Cellular senescence is a state of terminal growth arrest that is often associated with increased cell size, enhanced expression of anti-proliferative genes [such as the cyclin-dependent kinase (cdk) inhibitor p21 and the tumor suppressors p53 and p16], a distinct secretory phenotype and elevated expression of a senescence-associated β-galactosidase activity. Cellular senescence can arise from erosion of the chromosome ends, the telomeres, after successive cycles of replication (replicative senescence) or prematurely from loss or gain of function of some genes (genetically induced senescence) or from exposure to damaging stimuli (stress-induced senescence). Despite losing their ability to divide, senescent cells remain metabolically active and profoundly influence physiologic and pathologic processes in surrounding and distant tissues.1
In recent years, cellular, molecular and genetic studies have provided strong support for the notion that senescence represents a tumor suppressor mechanism.1 In keeping with this view, DNA damage arising from eroded telomeres or genotoxins, oxidative injury, activation of oncogenes and reduced function of tumor suppressors, all can potently trigger senescence and thereby avoid tumorigenesis. The specific molecular processes by which senescence is implemented are only partially known, but they include critical changes in gene expression programs. Such changes are mediated via both transcriptional and posttranscriptional events.
In a recent article in Aging, we surveyed microRNAs differentially expressed in dividing (early-passage) human diploid fibroblasts compared with terminally arrested (late-passage) fibroblasts that had reached replicative senescence.2 MicroRNAs constitute a large family of small (∼22-nt long) noncoding RNAs that modulate gene expression post-transcriptionally. MicroRNAs associated with Argonaute and other protein components of the RNA-induced silencing complex (RISC) interact with target mRNAs with partial complementarity, and typically reduce the stability of the mRNA, its translation, or both of these processes.3 In keeping with the major changes in gene expression that characterize senescence,4 we observed significant differences in the abundance of microRNAs expressed in senescent fibroblasts relative to proliferating fibroblasts.
Among the microRNAs that were preferentially upregulated in senescent cells, several were identified as tumor-suppressive microRNAs (ts-miRs). These included miR-519 and miR-34a, two microRNAs that were reported to suppress tumorigenesis and induce senescence, and were expressed in low levels in cancer cells.5,6 miR-34a suppresses tumor growth at least in part by repressing the production of several proliferative proteins (including E2F, c-Myc, cyclin D1, cyclin E, cdk4 and cdk6) and anti-apoptotic proteins (e.g., Bcl-2 and SIRT1).5 miR-519 reduces tumor growth at least partly by repressing the production of HuR, an RNA-binding protein that promotes the expression of angiogenic factors (e.g., hypoxia-inducible factor 1α and vascular endothelial growth factor) as well as proliferative proteins (e.g., c-fos and cyclins D1, E, B1, A2) and anti-apoptotic proteins (e.g., Bcl-2, SIRT1, Mcl-1 and prothymosin α).7 Thus, these microRNAs likely contribute to an anti-tumor program by triggering senescence.
Conversely, a subset of microRNAs with oncogenic function (oncomiRs) were found to be selectively reduced in senescent cells.8 For example, the oncomiRs miR-15b and miR-141 showed lower abundance in senescence populations.8 miR-15b exerts its pro-oncogenic function in part by enhancing cell proliferation and reducing apoptosis, while miR-141 reduces the production of pro-apoptotic and tumor-suppressor proteins (e.g., YAP-1 and PTEN), and facilitates endothelial-to-mesenchymal transition, an early step in tumor metastasis.9,10 The favored reduction of oncomiRs with senescence further underscores the contribution of microRNAs to a cellular program that helps to evade tumorigenesis.
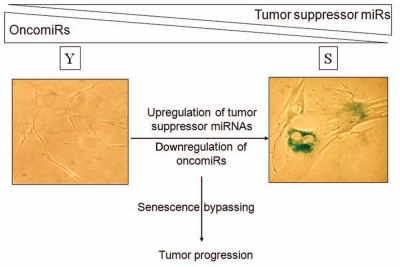
In conclusion, senescent cells express distinct collections of microRNAs which play a central role in the senescenceelicited tumor suppression (Fig. 1). MicroRNAs upregulated with senescence include ts-miRs such as miR-34a and miR-519, which reduce tumorigenesis, while microRNAs downregulated during senescence include oncomiRs like miR-15b and miR-141, which promote tumor development. Studies to gain a more comprehensive knowledge of microRNAs at the intersection of senescence- and cancer-associated processes are warranted. Understanding their function, their target mRNAs, the specific pathways that modulate their abundance, and their impact on senescence and cancer are all critical areas to explore. The insight gained from these efforts will enable us to exploit the diagnostic and therapeutic value of microRNAs, particularly microRNAs that influence diseases like cancer, which are strongly influenced by aberrant cellular senescence.
Figure 1.
miRNA expression programs jointly affect senescence and tumorigenesis. As cells become senescent, they express reduced levels of oncomiRs and higher levels of ts-miRs. This pattern of microRNA expression mirrors the expression needed to suppress tumorigenesis. We propose that aberrant changes in senescence-associated microRNA expression programs contribute to malignancy.
Comment on: Marasa BS, et al. Aging. 2010;2:333–343. doi: 10.18632/aging.100159.
References
- 1.Kuilman T, et al. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marasa BS, et al. Aging. 2010;2:333–343. doi: 10.18632/aging.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabian MR, et al. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 4.Fridman AL, et al. Oncogene. 2008;27:5975–5987. doi: 10.1038/onc.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermeking H. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 6.Gorospe M, et al. Trends Genet. 2011;27:233–241. doi: 10.1016/j.tig.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelmohsen K, et al. WIRES RNA. 2010;1:214–229. doi: 10.1002/wrna.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marasa BS, et al. Sci Signal. 2009;2:69. doi: 10.1126/scisignal.2000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satzger I, et al. Int J Cancer. 2010;126:2553–2562. doi: 10.1002/ijc.24960. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, et al. Carcinogenesis. 2010;31:559–566. doi: 10.1093/carcin/bgp335. [DOI] [PubMed] [Google Scholar]