Abstract
Background
Surgical-treatment outcomes for melanoma in African Americans are poorly characterized due to low incidence of melanoma among African Americans. We examined differences by race in overall and melanoma-specific survival, stratified by receipt of surgical treatment and by specific types of surgical treatment.
Methods
Data from the 1973–2004 public-use Surveillance, Epidemiology and End Results (SEER) Program were analyzed using Cox proportional hazard models to compare the effects of surgical treatments on overall and melanoma-specific survival in blacks, whites, and other race, controlling for confounding demographic and tumor-related variables.
Results
Of 151,154 patients with first primary melanoma (148,883 whites, 789 blacks and 1,532 other race), 142,653 (94.4%) received surgical treatment. Among patients who received surgical treatment, 10-year melanoma-specific survival was lower in blacks (73%) than in whites (88%) and other race (85%); black patients were at significantly higher risk of overall and melanoma-specific mortality when compared with white (hazard ratio [HR] = 1.64, 95% confidence interval [CI] 1.44–1.86, P < 0.0001 and HR = 1.50, 95% CI 1.25–1.79, P < 0.0001, respectively) and with other race (HR = 1.55, 95% CI 1.31–1.85, P < 0.0001 and HR = 1.49, 95% CI 1.16–1.91, P = 0.0017, respectively). Blacks who underwent biopsy, wide excision and surgery not otherwise specified were at higher risk of overall mortality compared with whites with the same treatment.
Conclusion
Overall and melanoma-specific survival was lower in blacks undergoing surgical treatment for melanoma compared to both whites and other race. Reasons for these disparities remain poorly understood.
Melanoma is the most lethal form of cutaneous malignancy, accounting for approximately 75% of all skin cancer deaths. Over the past three decades, the age-adjusted incidence of cutaneous melanoma increased from 7.89 per 100,000 in 1975 to 20.81 per 100,000 in 2007.[1] The average annual age-adjusted melanoma incidence (per 100,000 persons) is 1.0 for African Americans, 1.4 for Asians, 3.8 for Native Americans, and 4.5 for Hispanics, compared with 23.5 for whites.[1] Although, the incidence of melanoma is lower among racial/ethnic minorities compared with whites, a few studies have shown significant increases in invasive melanoma incidence in Hispanic populations since 1988.[2–4] Despite the lower incidence of cutaneous melanoma in these racial/ethnic minorities, it is the third most common skin cancer in minorities, with basal and squamous cell carcinoma the most common in Hispanics and African Americans, respectively.[5]
Several studies noted differences in the diagnosis and treatment of melanoma in racial/ethnic minorities compared with whites.[4, 6–8] In racial/ethnic minorities, melanoma is more frequently found on sun-protected skin and more likely to include acral, subungual, and mucosal histological subtypes [5] and to be diagnosed at advanced stages, portending a poor prognosis.[8] The primary treatment for melanoma is surgical excision. Systemic adjuvant therapies and radiotherapy may be considered for patients with high risk melanomas, including those with lymph node involvement and distant metastases.[9–13] However most systemic treatments have not been shown to improve survival, and survival is poor for regionally advanced (stage III) and metastatic (stage IV) melanoma.[10]
To the authors’ knowledge, this is the first large, population-based study to examine differences by race in overall and melanoma-specific survival, stratifying the analyses by receipt of surgical treatment and by specific types of surgical treatment, while controlling for demographic and tumor-related factors. Given the increases in racial/ethnic minority populations in the United States, [14] a better understanding of the impact of melanoma surgical treatment on survival in these patient populations is needed.
METHODS
Public-use Surveillance, Epidemiology, and End Results (SEER) Program data were used to conduct a retrospective, population-based cohort study of patients with a first primary diagnosis of cutaneous melanoma between January 1, 1973 and December 31, 2004.. To maximize the number of racial/ethnic minority patients with melanoma, data from all 17 SEER tumor registries [15] were included in the analysis. This study was approved by the Institutional Review Board at Washington University.
Patients with a first primary cutaneous melanoma were identified by the SEER code 25010 and a sequence number 00, indicating only one primary malignancy in the patient’s record. Patients were excluded if they had more than one record (indicating more than one cancer) or were diagnosed with melanoma after another cancer diagnosis or by death certificate or autopsy only.
Demographic variables included age at diagnosis, sex, race, and year of diagnosis. Using the SEER codes for race, three groups were created for analysis: white, black, and other race (including American Indian, Alaska Native, Asian, Pacific Islander and “other unspecified”); 9,582 patients whose race was coded as “unknown” were excluded. Since Hispanic ethnicity crosses all race codes in SEER, this variable was not included in the analysis.
Tumor-related variables included histologic subtype, SEER historic stage A, primary anatomic site, satellite nodules/tumors, skin ulceration and lymph node extension. Histologic subtypes for cutaneous melanoma were recorded by SEER according to the International
Classification of Diseases for Oncology (ICD-O) codes.[16] The SEER histology codes were grouped for analysis as follows: in situ, nodular melanoma, lentigo maligna melanoma, superficial spreading melanoma, acral lentiginous melanoma (ALM), and malignant melanoma not otherwise specified (NOS). The latter category included a heterogeneous group of histological subtypes that did not fit in one of the first five categories and had too few cases to be analyzed separately (Appendix A). SEER historic stage A was used to describe patients’ stage at diagnosis: in situ, local, regional, distant and unstaged. Use of SEER historic stage A allowed inclusion of all cutaneous melanoma patients as far back as 1973. Coding for extent of disease was inconsistent across the study period, and American Joint Committee for Cancer (AJCC) [17] staging for melanoma was not available in SEER until 2004 (SEER Web Technical Support, personal communication).
From 1973 to 1976, anatomic site was coded using the Manual of Tumor Nomenclature and Coding, [18] whereas cases from 1977–1991 were coded using the ICD-O-1976. From 1992–2004, primary anatomic site was derived from the ICD-O topography codes.[16] Primary anatomic site groupings included trunk, upper extremity, lower extremity, skin NOS, and head and neck.
Four SEER variables, “satellite tumors” (1973–1982), “Extension” (1983–2003), and “Clinical Stage (CS) Lymph Nodes” (2004) were recoded to form a 3-category satellite-nodule variable (having satellite nodules, not having satellite nodules, or unknown). Three SEER variables, “Type of melanoma” (1973–1982), “Extension” (1988–2003 r) and “CS Site Specific Factor 2 Ulceration” (2004) were used to form a 3-category skin-ulceration variable (having skin ulceration, not having skin ulceration, or unknown). Skin-ulceration status was not coded by SEER from 1983–1987; therefore all 1983–1987 cases were coded as ‘unknown’ for skin ulceration).[19] Five SEER variables, “Regional Lymph node involvement” (1973–1982), “Distant Lymph Nodes” (1973–1982), “Lymph Nodes” (1983–2003), and “CS Lymph Nodes” (2004) were recoded to categorize extent of lymph-node involvement (none, regional, distant, or unknown).
Two surgical treatment variables were analyzed. Two SEER variables, “site-specific surgery” (1973–1997) and “surgery of the primary site” (1998–2004), were recoded to form a dichotomous variable categorizing patients as receiving or not receiving any surgery on the primary site. Prior to 1983, type of surgery for melanoma was not recorded by SEER; therefore, patients diagnosed before 1983 were categorized as having “no surgery” (if the record indicated they did not have any surgery) or as “surgery NOS,” if the record indicated they received surgery). After 1982, type of surgery for melanoma was categorized as no surgery, biopsy, wide excision, amputation, and surgery NOS as recorded by the SEER registry (Appendix B).
Statistical Analysis
Survival (in months) was calculated using date of diagnosis and one of the following: date of death, date last known to be alive, or cut-off date for this file (December 31, 2004). The distributions of patient demographic, tumor- and treatment-related characteristics by race were compared using chi-square tests for categorical variables and analysis of variance for continuous variables. Two survival endpoints were examined: overall survival, defined as the time from diagnosis to death from any cause, and melanoma-specific survival, defined as the time from diagnosis to death from melanoma. Patients were stratified by receipt of surgery and by type of surgery to evaluate racial differences in survival after melanoma diagnosis. The Kaplan-Meier method was used to develop the survival curves, and the log-rank test was used to test for equality of survival curves. The Bonferroni correction for multiple comparisons was used in pairwise contrasts of the racial groups on the overall and melanoma-specific survival associated with receipt of surgery and with type of surgery. Cox proportional hazard regression models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of survival endpoints by race, for overall and melanoma-specific mortality risk, stratified by receipt of surgery and by surgery type; to assess differences in survival between each pair of racial groups, one set of models used “whites” as the reference group and another set of models used “other race” as the reference group. Each model was adjusted for age, sex, stage, anatomic site, histological subtype, tumor ulceration, presence of satellite nodules, lymph node involvement, and year of diagnosis. All analyses were conducted using SAS version 9.12 statistical software (SAS Institute, Cary, NC). Two-sided P values <.05 were considered significant.
RESULTS
Demographic and clinical data for the study cohort are presented in Table 1. Black patients were older at time of diagnosis. Tumor characteristics differed significantly by race. Although 55.6% of all cases were diagnosed with local cutaneous melanoma and 29.6% with in situ melanoma, a larger proportion of black patients were diagnosed with regional (17.7%) and distant (10.4%) disease than patients who were white or other race. Melanomas were largely found on the trunk (31.1%), head and neck (23.1%), and upper limbs/shoulders (23.0%); but 51.7% of all melanomas in black patients were found on the lower extremities. Black patients were more likely to have been diagnosed with ALM, tumor ulcerations, satellite nodules, and positive lymph nodes in regional and distant areas.
Table 1.
Characteristics of First Primary Melanoma Cases in the 1973–2004 SEER Registries, by Race
| White | Black | Other | Total | |
|---|---|---|---|---|
| n=148,833 No. (%) |
n=789 No. (%) |
n=1,532 No. (%) |
n=151,154 No. (%) |
|
| Mean age (SD)at diagnosisa | 55.6 (27.8) | 57.8 (18.9) | 55.0 (30.6) | 55.6 (27.8) |
| Sexb | ||||
| Male | 78,757 (52.9) | 330 (41.8) | 748 (48.8) | 79,835 (52.8) |
| Female | 70,076 (47.1) | 459 (58.2) | 784 (51.2) | 71,319 (47.2) |
| Stageb | ||||
| In situ | 44,149 (29.7) | 174 (22.1) | 463 (30.2) | 44,786 (29.6) |
| Local | 83,080 (55.8) | 339 (43.0) | 683 (44.6) | 84,102 (55.6) |
| Regional | 11,662 (7.8) | 140 (17.7) | 203 (13.3) | 12,005(7.9) |
| Distant | 4,203 (2.8) | 82 (10.4) | 106 (6.9) | 4,391 (2.9) |
| Unknown | 5,739 (3.9) | 54 (6.8) | 77 (5.0) | 5,870 (3.9) |
| Anatomic siteb | ||||
| Trunk | 46,503 (31.3) | 113 (14.3) | 360 (23.5) | 46,976 (31.1) |
| Upper extremity | 34,345 (23.1) | 116 (14.7) | 286 (18.7) | 34,747 (23.0) |
| Lower extremity | 27,835 (18.7) | 408 (51.7) | 493 (32.2) | 28,736 (19.0) |
| Skin, NOS | 5,662 (3.8) | 74 (9.4) | 120 (7.8) | 5,856 (3.9) |
| Head and Neck | 34–488 (23.2) | 78 (9.9) | 273 (17.8) | 34,839 (23.1) |
| Histologic Subtypeb | ||||
| In situ | 29,238 (19.6) | 128 (16.2) | 326 (21.3) | 29,692 (19.6) |
| MM | 51,903 (34.9) | 353 (44.7) | 641 (41.8) | 52,897 (35.0) |
| NM | 8,454 (5.7) | 42 (5.3) | 92 (6.0) | 8,588 (5.7) |
| LMM | 20,465 (13.8) | 70 (8.9) | 172 (11.2) | 20,707 (13.7) |
| SSM | 37,928 (25.5) | 106 (13.4) | 233 (15.2) | 38,267 (25.3) |
| ALM | 845 (0.6) | 90 (11.4) | 68 (4.4) | 1,003 (0.7) |
| Surgeryb | ||||
| No | 6,088 (4.1) | 70 (8.9) | 107 (7.0) | 6,265 (4.1) |
| Yes | 140,575 (94.5) | 683 (86.6) | 1,395 (91.1) | 142,653 (94.4) |
| Unknown | 2,170 (1.5) | 36 (4.6) | 30 (2.0) | 2,236 (1.5) |
| Type of surgeryb | ||||
| Biopsy | 46,436 (31.2) | 190 (24.1) | 461 (30.1) | 47,087 (31.1) |
| Wide excision | 77,419 (52.0) | 349 (44.2) | 752 (49.1) | 78,520 (51.9) |
| Amputation | 346 (0.2) | 27 (3.4) | 30 (2.0) | 403 (0.3) |
| NOS | 16,374 (11.0) | 117 (14.8) | 152 (9.9) | 16,643 (11.0) |
| Tumor ulcerationb | ||||
| No | 118,129 (85.4) | 506 (69.0) | 1132 (78.3) | 119,767 (85.3) |
| Yes | 6,952 (5.0) | 76 (10.4) | 106 (7.3) | 7,134 (5.1) |
| Unknown | 13,209 (9.6) | 151 (20.6) | 208 (14.4) | 13,568 (9.7) |
| Satellite nodulesb | ||||
| No | 135,172 (90.8) | 620 (78.6) | 1,298 (84.7) | 13,7090 (90.7) |
| Yes | 1,126 (0.8) | 19 (2.4) | 24 (1.6) | 1,169 (0.8) |
| Unknown | 12,535 (8.4) | 150 (19.0) | 210 (13.7) | 12,895 (8.5) |
| Lymph node extensionb | ||||
| No | 100,716 (67.7) | 443 (56.2) | 1,031 (67.3) | 102,190 (67.6) |
| Regional | 4,411 (3.0) | 48 (6.1) | 77 (5.0) | 4,536 (3.0) |
| Distant | 449 (0.3) | 8 (1.0) | 12 (0.8) | 469 (0.3) |
| Unknown | 43,257 (29.1) | 290 (36.8) | 412 (26.9) | 43,959 (29.1) |
Abbreviations: SEER= Surveillance Epidemiology End Results, MM = malignant melanoma, NM = nodular melanoma, LMM = lentigo maligna melanoma, SSM = superficial spreading melanoma, ALM = acral lentiginous melanoma
P = 0.0513.
P < 0.0001
Receipt of surgical treatment for melanoma and type of surgery also differed significantly by race. Compared with patients who were white and other race, a larger percentage of black patients were not surgically treated for melanoma. Of those patients who received surgical treatment, blacks were more likely than the other two groups to undergo amputation. Of those 6,265 patients who did not receive surgical treatment, 28.6% had distant disease. Demographic and clinical data stratified by type of surgery is displayed in Table 2.
Table 2.
Characteristics of First Primary Melanoma Cases in the 1973–2004 SEER Registries, by Type of Surgery
| No surgery | Biopsy | Wide Excision | Amputation | Surgery NOS | Total | |
|---|---|---|---|---|---|---|
| n=6,265 | n=47,087 | n=78,520 | n=403 | n=16,643 | ||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | 148,918a | |
| Mean age (SD) at diagnosisb | 60.0 (36.1) | 57.7 (32.7) | 54.6 (22.2) | 62.4 (16.0) | 51.5 (31.7) | 57.2 |
| Sexb | ||||||
| Male | 3,665 (58.5) | 24,894 (52.9) | 41,135 (52.4) | 209 (51.8) | 8,701 (52.3) | 78,604 (52.7) |
| Female | 2,600 (41.5) | 22,193 (47.1) | 37,385 (47.6) | 194 (48.2) | 7,942 (47.7) | 70,314 (47.2) |
| Stageb | ||||||
| In situ | 1,602 (25.6) | 21,793 (46.3) | 20,151 (25.6) | 24 (5.9) | 941 (5.6) | 44,511 (29.8) |
| Local | 1,268 (20.2) | 22,051 (46.8) | 49,764 (63.4) | 155 (38.4) | 10,377 (62.3) | 83,615 (56.1) |
| Regional | 480 (7.6) | 1,625 (3.4) | 6,856 (8.73) | 161(39.9) | 2,784 (16.7) | 11,906 (8.0) |
| Distant | 1,793 (28.6) | 541 (1.1) | 533 (.68) | 48 (11.9) | 1,026 (6.1) | 3,941 (2.6) |
| Unknown | 1,122 (17.9) | 1,077 (2.2) | 1,216 (1.5) | 15 (3.7) | 1,515 (9.1) | 4,945 (3.3) |
| Anatomic siteb | ||||||
| Trunk | 1,109 (17.7) | 14,037 (29.8) | 26,321 (33.5) | 17 (4.2) | 5,104 (30.6) | 46,588 (31.3) |
| Upper extremity | 810 (12.9) | 10,805 (23.0) | 19,059 (24.2) | 112 (27.8) | 3,719 (22.3) | 34,505 (23.1) |
| Lower extremity | 708 (11.3) | 7,746 (16.4) | 16,341 (20.8) | 234–58.1) | 3,455 (20.8) | 28,484 (19.1) |
| Skin, NOS | 2,487 (39.7) | 794 (1.7) | 398 (.51) | 7 (1.74) | 1,303 (7.8) | 4,989 (3.3) |
| Head and Neck | 1,151 (18.4) | 13,705 (29.1) | 16,401 (20.9) | 33 (8.2) | 3,062 (18.4) | 34,352 (23.1) |
| Histologic Subtypeb | ||||||
| In situ | 1,025 (16.6) | 13,632 (28.9) | 14,491 (18.4) | 22 (5.4) | 422 (2.5) | 29,592 (19.9) |
| MM | 3,757 (60.0) | 11,763 (25.0) | 27,284 (34.7) | 210 (52.1) | 8,315 (50.0) | 51,329 (34.5) |
| NM | 100 (1.6) | 1,477 (3.1) | 4,796 (6.1) | 40 (10.0) | 2,105 (12.6) | 8,515 (5.7) |
| LMM | 711 (11.3) | 10,170 (21.6) | 8,492 (10.8) | 8 (2.0) | 1,090 (6.5) | 20,471 (13.8) |
| SSM | 651 (10.4) | 9,890 (21.0) | 22,74 (29.0) | 37 (9.2) | 4,656 (28.0) | 38,008 (25.5) |
| ALM | 21 (.34) | 155 (.33) | 683 (.87) | 86 (21.3) | 55 (.33) | 1,000 (.67) |
Abbreviations: SEER= Surveillance Epidemiology End Results, MM = malignant melanoma, NM = nodular melanoma, LMM = lentigo maligna melanoma, SSM = superficial spreading melanoma, ALM = acral lentiginous melanoma
We did not include 2236 patients since their type of surgery was “unknown”.
P < 0.0001
Median duration of follow-up for the entire cohort was 46 months (whites, 46; blacks, 40; and other race, 41). Of the 151,154 cutaneous melanoma cases, 30,339 (20%) died during follow-up. Of these deaths, 13,577 (44.8%) were due to melanoma. Cause of death was unknown in 1,399 (4.6%) cases (4.6% white, 3.3% black, 5.6% other race). For the entire cohort, the 5-year and 10-year overall survival rates were 82.3% and 72.6%, respectively; the 5-year and 10-year melanoma-specific survival rates were 90.1% and 86.5%, respectively.
Racial Differences in Survival by Receipt of Surgery
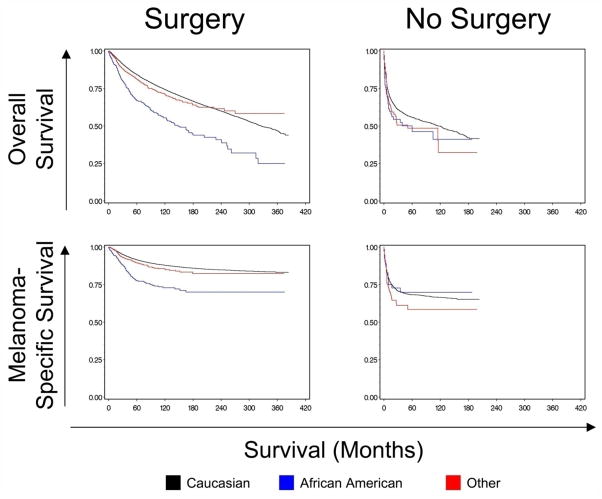
Table 3 shows the 5-year and 10-year overall and melanoma-specific survival estimates by race, stratified by receipt of surgical treatment and by surgery type. Among patients who underwent surgery, 10-year overall and melanoma-specific survival rates were poorer for blacks than for whites and other race. Figure 1 presents the Kaplan-Meier curves for overall and melanoma-specific survival by race for patients who did and did not receive surgical treatment. Among patients who underwent any surgery, whites and other race had higher overall and melanoma-specific survival rates than blacks (P < 0.0001).
Table 3.
Unadjusted 5- and 10-year Survival Rates of First Primary Melanoma Cases in the 1973–2004 SEER Registries, by Race and Stratified by Receipt of Surgical Treatment and by Surgery Type
| Overall survival
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whites | Blacks | Other race | Overall P valuea |
Whites v. Blacks P valuea |
Whites v. Other race P valuea |
Blacks v. Other race P valuea |
||||
| 5-year rate | 10-year rate | 5-year rate | 10-year rate | 5-year rate | 10-year rate | |||||
| Receipt of surgery | ||||||||||
| Any surgery | 84.2% | 74.3% | 66.8% | 55.4% | 81.1% | 71.0% | <0.0001 | <0.0001 | 0.0537 | <0.0001 |
| No surgery | 55.8% | 49.8% | 46.3% | 41.2% | 48.5% | 32.3% | 0.1405 | 0.1203 | 0.2100 | 0.7272 |
| b Log-rank test P value | <0.0001 | <0.0001 | <0.0001 | |||||||
| Type of surgery | ||||||||||
| Biopsy | 86.7% | 76.4% | 72.2% | 68.8% | 82.6% | 75.2% | 0.0014 | 0.0005 | 0.3018 | 0.0861 |
| Wide excision | 87.1% | 78.3% | 75.9% | 58.2% | 87.4% | 78.1% | 0.0001 | <0.0001 | 0.9453 | <0.0001 |
| Amputation | 48.4% | 34.4% | 36.9% | 24.6% | 36.9% | 19.7% | 0.0574 | 0.1518 | 0.1248 | 0.5434 |
| Surgery NOS | 69.7% | 59.1% | 45.1% | 36.6% | 60.0% | 47.2% | <0.0001 | <0.0001 | 0.1488 | 0.0022 |
| b Log-rank test P value | <0.0001 | <0.0001 | <.0001 | |||||||
| Melanoma-specific survival
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whites | Blacks | Other races | Overall P valuea | Whites v. Blacks P valuea | Whites v. Other race P valuea | Blacks v. Other race P valuea | ||||
| 5-year rate | 10-year rate | 5-year rate | 10-year rate | 5-year rate | 10-year rate | |||||
| Receipt of surgery | ||||||||||
| Any surgery | 91.5% | 87.9% | 77.6% | 73.0% | 89.5% | 85.3% | <0.0001 | <0.0001 | 0.0130 | <0.0001 |
| No surgery | 68.4% | 66.5% | 69.8% | 69.8% | 58.5% | 58.5% | 0.2595 | 0.9169 | 0.1013 | 0.2968 |
| b Log-rank test P value | <0.0001 | <0.0001 | <0.0001 | |||||||
| Type of surgery | ||||||||||
| Biopsy | 95.6% | 93.8% | 87.6% | 87.6% | 95.0% | 94.4% | 0.0007 | 0.0001 | 0.8641 | 0.0092 |
| Wide excision | 93.6% | 90.5% | 84.9% | 79.8% | 92.4% | 88.0% | <0.0001 | <0.0001 | 0.2814 | 0.0019 |
| Amputation | 60.8% | 53.0% | 43.4% | 36.2% | 54.6% | 36.4% | 0.0980 | 0.1203 | 0.1099 | 0.6762 |
| Surgery NOS | 76.7% | 70.7% | 53.8% | 47.5% | 71.1% | 63.0% | <0.0001 | <0.0001 | 0.0579 | 0.0111 |
| b Log-rank test P value | <0.0001 | <0.0001 | <0.0001 | |||||||
All P-values are corrected for multiple comparisons.
Log-rank test compares type of surgery within each race group, for both 5-year and 10-year rates
Figure 1.
Kaplan Meier analyses indicating differences in overall and melanoma-specific survival between patients who had surgery and patients who did not have surgery stratified by race. P values are log-rank tests. Overall survival: Surgery, P < 0.0001; No surgery, P = 0.1405. Melanoma-specific survival: Surgery, P < 0.0001; No surgery, P = 0.2595.
Multivariable Cox proportional hazard regression models were used to describe the association between race and risk of death during the study period while adjusting for demographic and tumor-related covariates. Table 4 shows the hazard ratio estimates of overall and melanoma-specific mortality by receipt of surgery. Among patients who did not receive surgical treatment, there were no significant differences by race in risk of overall or melanoma-specific mortality. However, among patients who received surgical treatment, black patients were at significantly higher risk of overall and melanoma-specific mortality (HR = 1.64 and 1.50, respectively) when compared with white patients. In addition, after surgical treatment, black patients were at higher risk of overall (HR = 1.55, 95% CI 1.31–1.85, P < 0.0001) and melanoma-specific mortality (HR = 1.49, 95% CI 1.16–1.91, P = 0.0017) compared with patients of other race (data not shown in Table 4).
Table 4.
Multivariable Cox Proportional Hazard Models Showing Risk of Overall and Melanoma-specific Mortality, by Receipt of Surgery
| Overall | Melanoma-specific | |||
|---|---|---|---|---|
| Characteristic | No Surgery | Surgery | No Surgery | Surgery |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Age | 1.007 (1.006–1.008)a | 1.005 (1.004–1.005)a | 1.00 (1.006–1.009)a | 1.00 (1.003–1.004)a |
| Sex | ||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 |
| Female | 0.90 (0.83–0.99)b | 0.71 (0.68–0.73)a | 0.87 (0.78–0.97)b | 0.65 (0.62–0.68)a |
| Race | ||||
| White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 1.16 (0.82–1.64) | 1.64 (1.44–1.86)a | 0.84 (0.51–1.38) | 1.50 (1.25–1.79)a |
| Others | 0.86 (0.64–1.15) | 1.05 (0.93–1.19) | 0.91 (0.64–1.29) | 1.01 (0.84–1.20) |
| Stage | ||||
| In situ | 1.00 | 1.00 | .26 (0.03–2.16) | 0.07 (0.05–0.10)a |
| Local | .87 (.59–1.30) | 1.31 (1.23–1.40)a | 1.00 | 1.00 |
| Regional | 3.03 (1.83–4.99)a | 3.99 (3.69–4.30)a | 7.03 (4.22–11.73)a | 4.51 (4.26–4.77)a |
| Distant | 12.59 (7.69–20.60)a | 11.00 (10.01–12.08)a | 30.49 (18.45–50.40)a | 13.51 (12.41–14.71)a |
| Unstaged | 2.57 (1.58–4.19)c | 2.34 (2.15–2.55)a | 5.67 (3.42–9.40)a | 2.56 (2.37–2.76)a |
| Anatomic site | ||||
| Trunk | 1.00 | 1.00 | 1.00 | 1.00 |
| Upper extremity | 1.03 (0.83–1.28) | 0.97 (0.94–1.01) | 0.84 (0.62–1.14) | 0.73 (0.69–0.77)a |
| Lower extremity | 0.99 (0.80–1.24) | 0.89 (0.86–0.93)a | 1.04 (0.79–1.38) | 0.84 (0.74–0.89)a |
| Overlap & NOS | 1.42 (1.21–1.68)a | 1.06 (0.99–1.14) | 1.20 (0.98–1.47) | 0.81 (0.74–0.88)a |
| Head and neck | 1.48 (1.22–1.77)a | 1.51 (1.46–1.56)a | 0.86 (0.65–1.13) | 1.03 (0.98–1.09) |
| Histologic subtype | ||||
| in situ | 1.00 | 1.00 | 0.25 (0.02–2.92) | 0.51 (0.32–0.82)c |
| MM | 2.52 (1.60–3.96)a | 1.75 (1.61–1.90)a | 1.00 | 1.00 |
| NM | 3.39 (1.98–5.78)a | 2.79 (2.55–3.05)a | 1.91 (1.33–2.75)c | 1.75 (1.66–1.85)a |
| LMM | 2.08(1.51–2.88)a | 2.11 (1.97–2.26)a | 0.21 (0.08–0.58)c | 0.44 (0.39–.51)a |
| SSM | 0.95 (0.58–1.58) | 1.20 (1.10–1.31)a | 0.28 (0.18–0.44)a | 0.63 (0.60–0.67)a |
| ALM | 3.44 (1.37–8.63)c | 2.75 (2.37–3.18)a | 1.35 (0.50–3.67) | 1.55 (1.32–1.82)a |
| Satellite Nodules | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 0.95 (0.68–1.33) | 1.43 (1.28–1.61)a | 1.00 (0.69–1.45) | 1.40 (1.23–1.58)a |
| Unknown | 1.00 (.75–1.33) | 0.85 (0.77–0.94)c | 1.10 (0.72–1.68) | 0.86 (0.76–0.98)b |
| LN Extension | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Regional | 1.07 (0.81–1.41) | 1.22 (1.14–1.30)a | 1.50 (0.99–2.26) | 1.48 (1.37–1.60)a |
| Distant | 1.21 (0.90–1.63) | 1.12 (0.97–1.29) | 1.82 (1.19–2.79)c | 1.21 (1.03–1.43)b |
| Unknown | 1.24 (0.98–1.57) | 1.09 (1.06–1.13)a | 1.63 (1.12–2.37)b | 1.10 (1.05–1.16)c |
| Ulceration Status | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.58 (1.05–2.38)b | 1.14 (1.08–1.19)a | 1.56 (0.91–2.68) | 1.12 (1.05–1.19)c |
| Unknown | 0.91 (0.70–1.19) | 1.30 (1.24–1.37)a | 1.06 (0.74–1.50) | 1.49 (1.39–1.60)a |
| Years of diagnosis | ||||
| 1973–1977 | 1.75 (1.59–1.93)a | 2.53 (2.20–2.91)a | ||
| 1978–1982 | 1.75 (1.60–1.92)a | 2.56 (2.24–2.92) a | ||
| 1983–1987 | 1.38 (1.25–1.52)a | 1.66 (1.44–1.91)a | ||
| 1988–1992 | 1.35 (1.16–1.57)a | 1.55 (1.42–1.69)a | 1.48 (1.22–1.78)a | 1.82 (1.59–2.07)a |
| 1993–1997 | 1.23 (1.07–1.42)c | 1.38 (1.27–1.51)a | 1.42 (1.20–1.69)a | 1.58 (1.39–1.80)a |
| 1998–2002 | 1.02 (0.91–1.15) | 1.10 (1.01–1.19)b | 1.04 (0.91–1.20) | 1.11 (0.98–1.26) |
| 2003–2004 | 1.00 | 1.00 | 1.00 | 1.00 |
P < 0.0001,
P < 0.05,
P < 0.01
Abbreviations: HR = Hazard Ratio, CI = confidence interval
MM = malignant melanoma, NM = nodular melanoma, LMM = lentigo maligna melanoma, SSM = superficial spreading melanoma, ALM = acral lentiginous melanoma, LN = Lymph Node
Differences in Survival by Type of Surgery
As shown in Table 3, among patients who underwent wide excision of their primary melanoma or surgery NOS, blacks had significantly worse overall and melanoma-specific survival compared to whites and other race (each P < 0.02); overall and melanoma-specific survival was similar for whites and other race. There were no significant differences by race in overall and melanoma-specific survival among patients who had undergone amputation. Table 5 shows the hazard ratio estimates of overall and melanoma-specific mortality by surgery type. Blacks who underwent biopsy, wide excision or surgery NOS were at significantly higher risk of overall mortality when compared to whites with these same treatments, whereas risk of overall mortality was similar between patients who were white and other race with these same treatments. Blacks who underwent surgery NOS also were at significantly higher risk of melanoma-specific mortality when compared to whites with the same treatment. In addition, blacks were at higher risk of overall mortality after wide excision (HR = 1.72, 95% CI 1.28–2.30, P = 0.0003) or surgery NOS (HR = 1.74, 95% CI, 1.29–2.33, P = 0.0003) and were at higher risk of melanoma-specific mortality after surgery NOS (HR = 1.70, 95% CI 1.16–2.50, P = 0.0065) when compared to other race with the same treatment (data not shown in Table 5).
Table 5.
Multivariable Cox Proportional Hazard Models Showing Risk of Overall and Melanoma-specific Mortality, by Surgery Type
| Overall | ||||
|---|---|---|---|---|
| Biopsy | Wide excision | Amputation | Surgery NOS | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Age | 1.004 (1.004–1.005)a | 1.005 (1.005–1.005)a | 1.02 (1.01–1.04)a | 1.004 (1.004–1.004)a |
| Sex | ||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 |
| Female | 0.72 (0.69–0.76)a | 0.69 (0.66–0.72)a | 0.86 (0.62–1.20) | 0.72 (0.68–0.75)a |
| Race | ||||
| White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 1.36 (1.01–1.83)b | 1.58 (1.27–1.95)a | 1.40 (0.81–2.43) | 1.76 (1.42–2.17)a |
| Other | 1.15 (.91–1.46) | 0.92 (0.75–1.13) | 1.28 (0.73–2.24) | 1.01 (.82–1.25) |
| Stage | ||||
| In situ | 1.00 | 1.00 | 1.00 | 1.00 |
| Local | 1.31 (1.18–1.47)a | 1.47 (1.32–1.64)a | 2.91 (0.13–67.96) | 1.22 (1.06–1.42)c |
| Regional | 4.69 (3.99–5.51)a | 5.01 (4.41–5.69)a | 7.10 (0.30–166.53) | 2.74 (2.34–3.20)a |
| Distant | 17.52 (14.35–21.39)a | 9.97 (8.23–12.07)a | 9.91 (0.39–247.72) | 6.81 (5.73–8.09)a |
| Unstaged | 1.84 (1.52–2.25)a | 1.47 (1.21–1.78)a | 4.12 (0.17–101.29) | 2.20 (1.88–2.58)a |
| Anatomic site | ||||
| Trunk | 1.00 | 0.99 (0.94–1.04) | 1.00 | 1.00 |
| Upper extremity | 1.04 (0.97–1.13) | 1.00 | 0.61 (0.26–1.46) | 0.90 (0.85–0.96)c |
| Lower extremity | 0.89 (0.81–0.97)b | 0.85 (0.79–0.90)a | 0.75 (0.31–1.79) | 0.92 (0.86–0.98)b |
| Overlap & NOS | 0.93 (0.80–1.07) | 1.07 (0.86–1.32) | 0.76 (0.17–3.25) | 1.20 (1.09–1.33)c |
| Head and neck | 1.66 (1.55–1.78)a | 1.55 (1.47–1.63)a | 1.26 (0.48–3.33) | 1.31 (1.23–1.39)a |
| Histologic subtype | ||||
| In situ | 1.00 | 1.00 | 1.00 | 1.00 |
| MM | 1.81 (1.58–2.08)a | 1.56 (1.36–1.78)a | 0.38 (.02–8.77) | 1.91 (1.49–2.46)a |
| NM | 3.19 (2.74–3.73)a | 2.73 (2.37–3.14)a | 0.82 (.03–19.42) | 2.48 (1.93–3.21)a |
| LMM | 2.07 (1.88–2.28)a | 1.87 (1.68–2.08)a | 0.70 (.03–16.21) | 2.38 (1.89–3.00)a |
| SSM | 1.13 (0.98–1.30) | 1.09 (0.96–1.25) | 0.46 (0.02–11.09) | 1.33 (1.04–1.72)b |
| ALM | 2.65 (1.85–3.81)a | 2.65 (2.17–3.24)a | 0.34 (0.01–7.94) | 2.78 (1.82–4.25)a |
| Satellite Nodules | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.01 (0.72–1.42) | 1.34 (1.07–1.68)b | 0.83(0.34–2.04) | 1.37 (1.17–1.59)a |
| Unknown | 0.67 (0.53–0.85)c | 0.71 (0.61–0.83)a | 0.69 (0.31–1.53) | 0.91 (0.75–1.10) |
| LN Extension | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Regional | 1.21 (0.98–1.50) | 1.35 (1.20–1.51)a | 1.41 (0.80–2.52) | 1.24 (1.12–1.37)a |
| Distant | 1.59 (1.20–2.12)c | 1.39 (1.03–1.89)b | 1.65 (0.51–5.36) | 0.97 (0.79–1.19) |
| Unknown | 1.03 (0.96–1.11) | 1.03 (0.99–1.08) | 1.42 (0.97–2.10) | 1.13 (1.06–1.22)c |
| Tumor ulceration | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.10 (0.95–1.29) | 1.05 (0.96–1.15) | 1.46 (0.90–2.38) | 1.08 (1.01–1.15)b |
| Unknown | 1.73 (1.49–2.01)a | 1.83 (1.61–2.09)a | 1.27 (0.65–2.50) | 1.01 (0.94–1.08) |
| Years of diagnosis | ||||
| 1973–1977 | 0.67 (0.40–1.14) | |||
| 1978–1982 | 0.59 (0.35–1.01) | |||
| 1983–1987 | 0.92 (0.76–1.11) | 0.93 (0.78–1.11) | 1.70 (0.62–4.67) | 0.65 (0.38–1.10) |
| 1988–1992 | 1.49 (1.30–1.70)a | 1.43 (1.26–1.62)a | 1.93 (0.84–4.41) | 0.64 (0.37–1.08) |
| 1993–1997 | 1.30 (1.14–1.48)c | 1.32 (1.16–1.49)a | 2.66 (1.21–5.89)b | 0.62 (0.36–1.06) |
| 1998–2002 | 1.14 (1.00–1.30)b | 1.08 (0.96–1.22) | 2.09 (0.96–4.58) | 0.59 (0.33–1.06) |
| 2003–2004 | 1.00 | 1.00 | 1.00 | 1.00 |
| Melanoma-specific | ||||
|---|---|---|---|---|
| Biopsy | Wide excision | Amputation | Surgery NOS | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Age | 1.003 (1.002–1.004)a | 1.004 (1.004–1.005)a | 1.002 (0.99–1.02) | 1.003 (1.003–1.004)a |
| Sex | ||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 |
| Female | 0.63 (0.57–0.70)a | .64 (0.60–0.68)a | 0.69 (0.45–1.06) | 0.67 (0.63–0.72)a |
| Race | ||||
| White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 1.09 (0.67–1.76) | 1.28 (0.94–1.76) | 1.16 (0.59–2.27) | 1.70 (1.30–2.23)c |
| Other | 0.85 (0.52–1.37) | 0.95 (0.71–1.27) | 1.23 (0.60–2.52) | 0.99 (0.76–1.32) |
| Stage | ||||
| In situ | 0.08 (0.04–0.18)a | 0.07 (0.04–0.14)a | 0.00 (0.00–0.00) | 0.12 (0.06–0.23)a |
| Local | 1.00 | 1.00 | 1.00 | 1.00 |
| Regional | 7.11 (5.98–8.46)a | 4.95 (4.49–5.45)a | 2.96 (1.65–5.28)c | 2.91 (2.67–3.17)a |
| Distant | 30.43 (24.38–37.99)a | 10.86 (8.98–13.13)a | 4.85 (1.94–12.14)c | 7.78 (6.92–8.76)a |
| Unstaged | 2.74 (2.19–3.44)a | 1.11 (0.90–1.39) | 1.75 (0.52–5.89) | 2.48 (2.25–2.73)a |
| Anatomic site | ||||
| Trunk | 1.00 | 1.00 | 1.00 | 1.00 |
| Upper extremity | 0.74 (0.64–0.85)a | 0.70 (0.65–0.77)a | 0.60 (0.21–1.73) | 0.72 (0.66–0.78)a |
| Lower extremity | 0.85 (0.73 –0.99)b | 0.82 (0.76–0.90)a | 0.88 (0.31–2.52) | 0.83 (0.76–0.90)a |
| Overlap & NOS | 0.67 (0.54–0.84)c | 0.74 (0.53–1.02) | 0.69(0.10–4.51) | 0.90 (0.80–1.01) |
| Head and neck | 0.96 (0.84–1.09) | 1.13 (1.04–1.22)c | 1.19 (0.36–3.93) | 0.91 (0.84–.99)b |
| Histologic subtype | ||||
| In situ | 0.42 (0.18–0.99)b | 0.51 (0.24–1.07) | 0.00 (0.00–0.00) | .68 (0.29–1.59) |
| MM | 1.00 | 1.00 | 1.00 | 1.00 |
| NM | 1.90 (1.67–2.17)a | 1.99 (1.85–2.16)a | 1.98 (1.10–3.55)b | 1.32 (1.21–1.43)a |
| LMM | 0.33 (0.24–0.45)a | 0.46 (0.38–0.56)a | 2.00 (0.37–10.91) | 0.58 (0.47–0.72)a |
| SSM | 0.53 (0.46–0.61)a | 0.69 (0.64–0.75)a | 1.16 (0.50–2.69) | .63 (0.58–0.68)a |
| ALM | 1.35 (0.80–2.28) | 1.63 (1.32–2.01)a | 0.96 (0.58–1.59) | 1.45 (0.98–2.15) |
| Satellite Nodules | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 0.94 (0.65–1.37) | 1.20 (0.94–1.54) | 0.83 (0.28–2.41) | 1.39 (1.18–1.65)c |
| Unknown | 0.81 (0.61–1.08) | 0.72 (0.59–0.87)c | 0.61 (0.23–1.61) | 0.76 (0.60–0.97)b |
| LN Extension | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Regional | 1.51 (1.17–1.96)c | 1.69 (1.48–1.93)a | 1.54 (0.75–3.13) | 1.30 (1.15–1.46)a |
| Distant | 1.85 (1.34–2.57)c | 1.56 (1.12–2.18)c | 1.10 (0.26–4.66) | 0.95 (0.75–1.20) |
| Unknown | 1.24 (1.09–1.41)c | 1.10 (1.02–1.17)b | 1.32 (0.79–2.19) | 1.02 (0.93–1.12) |
| Tumor ulceration | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.05 (0.84–1.29) | 1.06 (0.95–1.19) | 1.44 (0.79–2.60) | 1.02 (0.93–1.12) |
| Unknown | 1.82 (1.49–2.23)a | 2.25 (1.92–2.65)a | 1.43 (0.64–3.19) | 1.03 (0.94–1.14) |
| Years of diagnosis | ||||
| 1973–1977 | 1.01 (0.48–2.13) | |||
| 1978–1982 | 0.85 (0.40–1.80) | |||
| 1983–1987 | 1.23 (0.95–1.60) | 1.08 (0.86–1.37) | 1.39 (0.40–4.83) | 0.97 (.046–2.06) |
| 1988–1992 | 1.52 (1.21–1.91)c | 1.71 (1.41–2.06)a | 2.55 (0.92–7.03) | 0.96 (0.45–2.03) |
| 1993–1997 | 1.29 (1.04–1.61)b | 1.45 (1.21–1.75)a | 2.64 (0.98–7.15) | 0.90 (0.42–1.89) |
| 1998–2002 | 1.06 (0.86–1.32) | 1.08 (0.91–1.29) | 1.97 (0.74–5.27) | 0.59 (0.26–1.35) |
| 2003–2004 | 1.00 | 1.00 | 1.00 | 1.00 |
P < 0.0001.
P < 0.05.
P < 0.01.
Abbreviations: HR = Hazard Ratio, CI = confidence interval, MM = malignant melanoma, NM = nodular melanoma, LMM = lentigo maligna melanoma, SSM = superficial spreading melanoma, ALM = acral lentiginous melanoma, LN = Lymph Node.
Comparisons for each type of surgery were adjusted for all the variables shown in the table. Type of surgery was not coded in SEER prior to 1983; all surgeries were categorized as Surgery NOS for the 1973–1982 years of diagnosis.
DISCUSSION
Although cutaneous melanoma is rare among racial/ethnic minorities, [5, 6] this study and others [4, 8, 20, 21] indicate that blacks are at an increased risk of dying from melanoma compared with whites. Several factors have been studied in relation to higher cancer-related mortality rates among racial/ethnic minorities, including lower socioeconomic status, [8, 22–26] access to health care, [20, 26–28] more advanced stage at diagnosis, [1, 29–32] differences in tumor biology, [21, 33, 34] and in treatment.[35, 36] Such studies, however, have been limited by the small numbers of minority patients analyzed. The large number of patients captured in the SEER database allowed for sufficient statistical power for the analyses reported herein.
In this population-based study, racial/ethnic differences in overall and melanoma-specific survival were examined, comparing patients who did and did not receive surgical treatment and patients who received different types of surgical treatment. When blacks underwent surgical treatment for melanoma, they had shorter survival compared with whites and other race (Table 3) and were at greater risk of death after controlling for demographic and tumor-related characteristics (Table 4). Among patients who did not undergo surgery, however, neither risk of overall nor melanoma-specific mortality differed significantly by race. Black patients were less likely to have received surgery for melanoma. All patients who did not receive surgery were more likely to be diagnosed with distant disease.
Melanoma in black patients might follow a different, more aggressive course than in white patients. Blacks were more likely than whites to have ALM. ALM, unlike other melanoma subtypes, is found on non-sun-exposed palmoplantar surfaces, with a higher incidence in persons of color, especially black patients. This tumor subtype is associated with lower overall survival rates in minority populations and is associated with such factors as increased tumor thickness [37, 38] and advanced stage at presentation.[7, 37–39] Black patients were more likely to be diagnosed at more advanced stages of disease compared with whites, as reported elsewhere, [3, 4, 21, 38, 40] and were more likely to have tumor ulceration, satellite nodules and regional and distant metastases, [7, 38, 39, 41–44] all of which are associated with poor prognosis. These findings emphasize the importance of trying to detect a patient’s cutaneous melanoma earlier, when it is smaller and less invasive, leading to a greater chance of survival. Our study and others [4, 8] that included race, histological subtype, and stage in their multivariable models observed an elevated risk of death from melanoma for black compared to white patients.
Studies that reported on increasing melanoma incidence rates in white Hispanic populations [2, 3] and advanced stage at presentation in blacks and Hispanics [3, 40] suggest that unmeasured factors such as socioeconomic status, skin cancer awareness and cultural and social values affect melanoma stage at diagnosis. However, these studies did not examine mortality rates in these minority racial/ethnic groups. Poorer outcomes for black patients with melanoma, in particular, may have several explanations. First, melanoma tumors may be biologically different and/or more aggressive in blacks. The molecular or genetic mechanisms underlying potential differences in tumor biology among people of different races have not been determined. If such differences exist, they could have profound implications for the treatment of melanoma in different racial groups, such as a lower threshold for performing sentinel lymph node or complete lymph node dissection [45] or use of different systemic therapies. Alternatively, some non-white patients with melanoma might have lower access to care and experience disparities in receipt of other treatments not included in the SEER registry. However, a California Cancer Registry (CCR) study that included a small sample of 127 black patients and receipt of surgical treatment in their analysis as well as receipt of chemotherapy, radiation therapy, and immunotherapy observed poorer overall survival in black patients that was not explained by differences in treatment or socioeconomic status.[8] Also, much like the public-access SEER database, the CCR does not include information about comorbidity, insurance status or date of recurrence, all of which have prognostic significance.
The SEER data are further limited by lack of data regarding non-surgical treatment for melanoma, such as chemotherapy and biologic therapy, as well as data on psychosocial factors, margin status of biopsies, location of distant metastases, and recurrence, all of which might increase one’s mortality risk. Due to inconsistencies in the data recorded by SEER registries over the study period, we were not able to analyze the data by some known prognostic factors, such as tumor thickness and AJCC staging. Although findings from the present study and others [4, 7, 8, 20, 21, 38, 41–44, 46] indicate black patients, in particular, have lower overall and melanoma-specific survival, the potential impact of these unmeasured variables on survival could not be assessed. Population-based, retrospective cohort studies using these publicly available large databases are limited to some extent by lack of data about potential confounders. There also may be some limitations in generalizability of our findings due to selection bias, since SEER registries are more likely to sample from urban than from rural areas [47]. Finally, causal inferences from the associations we observed cannot be made. However, these findings can direct future research to determine the cause(s) of poorer outcomes in minority patients with melanoma.
Despite the limitations of this study, to the authors’ knowledge, this is the first study using the high quality, population-based SEER data [15] to demonstrate poorer survival outcomes for black patients with melanoma not only by receipt of surgical treatment but by type of surgical treatment. In conclusion, melanoma in black patients appears to have characteristics which require a different or more aggressive approach to treatment than in white patients. Certain tumor-biology or genetic characteristics may be implicit in melanoma in people of color, which is feasible given the higher concentrations of melanocytes and pigment in minority populations. Differences in tumor characteristics may lead to a different mechanism of tumor genesis, and in turn to different patterns of tumor growth and rates of metastases. Improving our understanding of these differences could have profound treatment and population-level public health implications.[45, 48] Given the low overall incidence of melanoma among African Americans, large population-based, observational studies such as this one can help us understand the relationships between various clinical and treatment factors and melanoma outcomes in black patients. However, future prospective studies aimed at discovering the biologic and/or genetic characteristics of melanoma tumors in this population are warranted to improve survival for black patients with melanoma.
Supplementary Material
Acknowledgments
Funding/Support: Funding for the study was provided by institutional/departmental funds and by the National Cancer Institute Cancer Center Support Grant to the Alvin J. Siteman Cancer Center (P30 CA91842).
The authors thank the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri, for the use of the Health Behavior, Communication and Outreach Core, especially James Struthers, BA, for data management services. The Core is supported in part by the National Cancer Institute Cancer Center Support Grant to the Alvin J. Siteman Cancer Center (P30 CA91842).
Footnotes
Financial Disclosures: None reported.
References
- 1.National Institutes of Health. SEER Cancer Statistics Review, 1975–2007. 2010 Available: http://seer.cancer.gov/csr/1975_2007/[July 15, 2010]
- 2.Cockburn M, Zadnick J, Deapen D. Developing epidemic of melanoma in the Hispanic population of California. Cancer. 2006;106:1162–1168. doi: 10.1002/cncr.21654. [DOI] [PubMed] [Google Scholar]
- 3.Hu S, Parmet Y, Allen G, Parker D, Ma F, Rouhani P, Kirsner R. Disparity in melanoma: A Trend analysis of melanoma incidence and stage at diagnosis among Whites, Hispanics and Blacks in Florida. Arch Dermatol. 2009;145:1369–1374. doi: 10.1001/archdermatol.2009.302. [DOI] [PubMed] [Google Scholar]
- 4.Cormier JN, Xing Y, Ding M, Lee JE, Mansfield PF, Gershenwald JE, Ross MI, Du XL. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907–1914. doi: 10.1001/archinte.166.17.1907. [DOI] [PubMed] [Google Scholar]
- 5.Shoo BA, Kashani-Sabet M. Melanoma Arising in African-, Asian-, Latino- and Native-American Populations. Semin Cutan Med Surg. 2009;28:96–102. doi: 10.1016/j.sder.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Hemmings DE, Johnson DS, Tominaga GT, Wong JH. Cutaneous melanoma in a multiethnic population: Is this a different disease? Arch Surg. 2004;139:968–973. doi: 10.1001/archsurg.139.9.968. [DOI] [PubMed] [Google Scholar]
- 7.Rahman Z, Taylor SC. Malignant melanoma in African Americans. Cutis. 2001;67:203–406. [PubMed] [Google Scholar]
- 8.Zell JA, Cinar P, Mobasher M, Ziogas A, Meyskens FL, Jr, Anton-Culver H. Survival for patients with invasive cutaneous melanoma among ethnic groups: The effects of socioeconomic status and treatment. J Clin Oncol. 2008;26:66–75. doi: 10.1200/JCO.2007.12.3604. [DOI] [PubMed] [Google Scholar]
- 9.Verma S, Quirt I, McCready D, Bak K, Charette M, Iscoe N. Systmatic review of systemic adjuvant therapy for patients at high risk for recurrent melanoma. Cancer. 2006;106:1431–1442. doi: 10.1002/cncr.21760. [DOI] [PubMed] [Google Scholar]
- 10.Eggermont A, Teston A, Marsden J, Henry P, Hersey P, Quirt I, Petralla T, Gogas H, Mackie R, Hauschild A. Utility of adjuvant systemic therapy in melanoma. Ann Oncol. 2009;20(Supp 6):30–34. doi: 10.1093/annonc/mdp250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: A systematic review and meta-analysis. JNCI. 2010;102:493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]
- 12.Testori A, Rutkowski P, Marsden J, Bastholt L, Chiariori-Sileni V, Hauschild A, Eggermont A. Surgery and radiotherapy in the treatment of cutaneous melanoma. Ann Oncol. 2009;20(Supp 6):vi, 22–29. doi: 10.1093/annonc/mdp257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendenhall W, Amdur R, Grobmeyer S, George T, Werning J, Hochwald S, Mendenhall N. Adjuvent radiotherapy for cutaneous melanoma. Cancer. 2008;112:1189–1196. doi: 10.1002/cncr.23306. [DOI] [PubMed] [Google Scholar]
- 14.America.gov. Increasing diversity predicted in US population: Hispanic and Asian populations both poised to triple. 2004 Available: http://www.america.gov/st/washfile-english/2004/March/20040318124311CMretroP0.4814264.html[July 15, 2010]
- 15.Merrill RM, Dearden KA. How representative are the surveillance, epidemiology, and end results (SEER) program cancer data of the United States? Cancer Causes Control. 2004;15:1027–1034. doi: 10.1007/s10552-004-1324-5. [DOI] [PubMed] [Google Scholar]
- 16.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S. International Classification of Diseases for Oncology. 3. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 17.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. American Joint Committee on Cancer Staging Manual. 6. New York, NY: Springer; 2002. [Google Scholar]
- 18.Manual for Tumor Nomenclature and Coding. New York: American Cancer Society; 1968. [Google Scholar]
- 19.Shambaugh E. SEER Extent of disease: Codes and coding instructions. National Cancer Institute; 1984. [Google Scholar]
- 20.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. JNCI. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 21.Bellows C, Bellafsy P, Fortang I, Beech D. Melanoma in African Americans: Trends in biological behavior and clinical characteristics over two decades. J Surg Onc. 2001;78:10–16. doi: 10.1002/jso.1116. [DOI] [PubMed] [Google Scholar]
- 22.Harrison RA, Haque AU, Roseman JM, Soong S-J. Socioeconomic characteristics and melanoma incidence. 1998;8:327–333. doi: 10.1016/s1047-2797(97)00231-7. [DOI] [PubMed] [Google Scholar]
- 23.Polednak A. Prostate cancer treatment in African American and Caucasian men: the need to consider both stage at diagnosis and socioeconomic status. J Natl Med Assoc. 1998;90:101–104. [PMC free article] [PubMed] [Google Scholar]
- 24.Howard G, Anderson RT, Russell G, Howard VJ, Burke GL. Race, socioeconomic status, and cause-specific mortality. Ann Epidemiol. 2000;10:214–223. doi: 10.1016/s1047-2797(00)00038-7. [DOI] [PubMed] [Google Scholar]
- 25.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 26.Neal R, Allgar V. Sociodemographic factors and delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS Patients: Cancer. Br J Cancer. 2005;92:1971–1975. doi: 10.1038/sj.bjc.6602623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bach P, Guadagnoli E, Schrag D, Schussler N, Warren J. Patient demographic and socioeconomic characteristics in the SEER-Medicare database: Applications and limitations. Med Care. 2002;40:IV19–IV25. doi: 10.1097/00005650-200208001-00003. [DOI] [PubMed] [Google Scholar]
- 28.Ayanian JZ, Chrischilles EA, Wallace RB, Fletcher RH, Fouad MN, Kiefe CI, Harrington DP, Weeks JC, Kahn KL, Malin JL, et al. Understanding cancer treatment and outcomes: The cancer care outcomes research and surveillance consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: Findings from community studies. Am J Public Health. 2003;93:200–208. doi: 10.2105/ajph.93.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morton R. Racial differences in adenocarcinoma of the prostate in North American men. Urology. 1994;44:637–645. doi: 10.1016/s0090-4295(94)80196-7. [DOI] [PubMed] [Google Scholar]
- 31.Optenberg SA, Thompson IM, Friedrichs P, Wojcik B, Stein CR, Kramer B. Race, treatment, and long-term survival from prostate cancer in an equal-access medical care delivery system. JAMA. 1995;274:1599–1605. [PubMed] [Google Scholar]
- 32.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 33.Shavers VL, Harlan LC, Winn D, Davis WW. Racial/ethnic patterns of care for cancers of the oral cavity, pharynx, larynx sinuses and salivary glands. Cancer Metastasis Rev. 2003;22:25–38. doi: 10.1023/a:1022255800411. [DOI] [PubMed] [Google Scholar]
- 34.Freedland S, Isaacs W. Explaining racial differences in prostate cancer in the United States: sociology or biology? Prostate. 2005;62:243–242. doi: 10.1002/pros.20052. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman U, Szedlak M, Rittgen W, Jung E, Schadendorf D. Primary staging and follow-up in melanoma patients-monocenter evaluation of methods, costs and patient survival. Br J Cancer. 2002;87:151–157. doi: 10.1038/sj.bjc.6600428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Streyerberg E, Earle C, Nevile B, Weeks J. Racial differences in surgical evaluation, treatment and outcome of locoregional esophageal cancer: A population-based analysis of elderly patients. J Clin Oncol. 2005;23:510–517. doi: 10.1200/JCO.2005.05.169. [DOI] [PubMed] [Google Scholar]
- 37.Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: Incidence and survival patterns in the United States, 1986–2005. Arch Dermatol. 2009;145:427–434. doi: 10.1001/archdermatol.2008.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byrd KM, Wilson DC, Hoyler SS, Peck GL. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21–24. doi: 10.1016/s0190-9622(03)02091-7. [DOI] [PubMed] [Google Scholar]
- 39.Albreski D, Sloan S. Melanoma of the feet: misdiagnosed and misunderstood. Clin Dermatol. 2009;27:556–563. doi: 10.1016/j.clindermatol.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Hu S, Parker D, Thomas A, Kirsner R. Advanced presentation of melanoma in African Americans: The Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031–1032. doi: 10.1016/j.jaad.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Cress R, Holly E. Incidence of cutaneous melanoma among non-Hispanic Whites, Hispanics, Asians and Blacks: an analysis of California Cancer Registry data, 1988–93. Cancer Causes and Control. 1997;8:246–252. doi: 10.1023/a:1018432632528. [DOI] [PubMed] [Google Scholar]
- 42.Hu S, Soza-Vento RM, Parker DF, Kirsner RS. Comparison of stage at diagnosis of melanoma among hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704–708. doi: 10.1001/archderm.142.6.704. [DOI] [PubMed] [Google Scholar]
- 43.Hutchenson A, McGowen J, Maize J, Cook J. Multiple primary and acral melanomas in African Americans: A case series and review of the literature. Dermatol Surg. 2007;33:1–10. doi: 10.1111/j.1524-4725.2007.33000.x. [DOI] [PubMed] [Google Scholar]
- 44.Johnson DS, Yamane S, Morita S, Yonehara C, wong JH. Malignant melanoma in non-caucasians: Experience from Hawaii. Surg Clin N Am. 2003;83:275–282. doi: 10.1016/S0039-6109(02)00093-2. [DOI] [PubMed] [Google Scholar]
- 45.Parmiani G, Castelli C, Santinami M, Rivoltini L. Melanoma immunology: past, present and future. Curr Opin Oncol. 2007;19:121–127. doi: 10.1097/CCO.0b013e32801497d7. [DOI] [PubMed] [Google Scholar]
- 46.Guidry J, Torrence W, Herbelin S. Closing the divide: Diverse populations and cancer survivorship. 2005;104:2577–2583. doi: 10.1002/cncr.21251. [DOI] [PubMed] [Google Scholar]
- 47.SEER: Population Characteristics. 2010 December 17; Available: http://seer.cancer.gov/registries/characteristics.html.
- 48.Haass N, Smalley K. Melanoma biomarkers: Current status and utility in diagnosis, prognosis and response to therapy. Mol Diag Ther. 2009;13:283–296. doi: 10.2165/11317270-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.