Abstract
Anxiety is characterized by exaggerated attention to threat. Several studies suggest that this threat bias plays a causal role in the development and maintenance of anxiety disorders. Furthermore, although the threat bias can be reduced in anxious individuals and induced in non-anxious individual, the attentional mechanisms underlying these changes remain unclear. To address this issue, 49 non-anxious adults were randomly assigned to either attentional training toward or training away from threat using a modified version of the dot probe task. Behavioral measures of attentional biases were also generated pre- and post-training using the dot probe task. Event-related potentials (ERPs) were generated to threat and non-threat face pairs and probes during pre- and post-training assessments. Effects of training on behavioral measures of the threat bias were significant, but only for those participants showing pre-training biases. Attention training also influenced early spatial attention, as measured by post-training P1 amplitudes to cues. Results illustrate the importance of taking pre-training attention biases in non-anxious individuals into account when evaluating the effects of attention training and tracking physiological changes in attention following training.
Keywords: attention training, dot probe, ERP
1. Introduction
Anxious individuals show an attentional bias towards threat (Mathews & Mackintosh, 1998; Mogg & Bradley, 1998). For example, using the dot probe task, several labs have demonstrated that anxious, but not non-anxious individuals, detect probes faster when they are preceded by threatening versus non-threatening stimuli (e.g., words, emotional faces, phobia-specific stimuli) (MacLeod, Mathews, & Tata, 1986; Mogg & Bradley, 2005; Mogg, Bradley, Miles, & Dixon, 2004; Wilson & MacLeod, 2003), a pattern confirmed by a recent meta-analysis (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007).
Several studies have now shown that this threat bias can be reduced in anxious participants using a modified version of the dot probe task in which participants are trained to avoid threat. Specifically, when participants are presented with a systematic contingency between cue and probe locations to induce a temporary bias away from threat (e.g., probes always appear in the location of the non-threatening stimulus), there are reductions in attentional bias to threat and symptoms of generalized anxiety disorder (Amir, Beard, Burns, & Bomyea, 2009), social anxiety disorder (Schmidt, Richey, Buckner, & Timpano, 2009), and social phobia (Amir, Beard, Taylor, et al., 2009). A recent meta-analysis demonstrated that this type of attention training, attentional bias modification (ABM), is effective in reducing anxiety and stress responsiveness at a medium effect size (d = .61) (Hakamata et al., 2010). However, while threat bias reduction is highly relevant for anxiety treatment, the complementary question of whether an attentional bias to threat can be induced may inform our understanding of the causal relationship between the threat bias and anxiety. This is a primary goal of the present study.
A small number of behavioral studies have addressed this by demonstrating that an attentional bias to threatening stimuli can be experimentally induced in non-anxious participants using a modified version of the dot probe task with a systematic contingency between the location of the threatening stimulus and probe. Specifically, participants trained towards threat in this manner show speeded response latencies to probes cued by threat as well as elevations in stress responsivity (Clarke, MacLeod, & Shirazee, 2008; Eldar, Ricon, & Bar-Haim, 2008; MacLeod, Rutherford, Campbell, Ebsworthy, & Holker, 2002). Taken together, these ABM studies suggest that there may be a causal link between the threat bias and anxiety, since reducing the threat bias also reduces symptoms and anxiety whereas inducing a threat bias increases emotional vulnerability to stress (Bar-Haim, 2010). Despite these promising early findings, little is known about the cognitive changes underlying ABM effects, and behavioral measures alone lack the sensitivity to capture subtle changes in distinct cognitive processes that may be influenced by attention training.
Although these behavioral studies have provided important information about the threat bias in anxiety and the utility of ABM in clinical and normative groups, they cannot measure very rapid attentional processing of threat. Scalp-recorded event-related potentials (ERPs), which can capture changes on the order of milliseconds, are particularly sensitive to attentional processes related to the threat bias. For example, four published studies have used ERPs during non-attention training versions of the dot probe task to track the time course of the threat processing in anxious relative to non-anxious participants (Eldar, Yankelevitch, Lamy, & Bar-Haim, 2010; Fox, Derakshan, & Shoker, 2008; Helfinstein, White, Bar-Haim, & Fox, 2008; Mueller et al., 2009). Fox et al. (2008) found greater N2pc amplitudes to angry faces for anxious relative to non-anxious individuals in a go/no-go version of the dot probe task, demonstrating early attentional capture by threatening stimuli in anxiety. Also using a go/no-go version of the dot probe task, Mueller et al. (2009) found greater P1 amplitudes to angry-neutral face pairs versus happy-neutral face pairs in participants with social anxiety disorder, providing further support for enhanced early and relatively automatic attention in the threat bias. In a primed version of the dot probe task, Helfinstein et al. (2008) found greater P1 and reduced N1 amplitudes to face pairs in socially anxious versus non-anxious individuals. Only angry-neutral face pairs were used in this study, however, thus precluding any conclusions about threat-specific emotional processing in social anxiety. In contrast, Eldar et al. (2010), by using the dot probe task with angry-neutral, happy-neutral, and neutral-neutral face pairs, were able to assess processing of threat relative to happy stimuli. They found that anxious individuals showed enhanced C1 amplitudes to angry-neutral face pairs and greater P2 amplitudes to all face pairs relative to controls. The authors argue that this pattern suggests enhanced automatic capture of attention (C1) as well as greater elaborated processing of emotional faces (P2) in anxious individuals. Taken together, these studies provide suggestive evidence for the hypothesis that the threat bias may be driven both by early attentional capture by threat as well as later, more elaborated processing.
One ABM study addressed this hypothesis directly by examining training effects on specific ERPs thought to reflect relatively early/automatic and later/elaborated cognitive processes (Eldar & Bar-Haim, 2010). In this study, anxious participants who were trained to attend to neutral faces showed reductions in P2 and enhanced N2 amplitudes to the face pairs on the post-training assessment. The authors suggest that this pattern of changes reflects reduced distraction by decreasing the processing of emotional stimuli (P2) and greater control of attentional resources (N2) since the N2 has been linked to activity of anterior cingulate cortex (van Veen & Carter, 2002). Furthermore, participants who were trained away from threat showed increases in P3 amplitudes to probes replacing neutral faces from angry-neutral pairs, regardless of anxiety level (Eldar & Bar-Haim, 2010). Taken together, results suggest that the ABM procedure may facilitate recruitment of both automatic and more elaborated/controlled cognitive functions during the dot probe task. This interpretation of the ERP findings is bolstered by recent fMRI studies demonstrating that lateral prefrontal cortical areas (Browning, Holmes, Murphy, Goodwin, & Harmer, 2010) and temporal occipital areas (Monk et al., 2004) mediate the modification of attention biases. It remains unclear, however, what cognitive processes might mediate the modification of attention biases in normative, non-clinical samples.
In addition to using ERPs to disambiguate the processes underlying attention changes following training, changes in behavioral components of the threat bias can be assessed as well. The proposed components of the threat bias are facilitated attention to threat, difficulty disengaging from threat, and strategic avoidance of threat (Cisler, Bacon, & Williams, 2009; Cisler & Koster, 2010). In its original format, the dot probe task can confirm the presence of the threat bias but cannot identify the mechanisms through which it occurs. A modification of the task includes an additional trial type, where two non-threatening stimuli are presented simultaneously and then followed by a probe in the location of one of the stimuli (Koster, Crombez, Verschuere, & De Houwer, 2004). Response latencies from these baseline trials are compared to the threat and non-threat cued trials: Faster responding to threat versus baseline cues suggests greater initial capture of attention by threat (i.e., vigilance toward the threatening location), while faster responding to baseline versus non-threat cues suggests greater attentional hold by threat (i.e., difficulty disengaging from the threatening location). Several studies using this adapted dot probe methodology have supported a difficulty disengaging model of attentional bias, but not a facilitated attention model (Koster et al., 2004; Salemink, van den Hout, & Kindt, 2007). Attentional avoidance, directing attention away from threatening stimuli, is generally found following initial vigilance for threat when stimulus presentation durations are prolonged (Koster, Crombez, Verschuere, Van Damme, & Wiersema, 2006; Mogg et al., 2004). In the present study we included angry and happy faces, consistent with an early attentional training study by Mathew and MacLeod (2002). Thus, in order to assess attentional capture and hold by threat (angry face) relative to non-threat (happy face) a pair of happy faces was used as the baseline trial. Happy faces were used instead of neutral faces in order to hold arousal constant while having both a threat-relevant and non-threat relevant stimulus.
Several studies have demonstrated that the pattern of bias toward or avoidance of threat depends on the duration of exposure to stimuli. Using both highly threatening (HT) and moderately threatening (MT) stimuli, Koster, Verschuere, Crombez, and Van Damme (2005) demonstrated that both anxious and non anxious individuals show a bias toward HT at 100 ms and 500 ms, but only anxious individuals show a bias toward MT at 500 ms. Among just non-anxious individuals, the threat bias is found at 100 ms but not at 500 ms (Koster, Crombez, Verschuere, Vanvolsem, & De Houwer, 2007; Mogg, Bradley, De Bono, & Painter, 1997). However, in another study the threat bias was found at 100 ms and an avoidance of threat at 500 ms for non-anxious participants (Cooper & Langton, 2006). Taken together, these studies suggest for non-anxious individuals that at shorter durations the threat bias is present while at longer durations it is either absent or reversed. However, a more recent study varied cue duration and the effects of ABM did not appear for the non-anxious sample of participants at the shorter durations (i.e., 30 and 100 ms) (Koster, Baert, Bockstaele, & De Raedt, 2008). Thus, in order to explore how the effects of presentation duration on attentional bias interact with ABM, the current study included short (100 ms) and long (500 ms) cue conditions.
In summary, the present study addressed important gaps in the research on ABM in several ways. First, we examined whether a bias towards threat can be induced or reduced in non-anxious adults using the dot probe task, by training participants to develop either an attentional bias towards angry faces (train toward threat group) or away from angry faces (train away from threat group). Second, we incorporated two modifications to the dot probe task, in order to assess changes in attentional capture and hold: (1) we included baseline trials (with two non-threatening stimuli, happy faces) in order to distinguish between vigilance for threat and difficulty disengaging from threat in the attentional bias; and (2) we included two presentation durations to assess changes in the threat bias in short and long exposure conditions. Third, we examined the neural processes underlying ABM by analyzing whether ABM influenced ERPs reflecting early and later stages of attentional processing.
We tested the following predictions for behavioral and ERP effects. Given the previous ABM study incorporating ERPs (Eldar & Bar-Haim, 2010), we predicted that individuals in the train away from versus train toward threat group will showed decreased attentional bias scores (attentional bias, vigilance, difficulty disengaging) and reduced attentional capture by threat as indicated by reduced ERP amplitudes at early (P1) and later, more elaborated stages (N170, P2, N2, P3) of attentional processing in response to threatening versus non-threatening cues and probe locations. In addition, we predicted that individuals in the train away from threat versus train toward threat group will show reduced subjective anxiety.
We also tested the hypothesis that effects of ABM will be greater for cues presented at short (100 ms) versus long (500) exposure durations since previous research suggests that the threat bias is most clearly detectable for non-anxious individuals when stimuli are briefly presented (Koster et al., 2007; Koster et al., 2005; Mogg et al., 1997).
Additionally, because the threat bias is more prevalent among anxious individuals (Bar-Haim et al., 2007), we expected that in this non-clinical sample attention training effects may only occur for those participants who already show a bias towards threat at baseline (for the train away from threat group) or a bias away from threat at baseline (for the train toward threat group), due to ceiling and floor effects, respectively.
Finally, in exploratory analyses, we examined whether relationships exist between ERP and behavioral biases following ABM. We predicted that greater neural processing of threat relative to non-threat at post-training will correlate with greater behavioral biases to threat, whereas greater neural processing of non-threat will correlate with reduced behavioral biases.
2. Method
2.1. Participants
Participants were 61 non-disordered adults recruited through the psychology participant research pool at Hunter College, The City University of New York. Prior to completing the tasks, participants were screened for psychological impairments (anxiety, depression) via self-report questionnaires. Twelve participants were excluded from analyses for the following reasons: participant refusal (f = 1), experimenter error during EEG recording (f = 2), too many incorrect responses during the dot probe task (f = 3), heavily artifacted EEG recording (f = 6). The final sample consisted of 49 non-disordered adults (20 males, 29 females) aged 18 to 38 (M = 20.92, SD = 4.30). Self-reported race/ethnicity was as follows: 18 White, 9 Hispanic, 15 Asian or Pacific Islander, 5 African American, and 2 “other.”
2.2. Procedure
Participants spent approximately three hours in the laboratory. After a brief questionnaire period, an elasticized nylon cap was fitted on the participants and scalp electrodes were applied. Participants were seated 65 cm from a 17 in monitor and instructed to remain still and not blink when the stimuli appeared on the screen to reduce the occurrence of muscle or ocular artifacts in the EEG recording. After passively viewing all faces used in the dot probe procedures (a procedure analyzed separately from the present study) participants completed the pre-training dot probe assessment, the dot probe training task, a brief mood questionnaire, the post-training dot probe assessment, and a brief mood questionnaire.1
2.2.1. Questionnaires
The State-Trait Anxiety Inventory is an 40-item questionnaire that measures participants’ perceptions of their current (state) and general (trait) level of nervousness, anxiety, and shyness (STAI; Spielberger, 1983). The sample average for state (M = 34.82, SD = 8.40) and trait (M = 40.18, SD = 7.57) anxiety were within the normative range before attention training. State anxiety was assessed two more times: immediately after the training task and approximately 30 minutes after training.
2.2.2. Emotional face stimuli
Stimuli were 24 black-and-white photographs of angry and happy faces (Tottenham et al., 2009)2. Faces were paired during the dot probe task so that angry-happy or happy-happy pairs were of the same person. There were equal numbers of males and females, as well as White and African American faces.
2.2.3. The dot probe task
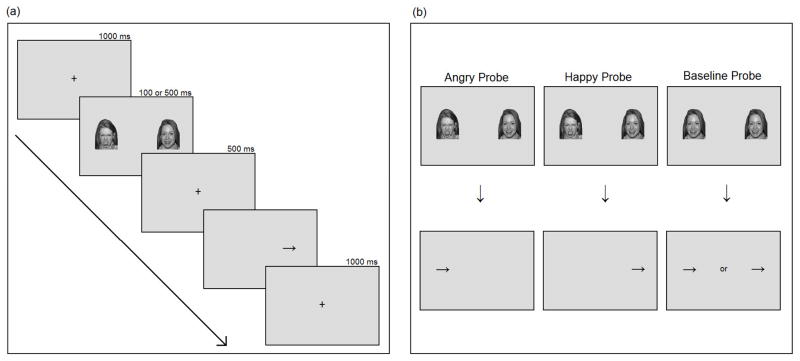
Stimuli were programmed using E-Prime version 1.1 (Schneider, Eschman, & Zuccolotto, 2002). Figure 1a displays the sequence of events for a trial of the dot probe task. Each trial begins with a fixation cross presented for 1000 ms, followed by a pair of cue stimuli (emotional faces) for either 100 or 500 ms. Face pairs were either angry-happy faces, happy-angry faces, or happy-happy faces. Each type of pair was presented an equal number of times so that angry and happy faces were equally presented on either side of the screen. An angry face probe is when the target replaces the angry face from a pair of angry and happy faces, while a happy face probe is when the target replaces the happy face from a pair of angry and happy faces. A baseline probe is when the target replaces either face from a pair of two happy faces (see Figure 1b). Face images subtended 7 cm ×10 cm and were presented equal distance to the right and left of the fixation cross. After the faces were removed a 500 ms delay occurred, followed by a probe (arrow) in the location previously occupied by one of the faces. Participants were asked to respond as quickly and as accurately as possible whether the arrow was pointing to the left or the right by clicking on the mouse. The inter-trial interval was 1000 ms.
Figure 1.
Sequence of events (a) and types of trials (b) in the dot probe task. Stimuli are taken from the NimStim set (Tottenham et al., 2009).
Reaction times (RT) were filtered by removing responses that were faster than −3SD from an individual’s mean and slower than +3SD from an individual’s mean. Following removal of these trials, mean RTs for each condition were either normally distributed across the sample or slightly positively skewed, as expected for RTs. Using correct trials only, the dot probe task yields three threat bias scores (attentional bias, vigilance, disengagement) by comparing reaction times (RT) following the different cues. The attentional bias score is calculated as RT happy probe – RT angry probe. Higher scores indicate an attentional bias toward threatening information, such that participants respond faster when the probe appears in the location of the angry face versus the happy face. Such a bias can be driven by the speed of attentional capture by threat (vigilance) or the length of attentional hold by threat (disengagement). The vigilance score is calculated as RT baseline probe – RT angry probe. Higher scores indicate greater attentional capture by threat, such that participants respond faster when the probe appears in the location of the angry face versus when no threatening face is presented. The disengagement score is calculated as RT happy probe – RT baseline probe. Higher scores indicate greater attentional hold by threat, such that participants are faster to respond when no threat is presented versus when they have to disengage and shift attention to the location of the happy face.
Participants received an equal number of angry, happy, and baseline probe trials during the pre- and post-training dot probe task, with a total of 288 trials each. The ABM task was a dot probe designed to either induce or reduce a bias toward threat. Half of the participants (n = 25) were randomly assigned to train toward threat group and the other half (n = 24) to the train away from threat group. The train toward threat group consisted of angry probes only while the train away from threat group consisted of happy probes only, with a total of 480 trials in each.
2.2.4. Electrophysiological recording and data reduction
EEG activity was recorded continuously via 64 Ag/AgCl scalp electrodes at a sampling rate of 512 Hz (BioSemi; Amsterdam, NL). The Biosemi system forms the ground electrode using Common Mode Sense active electrode and Driven Right Leg passive electrode during EEG acquisition; data were re-referenced offline to an average reference. Eye movements were monitored by electro-oculogram (EOG) signals from electrodes placed 1 cm above and below the left eye (to measure vertical eye movements) and 1 cm on the outer edge of each eye (to measure horizontal eye movements). All data preparation after recording was conducted using Brain Vision Analyzer (Version 2.2, GmbH; Munich, DE). The continuous EEG was filtered with a low cut-off frequency of .1 Hz and a high cut-off frequency of 30 Hz. Ocular artifacts were corrected using the Gratton method (Gratton, Coles, & Donchin, 1983). Stimulus-locked data were segmented into epochs from 200 ms before stimulus presentation to 600 ms after, with a 200 ms baseline correction. These raw EEG epochs were scanned for artifacts; data with voltage steps exceeding 75 μV, maximum changes exceeding 100 μV (within a given segment), maximum and minimum differences within a segment ±105 μV, and activity lower than 1 μV per 100 ms were excluded from analyses.
Peak amplitudes were generated to the face cues during the dot probe task separately for angry-happy and happy-angry pairs (threat face pairs) and happy-happy pairs (non-threat face pairs) for several ERP components: between 100 and 300 ms for P1 (maximal at approximately 120 ms) over PO7/O1/PO8/O2, N170 (maximal at approximately 170 ms) over PO7/P7/PO8/P8, and P2 (maximal at approximately 230 ms) over PO7/O1/PO8/O2; between 200 and 400 ms for N2 (maximal at approximately 310 ms) over Fz/FCz; between 325 and 475 ms for P3 (maximal at approximately 420 ms) over F3/FC3/F4/FC4. The average trial count for P1 was 43.75 (SD = 3.54), for N170 was 44.11 (SD = 2.70), for P2 was 43.75 (SD = 3.54), for N2 was 43.75 (SD = 3.55), and for P3 was 43.24 (SD = 3.56). Peak P1, N1, and P3 amplitudes were also generated separately to the angry, happy, and baseline probes. P1 amplitudes were generated between 100 and 200 ms (maximal at approximately 140 ms) over PO7/O1/PO8/P2. N1 amplitudes were generated between 150 and 250 ms (maximal at approximately 200 ms) over PO3/PO7/PO4/PO8. P3 amplitudes were generated between 325 and 475 ms (maximal at approximately 420 ms) over FCz/FC1/FC2. The average trial count for P1 to probes was 22.12 (SD = 1.91), for N1 to probes was 21.61 (SD = 2.60), and for P3 to probes was 44.69 (SD = 2.49).3 There were no significant differences between training groups on average trial counts. There were no group differences in the average trial counts between the train toward train away from threat groups, all ts < 1.5, ps > .10. See Figure 2 for waveforms of all ERPs from the pre-training dot probe assessment.
Figure 2.
Waveforms of target ERPs to cues (P1, N170, P2, N2, P3) and probes (P3) from the pre-training dot probe assessment.
3. Results
3.1. Descriptive Statistics
See Tables 1 and 2 for descriptive statistics of behavioral biases and ERP amplitudes at pre- and post-training. There were no differences between the train toward train away from threat groups on either state anxiety [t(47) = −0.25, p = .80] or trait anxiety [t(47) = 1.07, p = .28]. For all analyses, outliers in the data were replaced with sample means.
Table 1.
Descriptive Statistics for Behavioral Biases
| Train Toward Threat
|
Train Away From Threat
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Training | Post-Training | Pre-Training | Post-Training | |||||
| 100 ms | 500 ms | 100 ms | 500 ms | 100 ms | 500 ms | 100 ms | 500 ms | |
| Attention Bias | 2.66 (29.87) | 7.21 (22.10) | 5.60 (25.47) | 9.76 (22.74) | 6.06 (34.39) | −4.47 (28.50) | −9.35 (29.42) | 12.38 (52.20) |
| Vigilance | 5.80 (24.36) | 1.34 (23.82) | 5.99 (25.18) | −3.62 (31.96) | 0.74 (30.23) | −18.47 (89.84) | −11.28 (36.18) | 6.41 (31.09) |
| Disengagement | −3.14 (23.83) | 5.87 (26.20) | −0.38 (22.60) | 13.38 (41.44) | 5.32 (21.80) | 14.00 (71.95) | 1.93 (22.57) | 5.97 (51.49) |
Note. Difference scores of response times are presented as means with standard deviations in parentheses.
Table 2.
Descriptive Statistics for ERP Amplitudes
| Train Toward Threat
|
Train Away From Threat
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Training | Post-Training | Pre-Training | Post-Training | |||||
| 100 ms | 500 ms | 100 ms | 500 ms | 100 ms | 500 ms | 100 ms | 500 ms | |
| ERPs to Cues | ||||||||
| P1 | ||||||||
| Threat | 5.58 (2.40) | 6.09 (2.88) | 5.29 (2.61) | 4.81 (2.38) | 4.98 (2.26) | 4.54 (2.25) | 4.30 (2.01) | 4.36 (1.94) |
| Non-Threat | 5.70 (2.49) | 5.47 (2.58) | 5.46 (2.84) | 5.24 (2.87) | 5.24 (2.38) | 4.64 (2.17) | 4.25 (1.99) | 4.26 (1.85) |
| N170 | ||||||||
| Threat | −2.86 (2.61) | −2.99 (2.17) | −2.09 (2.10) | −2.45 (2.05) | −3.05 (2.29) | −3.18 (2.14) | −1.87 (2.16) | −2.32 (1.96) |
| Non-Threat | −2.70 (2.40) | −3.38 (2.53) | −2.27 (2.27) | −2.71 (2.27) | −3.12 (2.15) | −3.25 (2.04) | −1.99 (2.26) | −2.15 (2.07) |
| P2 | ||||||||
| Threat | 5.10 (2.80) | 4.90 (2.65) | 5.19 (2.39) | 5.02 (2.95) | 4.33 (2.06) | 3.81 (2.16) | 5.06 (2.54) | 4.79 (2.38) |
| Non-Threat | 5.00 (2.96) | 4.93 (3.15) | 5.21 (2.79) | 5.40 (3.57) | 4.41 (2.79) | 4.04 (2.45) | 5.01 (2.58) | 4.85 (2.72) |
| N2 | ||||||||
| Threat | −4.17 (1.56) | −4.36 (1.22) | −3.49 (1.20) | −3.76 (1.38) | −3.72 (1.23) | −3.75 (1.49) | −3.47 (1.44) | −3.42 (1.33) |
| Non-Threat | −3.92 (1.46 | −4.52 (1.61) | −3.31 (1.57) | −3.98 (2.10) | −3.75 (1.43) | −4.13 (1.62) | −3.07 (1.56) | −3.52 (1.82) |
| P3 | ||||||||
| Threat | 0.47 (0.95) | −0.63 (0.89) | 0.27 (0.93) | −0.29 (1.10) | 0.28 (0.81) | −0.63 (0.91) | 0.38 (0.84) | −0.42 (0.98) |
| Non-Threat | 0.58 (0.82) | −0.70 (1.02) | 0.61 (1.04) | −0.21 (1.04) | 0.40 (0.90) | −0.49 (0.96) | 0.59 (0.88) | −0.19 (1.06) |
| ERPs to Probes | ||||||||
| P3 | ||||||||
| Angry | 1.69 (1.80) | 1.46 (1.75) | 1.81 (1.26) | 1.62 (1.58) | 1.27 (1.34) | 0.74 (1.26) | 1.61 (1.31) | 1.08 (1.24) |
| Happy | 1.35 (1.88) | 1.18 (1.43) | 1.75 (1.57) | 1.70 (1.79) | 1.13 (1.25) | 0.81 (1.31) | 1.55 (1.34) | 1.21 (1.55) |
| Baseline | 1.64 (1.87) | 1.21 (1.48) | 1.70 (1.30) | 1.53 (1.53) | 1.12 (1.39) | 0.77 (1.34) | 1.55 (1.55) | 1.02 (1.36) |
Note. Amplitudes are presented as means with standard deviations in parentheses.
Below, we present separate analyses for behavioral threat bias, subjective anxiety, and ERP outcome variables for the sample as a whole. Following that, we conduct the identical analyses for behavioral threat bias and subjective anxiety only for those participants showing a pre-training attention bias towards or away from threat prior to training. Additionally, we examine correlations between ERPs and behavioral bias scores on the post-training assessment for the entire sample, to explore associations between neural and behavioral biases following ABM.
3.2. Effect of ABM on Behavioral Threat Bias
We first tested the predictions that participants in the train away versus train toward threat group would show decreased behavioral attentional biases following ABM, and that these effects would be more prominent at 100 versus 500 ms. For each behavioral threat bias score (attentional bias, vigilance, disengagement), a 2(ABM Group: train toward threat, train away from threat) × 2(Test: pre-training, post-training) × 2(Duration: 100 ms, 500 ms) mixed design factorial ANOVA was conducted, with ABM Group as a between-subjects factor and Test and Duration as within-subjects factors.
No significant effects on any behavioral threat bias scores emerged.
3.3. Effect of ABM on State Anxiety
Next, we tested the prediction that participants in the train away versus train toward threat group would show reduced subjective anxiety following ABM. A 2(Test: pre-training, post-training) × 2(ABM Group: train toward threat, train away from threat) mixed design factorial ANOVA was conducted on state anxiety scores, with ABM Group as a between-subjects factor and Test as a within-subjects factor. The main effect of Test revealed that state anxiety scores decreased from pre-training (M = 38.98, SD = 10.57) to post training (M = 36.94, SD = 10.13) for all participants, F(1, 47) = 6.45, p = .01 partial η2 = .12. Thus, state anxiety decreased following training, regardless of training group.
3.4. Effects of ABM on Participants with Pre-Training Attentional Biases
This non-anxious sample of participants showed a wide range in attentional bias scores on the pre-training assessment (100 ms: −52.59 to 110.54; 500 ms: −87.17 to 75.20). As such, analyses examining effects of ABM on behavioral bias scores and anxiety were repeated using only participants with a bias away from threat (negative attention bias score) in the train toward threat group and a bias toward threat (positive attention bias score) in the train away from threat group. This prevents ceiling effects from occurring in the case of the train toward threat group (i.e., if participants already show an attentional bias toward threat) and floor effects from occurring in the train away from threat group (i.e., if participants already show an attentional bias away from threat). This method is also analogous to studies with anxious participants – who are presumed to have a pre-training threat bias – who are trained away from threat. Because some participants showed a bias toward threat at 100 ms and a bias away from threat at 500 ms, all subsequent analyses were performed separately for the two durations.
For the 100 ms condition there were 26 participants (train toward threat: n = 14, attentional bias range: −48.37 to −1.87; train away from threat: n = 12, attentional bias range: 4.32 to 110.54). There were no differences between the two groups at 100 ms on either state anxiety [t(24) = −1.03, p = .32] or trait anxiety [t(24) = 0.46, p = .65]. Consistent with our group selection procedure in which only those participants with pre-training attentional biases were included in the train away from threat group, and vice versa for the train toward threat group, on the pre-training assessment, participants in the train away versus train toward threat group had greater attentional bias scores (M = 30.63, SD = 29.82 versus M = −17.73, SD = 13.97), t(24) = −5.43, p < .001. Additionally, participants in the train away versus train toward threat group also had greater vigilance scores (M = 15.54, SD = 26.67 versus M = −2.80, SD = 16.70), t(24) = −2.14, p = .04. There were no differences in disengaging scores at 100 ms between the two ABM groups on the pre-training assessment.
For the 500 ms condition there were 24 participants (train toward threat: n = 11, attentional bias range: −23.98 to −3.25; train away from threat: n = 13, attentional bias range: 2.02 to 36.76). There were no differences between the two groups at 500 ms on either state anxiety [t(22) = −0.73, p = .47] or trait anxiety [t(22) = −0.04, p = .97]. Again, consistent with our group selection procedure, on the pre-training assessment the train away from threat group had greater attentional bias (M = 14.33, SD = 9.84) than the train toward threat group (M = −9.70, SD = 7.13), t(22) = −6.73, p < .001.
3.4.1. Behavioral Threat Bias
See Tables 3 and 4 for descriptive statistics of behavioral threat biases for the two samples of participants at the two durations. We again tested the prediction that participants in the train away versus train toward threat group would show decreased behavioral attentional bias following ABM. Since analyses were conducted separately for the two durations, we further predicted that these effects may only emerge for the shorter duration. For each behavioral threat bias score, a 2(ABM Group: train toward threat, train away from threat) × 2(Test: pre-training, post-training) mixed design factorial ANOVA was conducted, with ABM Group as a between-subjects factor and Test as a within-subjects factor.
Table 3.
Descriptive Statistics for Behavioral Biases at 100 ms
| Train Toward Threat
|
Train Away From Threat
|
|||
|---|---|---|---|---|
| Pre-Training | Post-Training | Pre-Training | Post-Training | |
| Attention Bias | −17.73 (13.97) | 4.72 (28.33) | 30.63 (29.82) | −6.43 (18.02) |
| Vigilance | −2.80 (16.70) | 6.86 (26.07) | 15.54 (26.67) | −10.49 (13.55) |
| Disengagement | −14.93 (21.08) | −2.14 (21.32) | 15.08 (20.19) | 4.07 (15.52) |
Note. Difference scores of response times are presented as means with standard deviations in parentheses. There were 26 participants with biases in the 100 ms condition (train toward threat: N = 14; train away from threat: N = 12).
Table 4.
Descriptive Statistics for Behavioral Biases at 500 ms
| Train Toward Threat
|
Train Away From Threat
|
|||
|---|---|---|---|---|
| Pre-Training | Post-Training | Pre-Training | Post-Training | |
| Attention Bias | −9.70 (7.13) | 3.79 (13.60) | 14.33 (9.84) | 4.57 (24.40) |
| Vigilance | −0.29 (14.48) | −5.03 (17.01) | 11.08 (13.32) | 8.29 (22.27) |
| Disengagement | −9.40 (16.37) | 8.82 (22.63) | 3.25 (17.73) | −3.72 (22.64) |
Note. Difference scores of response times are presented as means with standard deviations in parentheses. There were 24 participants with biases in the 500 ms condition (train toward threat: N = 11; train away from threat: N = 13).
3.4.1.1. 100 ms
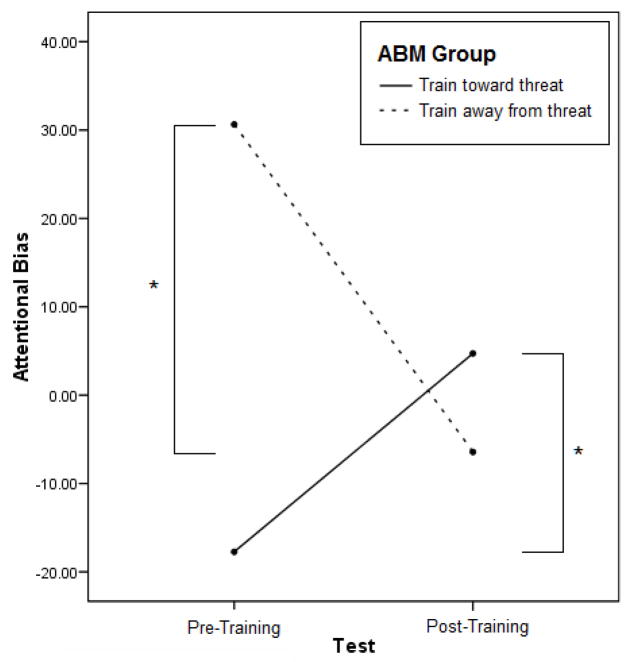
The two-way ABM Group × Test interaction was significant for attentional bias, F(1, 24) = 21.72, p < .001, partial η2 = .48. Consistent with predictions, the train toward threat group showed increases in attentional bias from pre-training (M = −17.73, SD = 13.97) to post-training (M = 4.72, SD = 28.33), t(13) = −2.47, p = .03, while the attend non-threat group showed decreases in attentional bias from pre-training (M = 30.63, SD = 29.82) to post-training (M = −6.43, SD = 18.02), t(11) = 4.20, p = .001 (see Figure 3).
Figure 3.
Attentional bias scores increased for the train toward threat group and decreased for the train away from threat group for participants who showed pre-training biases at 100 ms. (N = 26 with train toward threat N = 14 and train away from threat N = 12).
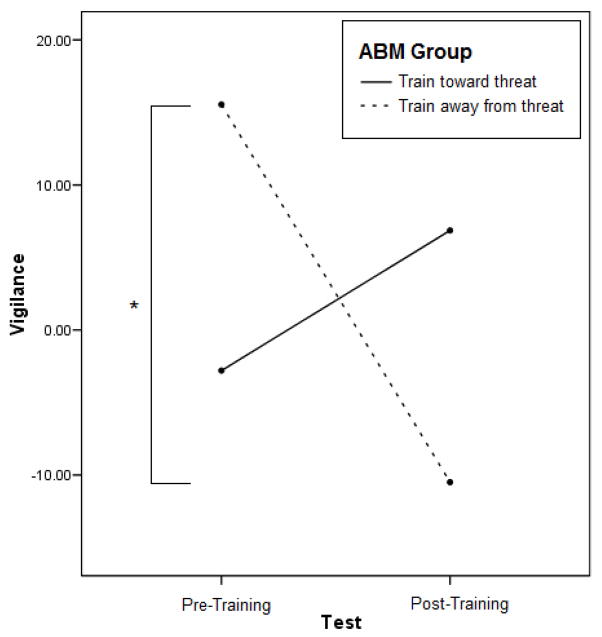
The two-way ABM Group x Test interaction was also significant for vigilance, F(1, 24) = 9.69, p = .005, partial η2 = .29.,Consistent with predictions, the train away from threat group should showed decreases in vigilance from pre-training (M = 15.54, SD = 26.67) to post-training (M = −10.49, SD = 13.55), t(11) = 3.16, p = .009. Additionally, on the post-training assessment, the train toward threat group had greater vigilance (M = 6.86, SD = 26.07) than the train away from threat group (M = −10.49, SD = 13.55), t(24) = 2.07, p = .049 (see Figure 4).
Figure 4.
Vigilance decreased for the train away from threat group for participants who showed pre-training biases at 500 ms. (N = 26 with train toward threat N = 14 and train away from threat N = 12).
There were no significant effects for difficulty disengaging.
3.4.1.2. 500 ms
The two-way ABM Group x Test interaction was significant for attentional bias, F(1, 22) = 7.84, p = .01, partial η2 = .26. Partially consistent with predictions, the train toward threat group showed increases in attentional bias from pre-training (M = −9.70, SD = 7.13) to post-training (M = 3.79, SD = 13.60), t(10) = −2.56, p = .03. There were no changes in attentional bias for the train away from threat group (see Figure 5).
Figure 5.
Attentional bias increased for the train toward threat group for participants who showed pre-training biases at 500 ms. (N = 24 with train toward threat N = 11 and train away from threat N = 13).
There were no significant effects for vigilance or difficulty disengaging.
In summary, when participants were selected based on having a pre-training attentional bias toward or away from threat, we found that, as predicted, the train toward threat group showed increases in attention bias at 100 ms and 500 ms while the train away from threat group showed decreases in attentional bias and vigilance at 100 ms only.
3.4.2. State anxiety
Last, we tested the prediction that participants in the train away versus train toward threat group would show reduced subjective anxiety following ABM, and that this effect would occur only at 100 ms. A 2(Test: pre-training, post-training) × 2(ABM Group: train toward threat, train away from threat) mixed design factorial ANOVA was conducted on state anxiety scores, with ABM Group as a between-subjects factor and Test as a within-subjects factor.
3.4.2.1. 100 ms
The main effect of Test revealed that state anxiety scores decreased from pre-training (M = 40.62, SD = 11.19) to post training (M = 39.00, SD = 10.58) for all participants, F(1, 24) = 4.37, p = .047, partial η2 = .15.
3.4.2.2. 500 ms
There were no significant effects.
3.4.5. Summary
In summary, when the sample was reduced such that only participants with attentional biases (away from threat for the train toward threat group and toward threat for the train away from threat group) were examined, behavioral effects consistent with predictions emerged. The train toward threat group showed increases in attentional bias at both 100 and 500 ms, while the train away from threat group showed decreases in attentional bias and vigilance at 100 ms only. Additionally, state anxiety again decreased following training, regardless of training group, but consistent with predictions this effect was limited to 100 ms.
3.5. Effect of ABM on ERPs to Face Pairs
Next, we tested the predictions that participants in the train away versus train toward threat group would show decreased ERP amplitudes to threat following ABM, and that these effects would be more prominent at 100 versus 500 ms. For each ERP time-locked to face pairs (P1, N170, P2, N2, P3) a 2(ABM Group: train toward threat, train away from threat) x 2(Test: pre-training, post-training) x 2(Duration: 100 ms, 500 ms) x 2(Face Pair: threat, non-threat) mixed design factorial ANOVA was conducted on ERP amplitudes, with ABM Group as a between-subjects factor and Test, Duration, and Face Pair as within-subjects factors. Bonferroni corrections were used for all follow-up t-test comparisons.
3.5.1. P1
The significant main effects of Test [F(1, 47) = 7.51, p = .009, partial η2 = .14] and Duration [F(1, 47) = 4.63, p = .04, partial η2 = .09] were subsumed under the significant ABM Group × Test × Duration interaction, F(1, 47) = 7.23, p = .01, partial η2 = .13. Participants in the train away from threat group showed pre- to post-training decreases in P1 amplitudes to all face pairs presented for 100 ms [pre-training M = 5.11, SD = 2.12; post-training M = 4.28, SD = 1.88), t(23) = 4.55, p < .001].
The three-way ABM Group × Test × Stimulus interaction was also significant, F(1, 47) = 4.19, p = .046, partial η2 = .08. Contrary to predictions, participants in the train away from threat group showed decreases in P1 amplitudes to non-threatening face pairs from pre-training (M = 4.94, SD = 2.15) to post-training (M = 4.26, SD = 1.77), t(23) = 2.95, p = .007.
3.5.2. N170
A significant main effect of Test revealed that N170 amplitudes decreased from pre-training (M = −3.07, SD = 2.20) to post-training (M = −2.23, SD = 2.03), F(1, 47) = 31.14, p < .001, partial η2 = .40. There was also a significant main effect of Duration, such that N170 amplitudes were greater to face pairs presented for 500 ms (M = −2.80, SD = 2.00) versus 100 ms (M = −2.49, SD = 2.14), F(1, 47) = 12.44, p = .001, partial η2 = .21.
3.5.3. P2
In contrast to the effects for N170, a significant main effect of Test revealed that P2 amplitudes increased from pre-training (M = 4.57, SD = 2.52) to post-training (M = 5.07, SD = 2.58), F(1, 47) = 7.58, p = .008, partial η2 = .14.
3.5.4. N2
Effects for N2 amplitudes were similar to those for N170 amplitudes. A significant main effect of Test revealed that N2 amplitudes decreased from pre-training (M = −4.05, SD = 1.22) to post-training (M = −3.50, SD = 1.33), F(1, 47) = 24.00, p < .001, partial η2 = .34. There was also a significant main effect of Duration, such that N2 amplitudes were greater to face pairs presented for 500 ms (M = −3.94, SD = 1.37) versus 100 ms (M = −3.61, SD = 1.20), F(1, 47) = 7.25, p = .01, partial η2 = .13. No other effects reached significance.
3.5.5. P3
Similar to the effects for both P1 and P2, a significant main effect of Test revealed that P3 amplitudes increased from pre-training (M = −.09, SD = 0.68) to post-training (M = .09, SD = 0.78), F(1, 47) = 5.25, p = .03, partial η2 = .10. Additionally, there was also a significant main effect of Duration, such that P3 amplitudes were greater to face pairs presented for 100 ms (M = 0.45, SD = 0.74) versus 500 ms (M = −0.45, SD = 0.87), F(1, 47) = 52.75, p < .001, partial η2 = .53. Last, a significant main effect of Face Pair revealed that P3 amplitudes were greater to non-threat face pairs (M = 0.07, SD = 0.69) versus threat face pairs (M = −0.07, SD = 0.73), F(1, 47) = 7.95, p = .007, partial η2 = .15. Neither the main effect of ABM Group nor any interactions reached significance.
In summary, counter to predictions, no training group differences in ERPs to face cues emerged. P1 amplitudes to face cues, however, were affected by training condition. Somewhat consistent with predictions, participants in the train away from threat group showed reductions in P1 amplitudes to all cues presented for 100 ms after training, regardless of stimulus type, and greater P1 amplitudes to non-threatening versus threatening face cues before training, regardless of duration. Additionally, N170 and N2 amplitudes were greater to cues presented for 500 versus 100 ms while P3 amplitudes were greater to cues presented for 100 versus 500 ms; however, these effects were across stimulus type and do not reflect any attentional biases as predicted.
3.6. Effect of ABM on P3 Amplitudes to Probes
Next, we tested the predictions that participants in the train away versus train toward threat group would show decreased ERP amplitudes to probes in the threatening location following ABM, and that these effects would be more prominent at 100 versus 500 ms.. A 2(ABM Group: train toward threat, train away from threat) × 2(Test: pre-training, post-training) × 2 (Duration: 100 ms, 500 ms) × 3(Probe: angry, happy, baseline) mixed design factorial ANOVA was conducted on P3 amplitudes, with ABM Group as a between-subjects factor and Test, Duration, and Probe as within-subjects factors.
A significant main effect of Test revealed that P3 amplitudes increased from pre-training (M = 1.20, SD = 1.45) to post-training (M = 1.51, SD = 1.36), F(1, 47) = 11.75, p = .001, partial η2 = .20. There was also a significant main effect of Duration, such that P3 amplitudes were greater to face pairs presented for 100 ms (M = 1.52, SD = 1.40) versus 500 ms (M = 1.20, SD = 1.38), F(1, 47) = 22.23, p < .001, partial η2 = .32.
3.7. Correlations
Correlations were conducted on post-training data separately for the two training groups. We predicted that greater ERP processing of threat relative to non-threat would be associated with greater behavioral biases on the post-training assessment for the train toward threat group, and vice versa for the train away from threat group. Effects emerged only for the train toward threat group at 100 ms. Consistent with predictions, greater P2 amplitudes to threat versus non-threat were associated with greater attentional bias (r = .50, p = .01) and vigilance (r = .47, p = .02) on the post-training assessment. Counter to predictions, however, greater N170 amplitudes to threat versus non-threat were associated with reduced attentional bias (r = −.57, p = .003) and vigilance (r = −.43, p = .03) on the post-training assessment. Thus, while there were no direct effects of ABM on behavioral biases, post-training correlations revealed associations between ERP amplitudes (i.e., P2 and N170) and behavioral biases.
4. Discussion
The goal of the present study was to examine the effects of ABM on behavioral attention biases and neurophysiological measures of early and later attention processing in non-anxious individuals. Results suggest training-related changes in the behavioral threat bias only emerged among a subset of participants who showed pre-training attention biases towards and away from threat. In addition, ABM also influenced ERP measures of early spatial attention to emotional face cues. Finally, post-training ERPs were correlated with behavioral threat biases – in particular, greater P2 amplitudes and reduced N170 amplitudes to threatening versus non-threatening cues were associated with greater attentional bias and vigilance for the group trained toward threat.
For the sample as a whole, ABM did not result in significant modification in behavioral attentional biases. When the sample was selected to only include those participants in each ABM condition with pre-training attentional biases, the predicted behavioral effects emerged. Specifically, participants in the train away from threat group showed reductions in attentional bias and vigilance, or attentional capture, at 100 ms. Furthermore, participants in the train toward threat condition showed increases in attentional bias at both 100 and 500 ms. Results underscore the importance of taking baseline threat bias into account when using the ABM task. It is likely that in this non-anxious sample no behavioral effects were found because of the wide range of biases present before attention training - selecting a sub-sample of those participants showing a pre-training bias reduced the potential for ceiling and floor effects.
In addition to these behavioral effects, ABM affected early spatial attention in this group of non-anxious individuals: Participants in the train away from threat group showed decreases in P1 amplitudes to all face pairs presented for 100 ms after training and decreases in P1 amplitudes to non-threatening face pairs after training. This pattern of reduced P1 suggests habituation effects such that training attention away from threat (and towards non-threat) in a normative group of participants actually serves to reduce early, automatic capture of attention of face cues – even to non-threatening ones. These effects are also interesting because, in contrast to the Eldar and Bar-Haim (2010) study with anxious participants, we found that ABM influences early spatial attention (P1) rather than later, more elaborated attentional processes (P2, N2) in non-anxious individuals. This hints at the possibility that anxious participants may need to recruit more effortful attentional processes to modify attention biases, whereas in non-anxious individuals shifts in more automatic and early attention are influenced by ABM.
Correlational analyses, however, suggest that for participants trained to attend to threat the degree to which post-training attention to threat at 100 ms is enhanced at later stages of attention (P2) is associated with greater behavioral measures of attention bias. Contrary to this pattern, enhanced processing of threatening versus non-threatening faces as measured by the N170 (at 100 ms) was associated with reduced behavioral attentional bias. This interesting dissociation may speak to the distinct processes reflected by the N170 and P2. While the N170 is an early-emerging face-specific component, thought to reflect structural encoding of faces (Bentin, Allison, Puce, Perez, & McCarthy, 1996; Holmes, Vuilleumier, & Eimer, 2003), the P2 has been linked to relatively more elaborated attentional processing of emotion (Carretié, Martín-Loeches, Hinojosa, & Mercado, 2001; Carretié, Mercado, Tapia, & Hinojosa, 2001; Eldar et al., 2010). Thus, enhanced face-specific processing of threat may actually hinder the effects of attention training toward threat, whereas the P2, which is more specific to emotion, may reflect greater attentional capture by threat due to attention training. Future research could tease apart these possibilities through the combined use of ERP (providing adequate temporal resolution) and fMRI (providing information on anatomical and functional specificity).
State anxiety scores were reduced following ABM regardless of training condition. This effect should perhaps not be surprising, however, since previous research suggests that emotional vulnerability, or stress reactivity, is altered by ABM rather than mood (Eldar et al., 2008; MacLeod et al., 2002). Indeed, it appeared that taking part in the dot probe task had general anxiety-reducing effects, perhaps due to its predictable and repetitive nature. Future research should include a measure of emotional vulnerability before and after ABM such as stress reactivity; including this measurement along with ERPs would allow for examination of how changes in stress reactivity are related to changes in physiological measures of the threat bias following attention training.
Results also underscore the importance of stimulus duration when considering the effects of ABM on behavior and ERP measures of attention. Although behavioral studies suggest that non-anxious individuals show an attentional bias to threat at shorter stimulus durations such as 100 ms (Cooper & Langton, 2006; Koster et al., 2007; Koster et al., 2005; Mogg et al., 1997), the present study suggests that non-anxious individuals show sensitivity to a range of stimulus durations. For example, at 100 ms both the train toward and away from threat conditions were successful in altering behavioral biases, while at 500 ms only the train toward threat condition had an effect. ERP amplitudes to both face cues and probes were sensitive to presentation duration. N170 and N2 amplitudes were greater to face cues presented for 500 versus 100 ms, while P3 amplitudes were greater to both face cues and probes presented for 100 versus 500 ms. Many of these duration effects, however, are independent of stimulus type (i.e., threatening versus non-threatening). Thus, it appears that both early- and later-developing attentional processes are implicated in threat processing in non-anxious individuals. Further research is required to track the time course of attention to threatening versus non-threatening stimuli presented for brief versus longer durations (O’Toole, DeCicco, Hong, & Dennis, in press).
Limitations of the current study include the duration of the ISI between cues and probes, the use of happy faces instead of neutral faces, and the effects of response slowing during the dot probe task. First, the somewhat long duration of the ISI (500 ms) between cues and probes may have allowed effects of the cues to extinguish before probes appeared. Furthermore, research on the inhibition of return demonstrates a suppression of attentional orienting toward previously cued locations, beginning around 500 ms after the stimulus (Klein, 2000). Thus, measurement of the attentional bias to toward threat via RTs to probes in the dot probe task may be inaccurate with such a long ISI. In future studies a shorter ISI should be employed, such as a jittered duration between 100 and 300 (Mueller et al., 2009). Second, the use of happy faces rather than neutral faces may have altered the interpretation of the vigilance and disengagement scores – although the inclusion of happy faces strengthens the inference that effects are specific to threat rather than emotionally arousing stimuli (Mathews & MacLeod, 2002). Third, a recent study suggests that high anxious individuals show response slowing following threat cues (angry-neutral) and that both high and low anxious individuals show response slowing after happy cues (happy-neutral pairs) (Mogg, Holmes, Garner, & Bradley, 2008). However, due to methodological and participant differences between the current student and Mogg et al.’s (2008), future studies are needed to assess the effect of response slowing on attentional bias and ABM in non-anxious individuals.
To further explore the processes underlying attention bias modification, other types of interventions should be explored. Mindfulness meditation involves both the self-regulation of attention as well as changes in the higher-order evaluation of emotional experiences (e.g., non-judgment; Bishop et al., 2004; Shapiro, Carlson, Astin, & Freedman, 2006). Several studies have demonstrated that mindfulness meditation techniques promote enhanced attentional stability and flexibility (Lutz, Slagter, Dunne, & Davidson, 2008; Lutz et al., 2009; MacLean et al., 2010). Future research should compare the effectiveness of attention training versus mindfulness in modifying attentional biases, and examine similarities or differences in their neural underpinnings.
In summary, identifying physiological changes following ABM can inform the processes underlying both the induction and reduction of the threat bias. Furthermore, the current results suggest that ABM may only be effective for non-anxious participants who show biases before training. Future studies should examine the link between the mechanisms through which attention training functions and changes in anxiety over time, as well as the effect of pre-training biases in anxious individuals.
Behavioral effects of attention training depend on pre-training biases.
Early spatial attention to emotional stimuli is affected by attention training.
Physiological changes in attention are associated with behavior after training.
Acknowledgments
This research was supported by grants from the National Institutes of Mental Health (NIMH) grant K01MH075764 and S06GM060654 awarded to Tracy A. Dennis. This research was also made possible by Grant RR03037 form the National Center for Research Resources (NCRR), a component of the National Institutes of Health.
Footnotes
Because participants first passively viewed all faces before the dot probe and ABM tasks there may be potential habituation effects. However, other studies have successfully employed the same task with a similar number of stimuli (Eldar & Bar-Haim, 2010; Eldar et al., 2008).
Actors: 01F, 07F, 10F, 12F, 13F, 14F, 20M, 21M, 23M, 38M, 39M, 43M
There were no significant effects for P1 and N1 amplitudes to probes. Thus, waveforms, descriptive statistics, and analyses are reported for P3 amplitudes only.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amir N, Beard C, Burns M, Bomyea J. Attention modification program in individuals with generalized anxiety disorder. Journal of Abnormal Psychology. 2009;118(1):28–33. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Beard C, Taylor CT, Klumpp H, Elias J, Burns M, Xi C. Attention training in individuals with generalized social phobia: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2009;77(5):961–973. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y. Research review: Attention bias modification (ABM): A novel treatment for anxiety disorders. The Journal of Child Psychology and Psychiatry. 2010;51(8):859–870. doi: 10.1111/j.1469-7610.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SR, Lau MA, Shapiro S, Carlson L, Anderson ND, Carmody J, Devins G. Mindfulness: A proposed operational definition. Clinical Psychology: Science and Practice. 2004;11(3):230–241. doi: 10.1093/clipsy/bph077. [DOI] [Google Scholar]
- Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer CJ. Lateral prefrontal cortex mediates the cognitive modification of attenitonal bias. Biological Psychiatry. 2010;67(10):919–925. doi: 10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L, Martín-Loeches M, Hinojosa JA, Mercado F. Emotion and attention interaction studied through event-related potentials. Journal of Cognitive Neuroscience. 2001;13(8):1109–1128. doi: 10.1162/089892901753294400. [DOI] [PubMed] [Google Scholar]
- Carretié L, Mercado F, Tapia M, Hinojosa JA. Emotion, attention, and the ‘negativity bias’, studied through event-related potentials. International Journal of Psychophysiology. 2001;41:75–85. doi: 10.1016/S0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Bacon AK, Williams NL. Phenomenological characteristics of attentional biases towards threat: A critical review. Cognitive Therapy and Research. 2009;33:221–234. doi: 10.1007/s10608-007-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P, MacLeod C, Shirazee N. Prepared for the worst: Readiness to aquire threat bias and susceptibility to elevate trait anxiety. Emotion. 2008;8(1):47–57. doi: 10.1037/1528-3542.8.1.47. [DOI] [PubMed] [Google Scholar]
- Cooper RM, Langton SRH. Attentional bias to angry faces using the dot-probe task? It depends when you look for it. Behaviour Research and Therapy. 2006;44:1321–1329. doi: 10.1016/j.brat.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Eldar S, Bar-Haim Y. Neural plasticity in response to attention training in anxiety. Psychological Medicine. 2010;40:667–677. doi: 10.1017/S0033291709990766. [DOI] [PubMed] [Google Scholar]
- Eldar S, Ricon T, Bar-Haim Y. Plasticity in attention: Implications for stress response in children. Behaviour Research and Therapy. 2008;46:450–461. doi: 10.1016/j.brat.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Eldar S, Yankelevitch R, Lamy D, Bar-Haim Y. Enhanced neural reactivity and selective attention to threat in anxiety. Biological Psychology. 2010;85:252–257. doi: 10.1016/j.biopsycho.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Fox E, Derakshan N, Shoker L. Trait anxiety modulates the electrophysiological indices of rapid spatial orienting towards angry faces. NeuroReport: For Rapid Communication of Neuroscience Research. 2008;19(3):259–263. doi: 10.1097/WNR.0b013e3282f53d2a. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifacts. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Lieibenluft E, Pine DS. Attention bias modification treatment: A meta-analysis toward the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein SM, White LK, Bar-Haim Y, Fox NA. Affective primes suppress attention bias to threat in socially anxious individuals. Behaviour Research and Therapy. 2008;46(7):799–810. doi: 10.1016/j.brat.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Vuilleumier P, Eimer M. The processing of emotional facial expression is gated by spatial attention: Evidence from event-related brain potentials. Cognitive Brain Research. 2003;16:174–184. doi: 10.1016/S0926-6410(02)00268-9. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends in Cognitive Sciences. 2000;4(4):138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Baert S, Bockstaele M, De Raedt R. Attentional retraining procedures: Manipulating early or late components of attentional bias? Emotion. 2008;10(2):230–236. doi: 10.1037/a0018424. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Verschuere B, De Houwer J. Selective attention to threat in the dot probe paradigm: Differentiating vigilance and difficulty to disengage. Behaviour Research and Therapy. 2004;42:1183–1192. doi: 10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Verschuere B, Van Damme S, Wiersema JR. Components of attentional bias to threat in high trait anxiety: Facilitated engagement, impaired disengagement, and attentional avoidance. Behaviour Research and Therapy. 2006;44:1757–1771. doi: 10.1016/j.brat.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Verschuere B, Vanvolsem P, De Houwer J. A time-course analysis of attentional cueing by threatening scenes. Experimental Psychology. 2007;54(2):161–171. doi: 10.1027/1618-3169.54.2.161. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Verschuere B, Crombez G, Van Damme S. Time-course of attention for threatening pictures in high and low trait anxiety. Behaviour Research and Therapy. 2005;43:1087–1098. doi: 10.1016/j.brat.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in attention. Trends in Cognitive Sciences. 2008;12(4):163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Rawlings NB, Francis AD, Greischar LL, Davidson RJ. Mental training enhances attentional stability: Neural and behavioral evidence. The Journal of Neuroscience. 2009;29(42):13418–13427. doi: 10.1523/JNEUROSCI.1614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean KA, Ferrer E, Aichele SR, Bridwell DA, Zanesco AP, Jacobs TL, Saron CD. Intensive meditation training improves perceptual discrimination and sustained attention. Psychological Science. 2010;21(6):829–839. doi: 10.1177/0956797610371339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037/0021-843X.95.1.15. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111(1):107–123. doi: 10.1037//0021-843X.111.1.107. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognitive Therapy and Research. 1998;22(6):539–560. doi: 10.1023/A:1018738019346. [DOI] [Google Scholar]
- Mathews A, MacLeod C. Induced processing biases have causal effects on anxiety. Cognition and Emotion. 2002;16(3):331–354. doi: 10.1080/02699930143000518. [DOI] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/S0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Attentional bias in generalized anxiety disorder versus depressive disorder. Cognitive Therapy and Research. 2005;29(1):29–45. doi: 10.1007/s10608-005-1646-y. [DOI] [Google Scholar]
- Mogg K, Bradley BP, De Bono J, Painter M. Time course of attentional bias for threat information in non-clinical anxiety. Behaviour Research and Therapy. 1997;35(4):297–303. doi: 10.1016/s0005-7967(96)00109-x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Miles F, Dixon R. Time course of attentional bias for threat scenes: Testing the vigilance-avoidance hypothesis. Cognition and Emotion. 2004;18(5):689–700. doi: 10.1080/02699930341000158. [DOI] [Google Scholar]
- Mogg K, Holmes A, Garner M, Bradley BP. Effect of threat cues on attentional shifting, disengagement and response slowing in anxious individuals. Behaviour Research and Therapy. 2008;46:656–667. doi: 10.1016/j.brat.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, Woldehawariat G, Montgomery LA, Zarahn E, McClure EB, Pine DS. Experience-dependent plasticity for attention to threat: Behavioral and neurophysiological evidence in humans. Biological Psychiatry. 2004;56(8):607–610. doi: 10.1016/j.biopsych.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Mueller EM, Hofmann SG, Santesso DL, Meuret AE, Bitran S, Pizzagalli DA. Electrophysiological evidence of attentional biases in social anxiety disorder. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 2009;39(7):1141–1152. doi: 10.1017/S0033291708004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole LJ, DeCicco JM, Hong M, Dennis TA. The impact of task-irrelevant emotional stimuli on attention in three domains. Emotion. doi: 10.1037/a0024369. in press. [DOI] [PubMed] [Google Scholar]
- Salemink E, van den Hout MA, Kindt M. Selective attention and threat: Quick orienting versus slow disengagement and two versions of the dot probe task. Behaviour Research and Therapy. 2007;45:607–615. doi: 10.1016./j.brat.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Richey JA, Buckner JD, Timpano KR. Attention training for generalized social anxiety disorder. Journal of Abnormal Psychology. 2009;118(1):5–14. doi: 10.1037/a0013643. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user’s guide. Pittsburgh, Pa: Psychology Software Tools, Inc; 2002. [Google Scholar]
- Shapiro SL, Carlson LE, Astin JA, Freedman B. Mechanisms of mindfulness. Journal of Clinical Psychology. 2006;62(3):373–386. doi: 10.1002/jclp.20237. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-trait anxiety inventory manual. Redwood City, CA: Mind Garden, Inc; 1983. [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior. 2002;77:477–482. doi: 10.1016/S0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Wilson E, MacLeod C. Contrasting two accounts of anxiety-linked attentional bias: Selective attention to varying levels of stimulus threat intensity. Journal of Abnormal Psychology. 2003;112(2):212–218. doi: 10.1037/0021-843X.112.2.212. [DOI] [PubMed] [Google Scholar]