilstrup JTVJT, Ho SYW, Foote AD, Morin PA, Kreb D, et al. Mitogenomic phylogenetic analyses of the Delphinidae with an emphasis on the Globicephalinae. Bmc Evolutionary Biology. 2011;11 doi: 10.1186/1471-2148-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishino H HM. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in hominoidea. J Mol Evol. 1989;29:170–179. doi: 10.1007/BF02100115. [DOI] [PubMed] [Google Scholar]
- 30.Avise JC. Phylogeography: retrospect and prospect. Journal of Biogeography. 2009;36:3–15. [Google Scholar]
- 31.Slater GJ, Price SA, Santini F, Alfaro ME. Diversity versus disparity and the radiation of modern cetaceans. Proceedings of the Royal Society B-Biological Sciences. 2010;277:3097–3104. doi: 10.1098/rspb.2010.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fordyce REMC. Mazin JMB, V, editors. Evolutionary history of the cetaceans: a review. Secondary adaptation of tetrapods to life in water. 2001. pp. 169–233.
- 33.Bianucci G. The Odontoceti (Mammalia, Cetacea) from Italian Pliocene. Systematics and phylogenesis of Delphinidae. Palaeontolographia Italica. 1996;83:73–167. [Google Scholar]
- 34.Perrin WF. Dolphins, porpoises, and whales. 1989. 27 An action plan for the conser-vation of biological diversity: 1988–1992.: IUCN.
- 35.Morin PA, Archer FI, Foote AD, Vilstrup J, Allen EE, et al. Complete mitochondrial genome phylogeographic analysis of killer whales (Orcinus orca) indicates multiple species. Genome Research. 2010;20:908–916. doi: 10.1101/gr.102954.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geisler JHMM, Yang G, Gatesy J. A supermatrix analysis of genomic, morphological, and paleontological data from crown Cetacea. BMC Evolutionary Biology. 2011;11:112. doi: 10.1186/1471-2148-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harlin-Cognato AD, Honeycutt RL. Multi-locus phylogeny of dolphins in the subfamily Lissodelphininae: character synergy improves phylogenetic resolution. Bmc Evolutionary Biology. 2006;6 doi: 10.1186/1471-2148-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoelzel AR, Hancock JM, Dover GA. Evolution of the Cetacean Mitochondrial D-Loop Region. Molecular Biology and Evolution. 1991;8:475–493. doi: 10.1093/oxfordjournals.molbev.a040662. [DOI] [PubMed] [Google Scholar]
- 39.Irwin DM, Kocher TD, Wilson AC. Evolution of the Cytochrome-B Gene of Mammals. Journal of Molecular Evolution. 1991;32:128–144. doi: 10.1007/BF02515385. [DOI] [PubMed] [Google Scholar]
- 40.Campbell KE, Frailey CD, Romero-Pittman L. The Pan-Amazonian Ucayali Peneplain, late Neogene sedimentation in Amazonia, and the birth of the modern Amazon River system. Palaeogeography Palaeoclimatology Palaeoecology. 2006;239:166–219. [Google Scholar]
- 41.Figueiredo J, Hoorn C, van der Ven P, Soares E. Late Miocene onset of the Amazon River and the Amazon deep-sea fan: Evidence from the Foz do Amazonas Basin. Geology. 2009;37:619–622. [Google Scholar]
- 42.Haq BU, Hardenbol J, Vail PR. Chronology of Fluctuating Sea Levels Since the Triassic. Science. 1987;235:1156–1167. doi: 10.1126/science.235.4793.1156. [DOI] [PubMed] [Google Scholar]
- 43.Hoorn C, Guerrero J, Sarmiento GA, Lorente MA. Andean Tectonics as a Cause for Changing Drainage Patterns in Miocene Northern South-America. Geology. 1995;23:237–240. [Google Scholar]
- 44.Días de Gamero ML. The changing course of the Orinoco River during the Neogene: a review. Palaeogeography, Palaeoclimatology, Palaeoecology. 1996;123:385–402. [Google Scholar]
- 45.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular Cloning: a Laboratory Manual: Cold Spring Harbor Lab. Press.
- 46.Sasaki T, Nikaido M, Hamilton H, Goto M, Kato H, et al. Mitochondrial phylogenetics and evolution of mysticete whales. Systematic Biology. 2005;54:77–90. doi: 10.1080/10635150590905939. [DOI] [PubMed] [Google Scholar]
- 47.Thompson JDHD, Gibson TJ. Clustal-W - Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang ZHYA. Comparison of likelihood and Bayesian methods for estimating divergence times using multiple gene loci and calibration points, with application to a radiation of cute-looking mouse lemur species. Systematic Biology. 2003;52:705–716. doi: 10.1080/10635150390235557. [DOI] [PubMed] [Google Scholar]
- 49.Ronquist FHJ. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 50.Guindon SGO. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 51.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 52.Anisimova MGO. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Systematic Biology. 2006;55:539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- 53.Drummond AJRA. BEAST: Bayesian evolutionary analysis by sampling trees. Bmc Evolutionary Biology. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fordyce RE, Barnes LG. The Evolutionary History of Whales and Dolphins. Annual Review of Earth and Planetary Sciences. 1994;22:419–455. [Google Scholar]
- 55.Fordyce RE. Cetacean Fossil Record. In: Perrin WF, Wursig B, Thewissen JGM, editors. Encyclopedia of Marine Mammals. Academic Press; 2008. pp. 207–214. [Google Scholar]
- 56.Fordyce R. Simocetus rayi (Odontoceti: Simocetidae, New Family): a bizarre new archaic oligocene dolphin from the Eastern North Pacific. Smithsonian Contributions to Paleobiology. 2002:185–222. [Google Scholar]
- 57.Berta A SJ, Kovacs KM. 2006. Marine Mammals: Evolutionary Biology: Academic Press.
- 58.Barnes LG. Evolution, Taxonomy and Antitropical Distributions of the Porpoises (Phocoenidae, Mammalia). Marine Mammal Science. 1985;1:149–165. [Google Scholar]
- 59.Barnes LG. Outline of Eastern North Pacific Fossil Cetacean Assemblages. Systematic Zoology. 1976;25:321–343. [Google Scholar]
- 60.Cozzuol MA. The record of the aquatic mammals in southern South America. Münchner Geowiss Abh. 1996;30:321–342. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional primers designed to enable complete sequencing of the delphinid mitochondrial genome.
(DOC)
Accession numbers of the species used in this study.
(DOC)