Abstract
In embryonic Eda mutant (“Tabby”) mice, the development of one of the two major types of hair, “primary” hair fails, but other “secondary” hairs develop in normal numbers, though shorter and slightly aberrant. In Tabby mice, Shh is undetectable in skin early on, but is activated during secondary hair formation. We inferred that Shh may be involved in primary hair formation, activated normally by Eda, and also possibly in secondary hair formation, activated by an Eda-independent pathway. Varying the dosage of Shh now supports these inferences. In Shh knockout mice, mice were totally hairless: primary and secondary hair follicle germs were formed, but further progression failed. Consistent with these findings, when Shh loss was restricted to the skin, secondary hair follicle germs were initiated on time in Tabby mice, but their subsequent development (down-growth) failed. An Shh transgene expressed in Tabby skin could not restore induction of primary hair follicles, but restored normal length to the somewhat aberrant secondary hair that was formed and prolonged the anagen phase of hair cycling. Thus, Shh is required for primary and secondary hair downgrowth and full secondary hair length, but is not itself sufficient to replace Eda or make fully normal secondary hair.
Key words: Eda, Shh, Wnt, hair follicle subtypes, Tabby
Introduction
Anhidrotic ectodermal dysplasia (EDA), a hereditary genetic disorder, profoundly affects the development of skin appendages, including hair follicles (OMIM305100). When the orthologous gene Eda was inactivated in a mouse model, however, it became clear that the major classes of mouse hair have distinctive developmental mechanisms, which respond differentially to Eda. Primary hair follicles, which form in early embryonic life and create a protective outer coat, are absent in the Eda-(“Tabby”) mice. By contrast, the secondary hair follicles, which develop later as a thermoregulatory undercoat, still form in normal numbers in the absence of Eda; but the secondary hairs formed are aberrantly short, thin and straight.2,3
Eda loss interrupts its specific signaling pathway.4–7 Along with its receptor Edar and receptor adaptor Edaradd, Eda acts to turn on NFκB-mediated transcription.8 The Eda pathway was found to regulate a set of candidate target genes including Shh, LTb, Dkk4, Cyclin D, Ctgf and BMP4/7.9–11 Among these Shh is the gene most strikingly affected in Tabby skin.9,12 Suggestively, although Shh is undetectable before and during the period when primary hair follicle induction fails, it is nevertheless subsequently activated in Tabby during secondary hair follicle development.5,13,14 The data were consistent with previous findings of Shh involvement in the development of various skin appendages,15 and further led to the inference that Shh was correlated with formation of both hair types, but was apparently activated in different ways for the two—perhaps with differential consequences.
Observations of Shh knockout embryos revealed that the gene was not required for primary hair follicle induction, but was indispensible for the subsequent developmental elongation of the follicle.16,17 However, because conventional Shh knockout mice are embryonic lethal, Shh function in secondary hair follicles, most of which start to form just before term, has been more difficult to study. Furthermore, there were hints that Shh may function in the induction phase as well as later for some skin appendages. For example, β-catenin overexpression resulted in excessive hair follicle placode formation that was shown to be mediated by Shh.18 We found that Shh was also implicated in secondary hair follicle initiation downstream of Eda and Wnt/Dkk4.1 And ablation of Noggin, which encodes a BMP antagonist, had been determined to selectively abolish secondary hair follicle induction by suppressing Wnt and Shh expression.19 Finally, in an ex vivo system, ectopically applied recombinant Shh protein partially rescued the induction of salivary glands, another skin appendage affected in Tabby mice.20,21
Here we have further analyzed the role of Shh in Tabby (secondary) hair follicle formation by modulating the level of activity of Shh up or down. By generating skin-restricted Shh transgenic and knockout mice in a Tabby background, where only secondary follicles form, we show that Shh expression is itself not sufficient to restore primary hair follicle induction; and in agreement with the findings for primary follicles, Shh is required for elongation but not for the first inductive phase of secondary hair.
Results
Skin-specific Shh knockout mice are hairless: hair follicles were initiated but further progression failed.
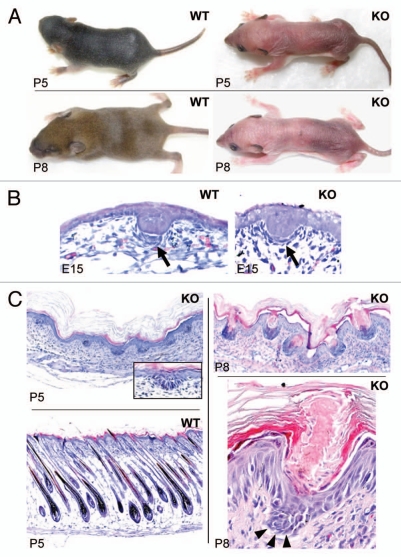
To assess Shh function in hair follicles, we generated skin-specific Shh knockout mice by crossing ShhloxP/loxP mice with mice bearing a Cre transgene driven by the Krt14 promoter to direct the ablation activity of Cre to skin. We aimed to observe the extent to which Shh knockout mice resemble Tabby mice during induction and subsequent progression stages of primary and secondary hair follicles. Most of the skin-specific Shh knockout mice died at P4-P5, but some survived until P8, reaching the time at which hair follicles complete their development in wild-type mice. Homozygous Shh knockout mice completely lack all types of visible hair including whiskers (Fig. 1A). Histological studies revealed that the primary hair germs were formed at E15 in the skin-specific knockout mice, but dermal condensation, involved in the next phase of hair follicle formation, was rudimentary compared with wild-type littermates (Fig. 1B, arrows). At P5, hair follicles in the wild-type pups produced hair shafts, but the knockout mice still remained at the earlier stage 1–2, and dermal condensations had completely disappeared (Fig. 1C, left parts). At P8, hair germs were comparable to those at P5, but the epidermis above the hair follicle germs had invaginated massively, and instead of hair follicles forming, striking follicular keratosis had occurred in Shh knockout mice. Both hyperkeratosis and parakeratosis were seen in the keratotic plugs (Fig. 1C, right parts), suggesting that Shh is required for epidermal differentiation-at least for keratinocytes competent in hair follicle formation. Hair germs in the knockout mice numbered about 57% of hair follicles in wild-type siblings at P5 (Fig. 1C and data not shown), suggesting that most primary and secondary hair germs were induced in the knockout mice. These findings are consistent with phenotypes of conventional Shh knockout embryos and skin transplanted onto immune deficient mice, and extend the characterization of the defects, demonstrating that all phenotypes are intrinsic to the skin from the time of follicle germ formation onward.16,17
Figure 1.
Phenotypes of skin specific Shh knockout mice. (A) Note hairs protruding onto the skin surface in wild-type (WT) pups at P5 and hair coat forming at P8. Shh knockout (KO) pups showed a complete lack of visible hairs, including whiskers. (B) Primary hair germs were formed in the WT embryos at E15 with a clear dermal condensation under each germ (arrow). In Shh knockout pups, primary hair germs were formed on time, but dermal condensations were rudimentary. (C) WT mice started to make hair shafts at P5 (lower left part), but knockout mice still remained at the germ stage (upper left part). Dermal condensations had disappeared in the knockout mice at P5 (insert in P5 KO). At P8, knockout skin showed invaginations at the site of hair follicle germs (upper right part). Higher magnification showed follicular keratosis with keratotic plugging (lower right part). Arrowheads point to a hair germ.
Secondary hair follicles were induced on time, but further elongation was blocked in Tabby mice lacking Shh.
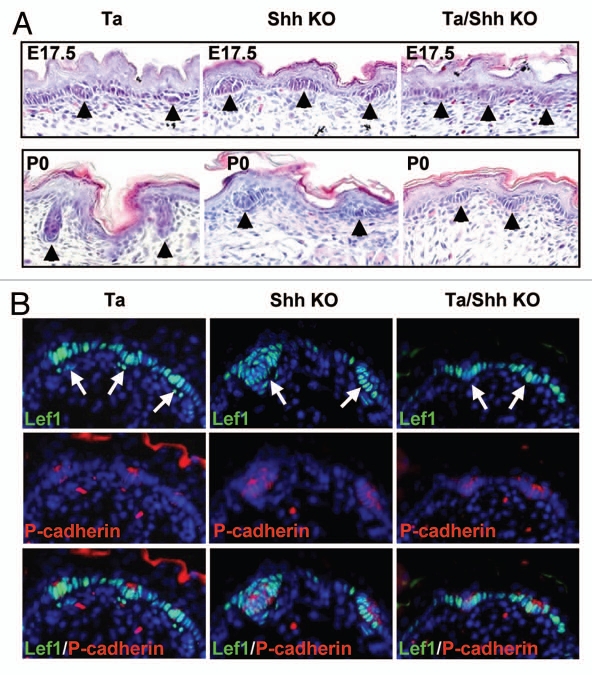
To focus on Shh function in secondary hair follicle development, we ablated Shh in the skin of Tabby mice, in which only secondary hair follicles form. The Shh knockout Tabby mice died shortly after birth, but we could recover samples from E17.5 and newborn animals. At E17.5, when Tabby mice started to form secondary hair follicles, Shh knockout mice in a wild-type background (Shh−/−Eda+/y) displayed both some larger and a majority of smaller hair follicle germs, most likely respectively representing primary and secondary hair follicle germs (Fig. 2A and B). Consistent with that notion, in the double mutant embryos (Shh−/−Eda−/y), uniformly small secondary hair germs were formed that were indistinguishable from those in Tabby mice in size and number, revealing that both Shh and Eda are dispensable for secondary hair follicle induction (Fig. 2A). Immunostaining with p-cadherin, a hair germ marker, and Lef1, a Wnt pathway transcription factor, further confirmed hair germ formation in all 3 mutant strains (Fig. 2B). In fact, the hair germs formed in embryos in each of the 3 mutants expressed Lef1 normally (Fig. 2B), as expected with both Shh and Eda located downstream of Wnt action. At P0, secondary hair germs had entered developmental stages 3–4 in Tabby (Fig. 2A, lower parts). By contrast, both primary and secondary hair germs remained at stage 1–2 in Shh knockout mice in wild-type background. In the double mutant mice, in sharp contrast to Tabby, hair germs at P0 were indistinguishable from those at E17.5, demonstrating that Shh is required for elongation (down-growth) of secondary hair follicles in Tabby mice.
Figure 2.
Induction of secondary hair follicles in Shh knockout Tabby mice. (A) Stage 1–2 hair germs were formed in Tabby, Shh knockout and Shh knockout Tabby mice at E17.5 (arrowheads). At P0, Tabby hair follicles were in stage 3–4, but hair germs in Shh knockout and Shh knockout Tabby mice remained at stage 1–2. (B) Immunostaining with Lef1 and P-cadherin revealed hair germ formation in the mutant strains (arrows). Note the larger and smaller hair germs formed in Shh knockout mice.
Restoration of hair length, but neither primary hair follicles nor normal secondary hair, in Shh transgenic Tabby mice.
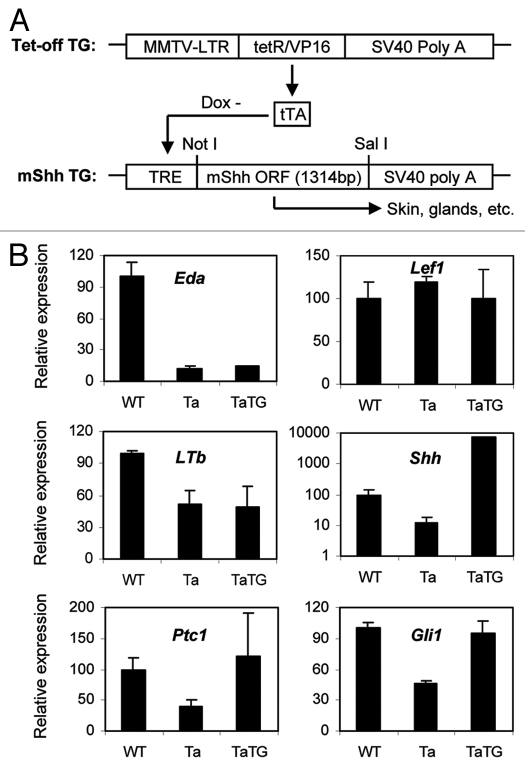
Given the position of Shh action downstream of Eda, one can ask if it is capable of replacing some or all of the action of Eda? If so, Shh supplementation in Tabby mice would restore wild-type phenotypes. We next generated Shh transgenic mice in a Tabby background (TaTG mice) (Fig. 3A and Materials and Methods). Q-PCR assays revealed that, consistent with its location downstream of Eda and Wnt, the Shh transgene did not affect expression levels of Eda and Lef1 in Tabby skin (Fig. 3B). Another putative target of Eda, LTb, is also unaffected by the transgene (Fig. 3B). We next verified the dramatic upregulation of Shh expression in the transgenic Tabby skin compared with wild-type and Tabby littermates at E14.5 (Fig. 3B). Also, expression of the Shh pathway and target genes Ptch1 and Gli1 was restored to wild-type levels in the transgenic embryos. Thus, the transgene indeed activated the Shh pathway in Tabby skin (Fig. 3B).
Figure 3.
Shh transgene expression system and expression levels. (A) A transactivator, tTA, driven by an MMTV promoter, is constantly expressed in mouse skin. tTA binds to the TRE promoter region of Shh transgene in the absence of doxycycline (Dox-), and activates transcription of Shh in skin and exocrine glands. (B) Expression levels of Shh pathway genes and some hair follicle markers at E14.5 in Tabby male mice bearing an Shh transgene. Shh was dramatically upregulated in the transgenic mice (note the log scale for Shh), and Ptc1 and Gli1 were restored to wild-type levels in the transgenic skin. Eda and Lef1 and LTb were unchanged in the transgenic skin.
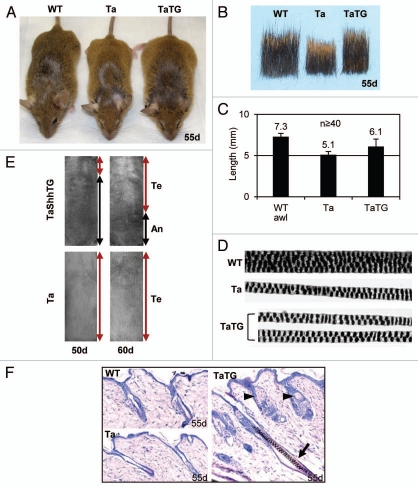
As for their phenotypes, Shh transgenic Tabby mice were slightly larger, and their hair coats appreciably longer, than Tabby littermates, though they still lacked hair behind ears and on tails (Fig. 4A and B). The length of secondary awl hair was about 7.3 mm in wild-type (ranging from 6.2–8.2 mm) and 5.1 mm in Tabby (4.0–6.0 mm). In the transgenic Tabby mice, average hair length was 6.1 mm (4.2–8.1 mm), a significant restoration by the transgene (Fig. 4C). Nevertheless, the restored hairs were still thin and straight, and the medullary granules (air cells) were fewer in number than in wild-type mice, and abnormally arranged, as in Tabby (Fig. 4D). Hair cycling was also altered in the transgenic mice confirming previous reports in reference 22 and 23. Hair follicles in Tabby and wildtype mice were uniformly in the telogen phase at 50 d and 60 d, however, about 70% of back skin was in the anagen phase at 50 d, and about 40% of lower back skin was in the anagen phase at 60 d in most transgenic mice (Fig. 4E, left; skin pigmentation represents anagen phase). Histological analysis revealed that both anagen and telogen follicles exist in the middle back skin in the transgenic mice, but only telogen follicles were observed in Tabby or wild-type mice at 55 d (Fig. 4F). From these observations we infer that restoration of hair length in the transgenic Tabby mice was presumably attributable to prolonged occupancy of the anagen phase.
Figure 4.
Gross phenotypes of Shh transgenic Tabby mice. (A) Shh transgenic Tabby mice were larger, and had longer hair coats than Tabby controls. (B) Hair samples from back skin showed restoration of hair length in Shh transgenic Tabby mice. (C) Awl hair in WT was about 7.3 mm, awl-like hair in Tabby was about 5.1 mm, and in Shh transgenic Tabby was about 6.1 mm. (D) Hair structure in Shh transgenic Tabby mice was comparable to Tabby controls. (E) Tabby mice were uniformly in the telogen phase at 50- and 60-d old age. In contrast, about 70 and 40% of lower back skin was still in the anagen phase in the Shh transgenic Tabby mice at 50 d and 60 d of age, respectively. Te, telogen phase; An, anagen phase.
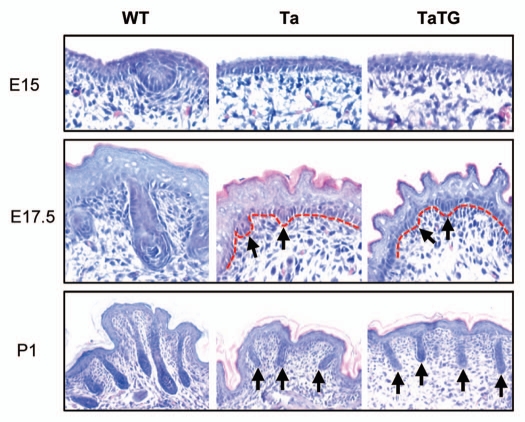
The Shh transgene was also unable to rescue primary hair follicle induction in the Tabby mice. At E15, primary hair follicle germs were evident in wild-type embryos, but not in Tabby or Shh transgenic Tabby embryos (Fig. 5; E15). At E17.5, primary hair follicles in wild-type embryos formed dermal papillae (stage 5–6), and both Tabby and transgenic Tabby mice just started to form secondary hair follicle germs (stage 1–2) (Fig. 5; E17.5). At P1, primary hair follicles in the wild-type pups had begun to make hair shafts (stage 7–8), but only stage 3–4 uniformly short follicles were detected in Tabby and Shh transgenic Tabby mice (Fig. 5; P1).
Figure 5.
Shh transgene does not rescue primary hair follicle induction in Tabby. In WT controls, primary hair germs were clear at E15, had formed dermal papillae at E17.5, and were mostly mature at P1 (left parts). In Tabby, primary hair follicles were not induced, secondary hair follicle germs were emerged at E17.5 (arrows in middle part), and stage 3–4 follicles were detected at P1 (arrows in lower middle part). Shh transgenic Tabby mice showed identical developmental histology to Tabby.
Overall, the findings from the Shh transgenic mice (Figs. 4 and 5) thus confirmed the requirement of Shh for the elongation of hair follicles rather than for the induction of primary and secondary hair follicles.
Sebaceous gland hyperplasia and basal cell carcinomalike tumors in Shh transgenic Tabby mice.
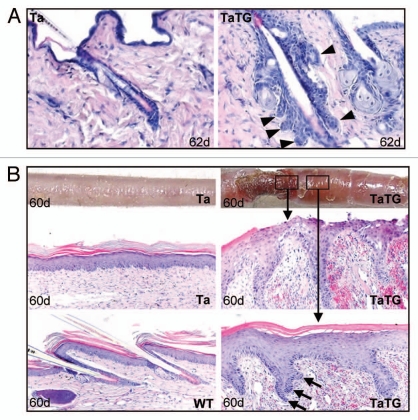
Consistent with previous findings,24 enlarged sebaceous glands were readily detectable in all hair follicles in the Shh transgenic Tabby mice (Fig. 4F, arrowheads and Fig. 6A). In addition, we found occasional formation of basal cell carcinoma-like tumors25 in hairy back skin and non-hairy tail skin of transgenic Tabby mice. Figure 6A shows basal cell carcinoma-like tumors that clearly originated from hair follicle epithelium (arrowheads, in TaTG). We also found severe skin erosion in areas of the tails of some transgenic Tabby mice about 2 mo old (Fig. 6B, upper parts). Histological analysis revealed hair follicle-like downgrowths in the lesional and adjacent skin (Fig. 6B, middle and lower right parts). Cells at the tips of the ingrowths had a basal cell-like columnar shape and tended to form branching cords (Fig. 6B, arrows in lower right part), suggesting that basal cell carcinoma-like tumors can be formed both in hair follicles and non-hairy epithelium, each of which had been proposed by different groups as the sole site of basal cell carcinoma formation.26 Thus, as reported in wild-type mice, an Shh transgene in Tabby mice had comparable effects on sebaceous glands and tumorigenesis.
Figure 6.
Basal cell carcinoma-like tumor formation in Shh transgenic Tabby mice. (A) BCC-like tumor formation occasionally seen in transgenic hair follicles. Arrowheads indicate BCC-like island formation in the transgenic skin. (B) Skin erosion was found in some transgenic Tabby tails, where no hair follicles were formed. Massive proliferation of keratinocytes was seen in the lesional and non-lesional skin. Keratinocytes at the tip of proliferation showed columnar shape and tend to form “islands” (arrows in lower right part).
Discussion
Skin appendages have characteristic structures and functions. Their developmental processes nevertheless share features, especially during early embryonic stages. For all skin appendages, formation starts from mesenchymal-epithelial interactions followed by massive proliferation of epithelial cells to form the major appendage masses—as is observed for hair follicles, feathers, teeth and sweat glands in a variety of species. However, although the various appendages only diverge prominently thereafter to form distinctive end products, recent findings have suggested that distinctive molecular mechanisms may be involved even at early developmental stages.1,19,27–29
A striking illustration was seen in Eda mutant Tabby mice, in which primary hair follicles were not induced but secondary hair follicles were formed on time. The molecular mechanism underlying this sharp difference is unclear, but Shh showed a unique expression pattern correlated with hair production (see Introduction). In the present study, with a more discriminating tissue-specific animal model, we could essentially confirm previous findings of Shh function in early stage embryos, transplanted skin or tissue cultures,11,16,17 and extended them. By varying the dosage of Shh in Tabby mice, we have further explicated its role in primary and secondary hair follicle development. Eda is required not only for initiation, but also for initial down growth of primary hair follicles.3 Because Shh is not expressed in Tabby skin before and during primary hair follicle induction, it was inferred to affect primary hair formation as a target of Eda.5,9 However, the ensemble of our data and previous reports reveal that primary hair follicle germs were not restored by supplementation of Shh in Tabby, and were normally formed in Shh knockout mice. Thus, the animal model confirmed that Shh is dispensable at the initial germ formation stage. Sharp downregulation of Shh in Tabby from an early stage rather suggests its involvement in down-growth of primary hair follicles, under Eda regulation. Consistent with this notion, primary hair germ progression is arrested at about E15.5 in Eda+ Shh knockout mice, so that downgrowth of primary hair follicle germs in Eda-A1 transgenic Tabby mice when the transgene is shutdown is attributable at least in part to downregulation of Shh.3
Secondary hair follicles start to form when primary hair follicles have already begun to form the critical dermal papillae. Therefore, in wild-type animals, it is awkward to try to discriminate Shh function in secondary hair follicle development separately from its action in primary hair follicles. However, Tabby mice provided a model to focus on Shh function in secondary hair follicle development. The Shh transgene did not affect secondary hair follicle induction. Notably, secondary hair follicles were still induced on time in an Shh knockout in Tabby mice (double-mutant), further demonstrating that both Shh and Eda are dispensable for secondary hair follicle induction. The Shh transgene did partially restore hair length in Tabby, but when Shh was ablated in either an Eda+ or a Tabby background, secondary hair germs were unable to grow down to form hair follicles. Shh is thus required for progression of secondary hair follicles, comparable to its role in primary hair follicle development (Table 1).
Table 1.
Summary of hair phenotypes in mutant mouse strains
| Phenotypes | WT | Ta | Ta/ShhTG | ShhKO | Ta/ShhKO |
| PH germ | + | − | − | + | − |
| SH germ | + | + | + | + | + |
| PHF elongation | + | n.a. | n.a. | − | n.a. |
| SHF elongation | + | + | + | − | − |
| SH length | L | S | S + L | n.a. | n.a. |
| Air cells in straight SH | 3 or 4 | 1–2 | 1–2 | n.a. | n.a. |
PH, Primary hair; SH, secondary hair; L, long; S, short; n.a., not applicable.
Previous studies have suggested that the Wnt pathway initiates formation of primary hair follicles,30 and Eda is required for subsequent induction stages, with Shh responsible for hair germ elongation downstream of Eda. Thus, early stage primary hair follicle development is most likely governed by a Wnt-Eda-(Wnt)-Shh cascade.31 For secondary hair follicle development, a subset of Wnt signaling that can be blocked by Dkk4 in vivo likely plays an inductive role, and Eda plays only a minor role for example, partially involved in formation of secondary hair structures, as shown in our previous studies.1,3 Our animal models revealed that Shh indeed does not directly mediate induction of secondary or primary hair follicles. As shown in Figure 3B, transgenic Shh in a Tabby background was activated to levels comparable to wild-type, but spatiotemporal action of endogenous Shh cannot be simulated easily with the transgene. This could explain why completely normal secondary hair structures were not generated in Tabby. More likely, a critical mediator is still unidentified that operates in concert with Eda-Shh or Wnt/Dkk4-Shh to induce primary and secondary hair follicles. Among possible candidates for such a missing participant are Sox18, Sox2 and BMP's. Notably, ragged mice bearing a point mutation in the Sox18 gene selectively lack zigzag hair, the major subtype of secondary hair, and Troy mutant mice in a Tabby background lack awl hair follicles.28,29 More recently, it was shown that Sox2 expression was selectively absent in zigzag hair, thus further distinguishing zigzag from other hair types.27 How these genes might interact with the Wnt/Dkk4-Shh cascade or the BMP/Noggin axis to specify secondary hair follicle fate remains to be seen.
Materials and Methods
Generation of skin specific Shh knockout mice.
Homozygous 129-Shhtm2Amc/J mice, containing two loxP sites that flank Exon 2 of the Shh gene, and homozygous Tg(KRT14-cre)1Amc/J mice, expressing Cre recombinase in skin epidermis and hair follicles, were purchased from Jackson Laboratory (Bar Harbor, MA). Mice heterozygous for both genes were generated by crossing the two homozygous strains, and Cre positive Shh homozygous mice were obtained by crossing heterozygous mice. All animal study protocols were approved by the Animal Care and Use Committee of National Institute on Aging.
Generation of Shh knockout mice in Tabby background.
Mice heterozygous for Shh KO and K14-Cre were crossed with Tabby mice (C57BL/6J-Aw-j-Ta6j strain, Jackson Laboratory) to obtain heterozygous Shh knockout Tabby mice carrying a K14-Cre transgene. Homozygous male and female Shh knockout Tabby mice were obtained by further crosses between them.
Generation of conditional Shh transgenic mice in a Tabby background.
The full-length open reading frame (1,314 bp) of mouse Shh cDNA (NM_009170.3) was amplified from pCMV-SPORT6-Shh plasmids (Invitrogen) by PCR with a primer set containing a forward primer with a Not I site followed by Kozak sequence GCC ACC ATG and a reverse primer with a SalI site and a TGA stop codon. Forward: AAA AGC GGC CGC GCC ACC ATG CTG CTG CTG CTG GCC AG. Reverse: AAA AGT CGA CTC AGC TGG ACT TGA CCG CC. The transgene was subcloned into a pTRE-Tight vector (Clontech) using the Not I and SalI sites (Fig. 3A). A linear 1,903 bp fraction of the TRE-mShh-SV40 polyA was excised with Xho I and Pvu I, purified and microinjected into pronuclei of one-cell C57BL/6J mouse embryos. Microinjected embryos were implanted into pseudo-pregnant female mice. Genotyping was done by PCR with primers spanning Intron 2. Forward primer: CTG GGT CTA CTA TGA ATC CAA AGC. Reverse primer: CAG GAA GGT GAG GAA GTC GCT GTA. Potential founders were mated to C57BL/6J mice to identify those passing the transgene.
The Tabby genotype was scored by PCR amplification and enzyme digestion as described previously in reference 9. Tabby mice were mated with “tet-off” positive C57BL/6J-TgN (MMTV-tTA) mice (Jackson Laboratory),32 to generate tet-off positive Tabby females, which were then crossed with Shh transgenic male mice to activate Shh transgene transcription in Tabby male progeny. Because the Eda gene is X-linked, male mice showed unequivocal Eda+ or Tabby genotypes, and we selectively analyzed male mice that carried two transgenes, Shh and tet-off, in a Tabby background.
Timed-mating, quantitative PCR, histology and immunohistochemistry.
Timed matings were set up with Shh knockout, Shh knockout Tabby and Shh transgenic strains as indicated in the text to obtain embryos at E14.5, E15, E17.5, and newborn mice at P0, P1, P5 and P8. The morning after mating was designated as E0.5. Back skin samples were fixed in 10% formaldehyde (Ricca Chemical) or embedded in OCT and frozen on dry ice, and stored at −80°C until use. Livers were taken for genotyping, and sex and Ta mutation were determined by PCR-based genotyping.9
One-step real-time PCR (Q-PCR) with Taqman probe/primer sets was performed to analyze expression levels of Shh pathway and related genes (Applied Biosystems). Genes analyzed by Q-PCR included Eda, Ltb, Shh, Ptch1, Gli and Lef1. Total RNA from the back skin of E16.5 WT embryos was used to generate a standard curve. RNAs from two mice for each genotype were analyzed in triplicate by Q-PCR. Reactions were normalized to GAPDH.
Histology of hair follicles at various developmental stages was analyzed by hematoxylin/eosin staining on paraffin sections. Hair subtypes were scored for more than 500 hairs for each mouse, and their morphology was scored under a dissection microscope.
For immunofluorescent staining, paraffin sections (5 µm) were deparaffinized with xylene, hydrated and unmasked with Antigen Unmasking Solution (Vector Laboratories) for 2 min at 121°C. Unmasked sections were incubated with primary antibodies at 4°C overnight, followed by AlexaFluor secondary antibodies (Invitrogen), and were analyzed using a DeltaVision microscope. Anti-Pcadherin (Invitrogen, 1:100) and anti-Lef1 (Cell Signaling, 1:150) were used as primary antibodies.
Acknowledgments
The authors thank David Richardson and Anna Butler for helping with animal management. This work was entirely supported by the Intramural Research Program of National Institute on Aging, National Institutes of Health.
Abbreviations
- Eda
anhidrotic ectodermal dysplasia
- Shh
sonic hedgehog
- Q-PCR
quantitative real time PCR
- E15
embryonic day 15
Conflict of Interest
The authors state that they have no conflict of interest.
References
- 1.Cui CY, Kunisada M, Piao Y, Childress V, Ko MS, Schlessinger D. Dkk4 and Eda regulate distinctive developmental mechanisms for subtypes of mouse hair. PLoS ONE. 2010;5:10009. doi: 10.1371/journal.pone.0010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui CY, Durmowicz M, Ottolenghi C, Hashimoto T, Griggs B, Srivastava AK, et al. Inducible mEDA-A1 transgene mediates sebaceous gland hyperplasia and differential formation of two types of mouse hair follicles. Hum Mol Genet. 2003;12:2931–2940. doi: 10.1093/hmg/ddg325. [DOI] [PubMed] [Google Scholar]
- 3.Cui CY, Kunisada M, Esibizione D, Douglass EG, Schlessinger D. Analysis of the temporal requirement for eda in hair and sweat gland development. J Invest Dermatol. 2009;129:984–993. doi: 10.1038/jid.2008.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava AK, Pispa J, Hartung AJ, Du Y, Ezer S, Jenks T, et al. The Tabby phenotype is caused by mutation in a mouse homologue of the EDA gene that reveals novel mouse and human exons and encodes a protein (ectodysplasin-A) with collagenous domains. Proc Natl Acad Sci USA. 1997;94:13069–13074. doi: 10.1073/pnas.94.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat Genet. 1999;22:370–374. doi: 10.1038/11943. [DOI] [PubMed] [Google Scholar]
- 6.Headon DJ, Emmal SA, Ferguson BM, Tucker AS, Justice MJ, Sharpe PT, et al. Gene defect in ectodermal dysplasia implicates a death domain adapter in development. Nature. 2001;414:913–916. doi: 10.1038/414913a. [DOI] [PubMed] [Google Scholar]
- 7.Kere J, Srivastava AK, Montonen O, Zonana J, Thomas N, Ferguson B, et al. X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat Genet. 1996;13:409–416. doi: 10.1038/ng0895-409. [DOI] [PubMed] [Google Scholar]
- 8.Cui CY, Schlessinger D. EDA signaling and skin appendage development. Cell Cycle. 2006;5:2477–2483. doi: 10.4161/cc.5.21.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui CY, Hashimoto T, Grivennikov SI, Piao Y, Nedospasov SA, Schlessinger D. Ectodysplasin regulates the lymphotoxin-beta pathway for hair differentiation. Proc Natl Acad Sci USA. 2006;103:9142–9147. doi: 10.1073/pnas.0509678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mou C, Jackson B, Schneider P, Overbeek PA, Headon DJ. Generation of the primary hair follicle pattern. Proc Natl Acad Sci USA. 2006;103:9075–9080. doi: 10.1073/pnas.0600825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pummila M, Fliniaux I, Jaatinen R, James MJ, Laurikkala J, Schneider P, et al. Ectodysplasin has a dual role in ectodermal organogenesis: inhibition of Bmp activity and induction of Shh expression. Development. 2007;134:117–125. doi: 10.1242/dev.02708. [DOI] [PubMed] [Google Scholar]
- 12.Kunisada M, Cui CY, Piao Y, Ko MS, Schlessinger D. Requirement for Shh and Fox family genes at different stages in sweat gland development. Hum Mol Genet. 2009;18:1769–1778. doi: 10.1093/hmg/ddp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamago G, Takata Y, Furuta I, Urase K, Momoi T, Huh N. Suppression of hair follicle development inhibits induction of sonic hedgehog, patched and patched-2 in hair germs in mice. Arch Dermatol Res. 2001;293:435–441. doi: 10.1007/s004030100252. [DOI] [PubMed] [Google Scholar]
- 14.Laurikkala J, Pispa J, Jung HS, Nieminen P, Mikkola M, Wang X, et al. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development. 2002;129:2541–2553. doi: 10.1242/dev.129.10.2541. [DOI] [PubMed] [Google Scholar]
- 15.Chuong CM, Patel N, Lin J, Jung HS, Widelitz RB. Sonic hedgehog signaling pathway in vertebrate epithelial appendage morphogenesis: perspectives in development and evolution. Cell Mol Life Sci. 2000;57:1672–1681. doi: 10.1007/PL00000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, Robertson EK, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205:1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- 17.St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/S0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Yamaguchi Y, Villacorte M, Mihara K, Akiyama M, Shimizu H, et al. Embryonic hair follicle fate change by augmented beta-catenin through Shh and Bmp signaling. Development. 2009;136:367–372. doi: 10.1242/dev.021295. [DOI] [PubMed] [Google Scholar]
- 19.Botchkarev VA, Botchkareva NV, Sharov AA, Funa K, Huber O, Gilchrest BA. Modulation of BMP signaling by noggin is required for induction of the secondary (nontylotrich) hair follicles. J Invest Dermatol. 2002;118:3–10. doi: 10.1046/j.1523-747.2002.01645.x. [DOI] [PubMed] [Google Scholar]
- 20.Wells KL, Mou C, Headon DJ, Tucker AS. Recombinant EDA or Sonic Hedgehog rescue the branching defect in Ectodysplasin A pathway mutant salivary glands in vitro. Dev Dyn. 2010;239:2674–2684. doi: 10.1002/dvdy.22406. [DOI] [PubMed] [Google Scholar]
- 21.Wells KL, Mou C, Headon DJ, Tucker AS. Defects and rescue of the minor salivary glands in Eda pathway mutants. Dev Biol. 2011;349:137–146. doi: 10.1016/j.ydbio.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Paladini RD, Saleh J, Qian C, Xu GX, Rubin LL. Modulation of hair growth with small molecule agonists of the hedgehog signaling pathway. J Invest Dermatol. 2005;125:638–646. doi: 10.1111/j.0022-202X.2005.23867.x. [DOI] [PubMed] [Google Scholar]
- 23.Sato N, Leopold PL, Crystal RG. Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog. J Clin Invest. 1999;104:855–864. doi: 10.1172/JCI7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen M, Grachtchouk M, Sheng H, Grachtchouk V, Wang A, Wei L, et al. Hedgehog signaling regulates sebaceous gland development. Am J Pathol. 2003;163:2173–2178. doi: 10.1016/S0002-9440(10)63574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH Jr, Scott MP. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 26.Ridky TW, Khavari PA. The hair follicle bulge stem cell niche resists transformation by the hedgehog pathway. Cell Stem Cell. 2010;6:292–294. doi: 10.1016/j.stem.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pennisi D, Gardner J, Chambers D, Hosking B, Peters J, Muscat G, et al. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat Genet. 2000;24:434–437. doi: 10.1038/74301. [DOI] [PubMed] [Google Scholar]
- 29.Pispa J, Pummila M, Barker PA, Thesleff I, Mikkola ML. Edar and Troy signalling pathways act redundantly to regulate initiation of hair follicle development. Hum Mol Genet. 2008;17:3380–3391. doi: 10.1093/hmg/ddn232. [DOI] [PubMed] [Google Scholar]
- 30.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Tomann P, Andl T, Gallant NM, Huelsken J, Jerchow B, et al. Reciprocal requirements for EDA/EDAR/NFkappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev Cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hennighausen L, Wall RJ, Tillmann U, Li M, Furth PA. Conditional gene expression in secretory tissues and skin of transgenic mice using the MMTV-LTR and the tetracycline responsive system. J Cell Biochem. 1995;59:463–472. doi: 10.1002/jcb.240590407. [DOI] [PubMed] [Google Scholar]