Abstract
Objective
To pilot test the feasibility and acceptability of a family-based group behavioral intervention to improve medication adherence in adolescents diagnosed with Inflammatory Bowel Disease (IBD).
Methods
Participants were 40 adolescents age 11-18 years diagnosed with IBD and their primary caregivers, who were randomized to either a 4-session Family-Based Group Behavioral Treatment or Usual Care over a 6-week period. Adherence was measured using a mutli-method, multi-informant assessment involving caregiver- and patient-report, pill count data, and electronic monitoring.
Results
Adherence rates ranged from 66-89% for 6-MP/azathioprine and 51-93% for mesalamine across assessment methods. The intervention was feasible, as evidenced by the 99% treatment session attendance rate, and acceptable based on patient and caregiver report. Repeated measures ANOVA tests revealed nonsignificant differences between the conditions from baseline to post-treatment assessments for pill count, electronic monitor, and primary caregiver-reported adherence (p's> .05). There was a statistically significant improvement in patient-reported mesalamine adherence represented by a significant main effect for Condition (F = 22.24, p< .01; δ = .79) and Condition X Time interaction (F = 13.32, p< .05; δ = .69).
Conclusions
Findings suggest potential for use of behavioral intervention to improve medication adherence in this population. This intervention may be more effective with more complex regimens (e.g., multiple doses per day) such as those prescribed with mesalamine. Further research is needed to examine this type of intervention in more diverse samples with more active disease. Use of alternative adherence measurement approaches, including electronic pill boxes and/or real-time self-report (e.g., via text messaging, electronic diaries, etc.) is also recommended.
Keywords: Inflammatory bowel disease, adherence, self-management, medication
Introduction
Inflammatory bowel disease (i.e., Crohn's disease and ulcerative colitis, collectively IBD) affects approximately 71 in 100,000 individuals1, with approximately 25% of those diagnosed as children or adolescents2,3. Treatment regimens for these chronic, remittent, and unpredictable diseases can include multiple prescription medications and over the counter supplements with various doses and dosing schedules4. In addition, behavioral and psychological difficulties are prevalent in adolescent patients with IBD and their parents, with increased rates of depressive symptoms5-8, social dysfunction 9, and parenting stress10. These disease, treatment, and behavioral factors likely contribute to nonadherence in this population. Medication nonadherence prevalence rates in pediatric IBD are elevated and range from 64-88% for 6-mercaptopurine (6-MP)/azathioprine and mesalamine, respectively, with 38-49% of doses missed by patients4. Further, nonadherence has been associated with increased disease severity13. Nevertheless, there is scant treatment outcome research examining interventions to improve medication adherence in this population.
Treatment of nonadherence in pediatric chronic conditions has received some empirical attention, with clinical trials utilizing either individual or group-based intervention to improve adherence to medical regimens. Across intervention studies, the disease populations, intervention content, and timing of sessions have varied. Wysocki and colleagues14 used group-based treatment focused on problem-solving, communication, cognitive restructuring, and functional-structural family therapy to improve social support and adherence in adolescents diagnosed with type 1 diabetes or insulin-treated type 2 diabetes. Results demonstrated significant improvement in treatment adherence compared to standard care and education only groups. Stark and colleagues demonstrated significant improvements in dietary adherence (i.e., calorie consumption) in cystic fibrosis baseline to post-treatment15,16, and at two-year follow up17,18 using group-based behavioral intervention. The same treatment approach was used to increase dietary calcium intake in children with Juvenile Rheumatoid Arthritis (JRA) and IBD. Children with JRA in the behavioral intervention group demonstrated a net gain in dietary calcium intake of more than twice that of controls, with 92% of children who received the behavioral intervention achieving their adherence goals, compared to 19% of controls19,20. Children with IBD in the behavioral intervention group demonstrated a net gain in dietary calcium intake of more than three times that of controls, with 81% of children who received the behavioral intervention achieving their adherence goals, compared to only 19% of controls21. Nevertheless, these studies have targeted either young children, as opposed to adolescents, or a different chronic condition with, consequently, different treatment regimens. Thus, there is some broad applicability of these studies to intervention with adolescents diagnosed with IBD. However, treatment outcome research is needed to determine 1) the feasibility and acceptability of a treatment protocol for nonadherence in adolescents with IBD, 2) whether this approach is promising in this population, and 3) which specific treatments for IBD are most impacted.
We developed a family-based group behavioral treatment protocol to promote medication adherence in adolescents with IBD using empirically supported intervention techniques demonstrated in the pediatric adherence literature as well as data collected in a prior study with IBD patients to identify any additional targets of intervention22. The present study was a randomized controlled trial to evaluate this intervention utilizing Treatment and Usual Care conditions. Using a manualized treatment protocol, we tested the feasibility, acceptability, and preliminary efficacy of the intervention in adolescent patients with IBD and their caregivers. Both objective and subjective methods were used to measure medication adherence23 to account for potential technical errors in electronic measurement. We hypothesized that patients in the Treatment condition would demonstrate significant improvement in adherence rates from baseline to post-treatment compared to patients in the Usual Care condition. We also hypothesized that the intervention would be feasible, based on treatment session attendance, and acceptable, based on patient and parent ratings of aspects of the intervention components (i.e., format, duration, topics covered, etc.).
Methods
Participants
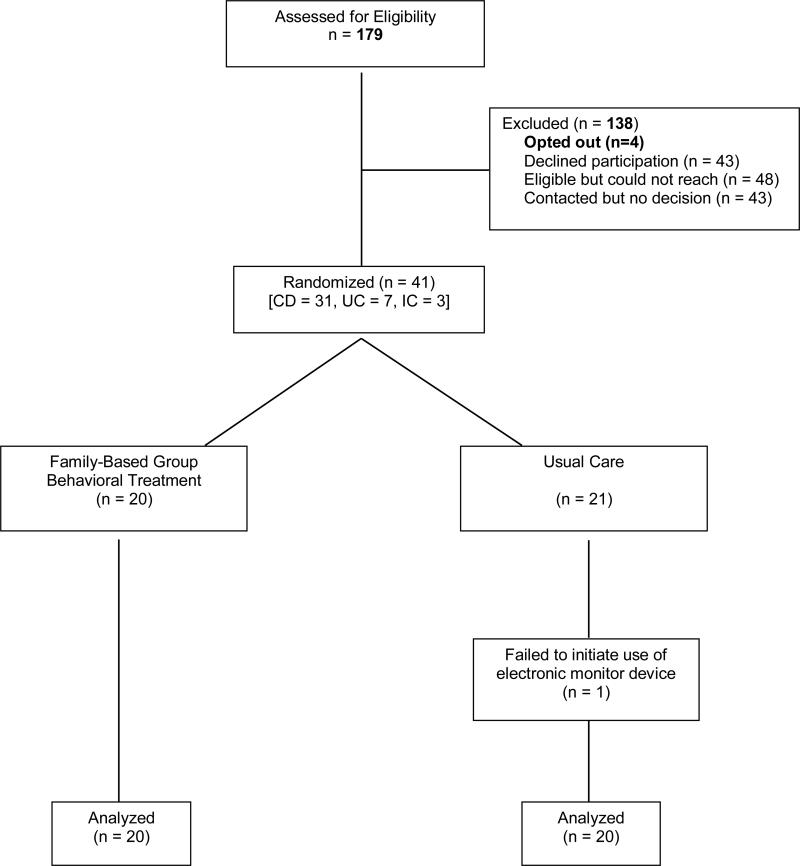
Patients were recruited from an outpatient gastroenterology clinic at a large pediatric hospital. Inclusion criteria for eligibility included 1) diagnosis of Crohn's disease, ulcerative colitis or indeterminate colitis, 2) aged 11 to 17 years, 3) current prescription of 6-mercaptopurine (6-MP)/azathioprine and/or mesalamine, and 4) English fluency for patient and caregiver. The study was approved by the hospital's Institutional Review Board. Prospective participants (N=179) were identified through chart review and mailed a recruitment letter. An opt out phone number was included for families who did not wish to be contacted with study details. A total of 4 patients opted out. Remaining families were contacted by telephone or approached during a regularly scheduled clinic visit. Of the 84 families contacted, 43 declined participation (21 – too busy/study was too much time commitment, 6 – live too far away, 6 – not interested in research, 6 – no concerns about adherence, 3 – patient did not want to talk about disease, 1 – patient going away to college). All remaining families agreed to participate. One participant failed to initiate use of the electronic monitor device and was subsequently excluded from analyses. Thus, the final evaluative sample consisted of 40 adolescents with IBD and at least one caregiver (Figure 1).
Figure 1.
Participant Flow Chart
Study Design and Procedure
This study was a randomized controlled clinical trial designed to obtain pilot data on a novel treatment approach for medication nonadherence. Participants, consisting of patients and their caregivers, were randomly assigned to a Family-Based Group Behavioral Treatment (N=20) or Usual Care (N=20) condition using a permuted block randomization with block size of two. During the baseline visit, informed consent/assent was obtained, and adherence and disease severity assessments were conducted. Two weeks after the baseline visit, participants assigned to the Treatment condition attended four weekly family-based group behavioral intervention sessions led by doctoral level clinical psychologists or postdoctoral psychology fellows (see Table 1 for description of session content). One week following the final group session, participants assigned to both conditions again completed study assessments. Participants in the Treatment condition completed a Feasibility and Acceptability Questionnaire to assess intervention delivery.
Table 1.
Treatment Session Content
| Session # | Session Length | Session Content |
|---|---|---|
| Session 1: | 60-90 Minutes | Educational and Organizational intervention (patients and parents meet separately): 1) education regarding IBD, medication action and side effects, 2) discussion of barriers to adherence, regimen simplification, perceived illness threat, and 3) discussion of organizational cues to prompt adherence. |
| Sessions 2 and 3: | 60-90 Minutes | Behavior Modification Problem Solving Skills and Monitoring Adherence (patients and parents meet separately): 1) training in behavioral contracts and goal setting, 2) positive reinforcement and extinction, 3) discussion/problem solving of goal setting, and 4) training in problem-solving skills, parental monitoring and adolescent self-monitoring of adherence. |
| Session 4: | 60-90 Minutes | Family Functioning (patients and parents meet together): 1) training in communication, negotiation, resolving conflict between family members. |
Measures
Demographics
Caregivers provided demographic information during the baseline assessment (e.g., caregiver age, education, marital status, patient ethnicity, and annual household income). Other demographic information (e.g., patient age) was obtained via medical record review.
Pill count
Pill counts were conducted at each assessment time point for 6-MP/azathioprine and/or mesalamine. Data collected from pill bottles included dosing instructions, prescription fill date, quantity filled, and current quantity. Adherence frequency was calculated as doses removed from bottle / doses prescribed × 100.
Treatment Regimen Adherence Questionnaire (TRAQ)
The TRAQ, developed for the purpose of this study and administered at each assessment time point, contains 16 items assessing adherence to treatments (i.e., medications, diet, tube feeding), barriers to medication adherence, organization of medications, and treatment responsibility. For the purpose of this study, only the medication adherence question was utilized in data analyses. This question was phrased:
“Children and adolescents often have difficulty taking medications. They may forget, have activities that conflict with the treatment, or just decide not to take a dose of medication. There may be other reasons too. All of these reasons are completely understandable.
Please tell us the number of medication doses you have missed in the past 2 weeks and which medication was missed:
Medication: _________________ Number of doses missed: _______
Medication: _________________ Number of doses missed: _______”
Adherence frequency was calculated as 100 – (reported missed number of doses / doses prescribed × 100).
Medication Event Monitoring System (MEMS)
The MEMS® TrackCaps, manufactured by AARDEX, are comprised of a standard plastic vial and a cap with a micro-electric circuit. The cap records the date, time, and frequency of openings. Data was downloaded from the caps at each assessment time point to track patient's adherence to 6-MP/azathioprine and/or mesalamine. Data were downloaded directly to compatible software then exported to Excel for data management. Upon completion, data was imported into PASW18.0 for statistical analysis.
Partial Harvey Bradshaw Index (PHBI)24
The PHBI is a 3-item disease severity assessment for patients diagnosed with Crohn's disease. Patients are asked to rate their general well-being, abdominal pain, and number of liquid stools for the past 7 days. Total scores range from 0-12, with a higher score denoting more active disease (i.e., 0 = inactive disease; 1-4 = mild disease; ≥ 5 = moderate to severe disease).The PHBI has demonstrated adequate reliability and validity in prior studies. Internal consistency was .71 for this sample. The PHBI was conducted at each assessment time point.
Pediatric Ulcerative Colitis Activity Index (PUCAI)25
The PUCAI is a disease severity assessment for patients with ulcerative colitis. Patients are assessed across 6 domains of disease severity including abdominal pain, rectal bleeding, stool consistency, number of stools per 24 hours, nocturnal stools, and activity level. Factors are assessed for the past 2 days, unless noted for the past 24 hours (i.e., number of stools per 24 hours). Total scores, representing a sum of the 6 items, range from 0-85 with higher scores indicating more active disease (i.e., 0-9 = inactive; 10-34 = mild; 35-64 = moderate; ≥ 65 = severe disease). The PUCAI has demonstrated adequate reliability and validity in prior research. Internal consistency reliability for this sample was .63. The PUCAI was conducted at each assessment time point.
Feasibility and Acceptability Questionnaire (FAQ)
The FAQ is a 22 item report for caregivers and patients to assess feasibility and acceptability of the family-based group behavioral intervention. The measure was developed specifically for the study and assesses factors including format, topics covered, length of study, duration of intervention sessions, convenience of attendance, use of skills learned, and impact sessions had on the patient's adherence. Items were assessed on a 7-point Likert scale. Families assigned to the Treatment condition completed the FAQ at post-treatment assessment.
Data Analyses
Data were entered into a secure database and data quality analysis was performed. All data analyses were conducted in PASW 18.0. Descriptive statistics were calculated for demographic, adherence, disease severity, and feasibility/acceptability variables. Independent samples t-tests were conducted to examine differences between Treatment and Usual Care conditions at baseline. Differences in mean medication adherence rates at post-treatment were analyzed using repeated measures ANOVA tests. All tests were considered significant at the p< .05 level.
Results
Descriptive Data
Participants included 30 patients with CD, 7 patients with UC, and 3 patients with indeterminate colitis. Of the patients with CD, 28% had inactive disease; 55% had mild disease; and 17% had moderate to severe disease. Forty percent of patients with UC or indeterminate colitis had inactive disease; 40% had mild disease; and 20% had moderate disease. Twenty-four patients were prescribed 6-MP/azathioprine, 21 patients were prescribed mesalamine, and 6 patients were prescribed both. Table 2 summarizes participant demographic, adherence, and disease parameters. Feasibility of the treatment was demonstrated by 99% treatment session attendance for all participants assigned to the intervention. Patient and parent ratings on the FAQ are summarized in Table 3 and suggest a high degree of acceptability.
Table 2.
Participant Demographic, Adherence, and Disease Characteristics
| Adolescent age (years) | 15.4 ± 1.5 | |
| Gender (% male) | 50% | |
| Ethnicity (% White, not Hispanic origin) | 90% | |
| Primary Caregiver: | ||
| Age (years) | 46.2 ± 4.9 | |
| Marital Status (% married) | 87.5% | |
| Education Level (% with at least a college degree) | 45% | |
| Annual Household Income (Median) | $100,001-125,000 | |
| IBD Diagnosis (%): | ||
| Crohn's Disease | 75.0% | |
| Ulcerative Colitis | 17.5% | |
| Indeterminate Colitis | 7.5% | |
| Disease Severity: | Baseline | Post-treatment |
| PHBI | 2.10 ± 2.27 | 2.87 ± 2.66 |
| PUCAI | 14.0 ± 15.06 | 12.0 ± 14.18 |
| Adherence: | ||
| Pill Count - 6-MP/azathioprine | 66% | 56% |
| Pill Count - mesalamine | 51% | 50% |
| Electronic monitor | 89% | 84% |
| Parent-reported - 6-MP/azathioprine | 83% | 81% |
| Parent-reported - mesalamine | 89% | 94% |
| Teen-reported - 6-MP/azathioprine | 87% | 87% |
| Teen-reported - mesalamine | 93% | 96% |
Table 3.
Descriptive Data for Parent- and Patient-Report Forms of the FAQ
| Parent | Child | |||
|---|---|---|---|---|
| % Rating in Ideal Range | Mean Rating | % Rating in Ideal Range | Mean Rating | |
| 1. I liked the group-based formata | 100% | 6.65 | 90% | 5.70 |
| 2. I thought the group-based format was helpfula | 94% | 6.41 | 85% | 5.75 |
| 3. Amount of informationb | 82% | 4.24 | 100% | 4.30 |
| 4. Treatment session lengthb | 76% | 4.35 | 90% | 4.55 |
| 5. Number of sessionsb | 76% | 3.53 | 80% | 3.55 |
| 6. Total time commitment for treatment (i.e., 4 weeks)b | 88% | 3.76 | 85% | 3.75 |
| 7. I thought attending sessions was convenienta | 88% | 5.88 | 65% | 4.95 |
| 8. I used the behavioral skills I learneda | 82% | 5.47 | 70% | 5.25 |
| 9. Treatment helped improve my (child's) adherencea | 82% | 5.41 | 75% | 5.25 |
Note. Ideal range is based on assumption that ratings in this range represent a high degree of acceptability for respondents.
Ideal range = 5-7 on 7-point likert scale.
Ideal range = 3-5 on 7-point likert scale.
Primary Analyses
Independent samples t-tests revealed no significant differences between conditions at baseline across demographic, disease, and adherence parameters (p's > .05). Repeated measures ANOVA tests were conducted to examine treatment effects on each adherence assessment. These analyses revealed nonsignificant differences between the Treatment and Usual Care conditions from baseline to post-treatment assessments across pill count assessment (Treatment = 4%, Usual Care = 2%, p = .95 for 6-MP/azathioprine; Treatment = 17%, Usual Care = 6%, p = .40 for mesalamine), electronic monitor assessment (Treatment = 7%, Usual Care = 3%, p = .73), and parent-reported adherence assessment (Treatment = 6%, Usual Care = 8%, p = .33 for 6-MP/azathioprine; Treatment = 19%, Usual Care = 2%, p = .50 for mesalamine). However, while patient-reported adherence was nonsignificant for 6-MP/azathioprine (Treatment = 6%, Usual Care = 10%, p = .76), there was a statistically significant improvement in mesalamine adherence as represented by a significant main effect for Condition (F = 22.24, p< .01; δ = .79) and Condition × Time interaction (F = 13.32, p< .05; δ = .69). Moreover, this translated to a substantial 25% increase in mesalamine adherence in the Treatment condition compared to only a 1% increase in the Usual Care condition.
Discussion
This study is the first known RCT of a family-based group behavioral intervention to promote treatment adherence in adolescents with IBD. Adherence is a significant behavioral health issue as apparent failure of oral medication to treat disease that is actually due to nonadherence can lead to erroneous escalation in therapy, which might include biologic agents that could have long-term ramifications for young patients. Moreover, there is evidence that nonadherence is associated with greater risk of relapse11 and increased health care costs12 in adults with IBD. In the present trial, adolescents reported a significant 25% increase in mesalamine but not in 6-MP/azathioprine adherence. Evaluation of treatment effects with other measures of adherence including pill counts and electronic monitors did not reveal significant improvements. Given the known measurement variability in adherence assessment tools, including that documented in pediatric IBD4,23, we planned a multi-method, multi-informant assessment approach in order to compensate for the limitations of each measure. However, there were some unforeseen challenges to using electronic monitor devices in our sample that may have affected the validity of that assessment tool. Fifty-six percent of the sample reported using pill boxes to organize their medications. When asked to use a pill bottle instead of the pill box for one medication (potentially further complicating an already complicated regimen), many participants reported that they did not put the medication into the bottle, but rather kept it in the pill box as usual and tried to remember to open the empty bottle each time they accessed the pill box for that medication. This resulted in the electronic monitors being used as a proxy measure. One might expect that this would result in lower electronic monitor adherence; however, based on the high electronic monitor adherence rates compared to pill counts, it appears that in our sample many patients were likely opening the bottle each time they accessed the pill box for all medications rather than just the one being examined. This is a critical measurement issue that is not often discussed in the literature. Although we know this occurred with a substantial number of participants, we do not know the exact number as we discovered this was happening too late in data collection. These issues notwithstanding, we found significant improvement in the type of assessment most often utilized in a clinical setting (i.e., patient self-report). Nevertheless, the scope of this study and findings suggest that it should be considered pilot/preliminary data that warrant further examination in larger trials. As this study is currently in follow-up phases, we plan to evaluate any long-term changes in adherence.
Because this is an initial investigation using group-based behavioral treatment to promote adherence in this population, we sought to examine the feasibility and acceptability of the intervention. Group-based treatment is appealing because it is generally cost-effective, efficient, and allows for social networking among patients and families. In this trial, excellent feasibility was demonstrated by 99% treatment session attendance by patients in the Treatment condition. The intervention was also perceived as highly acceptable to both patients and their caregivers as evidenced by 70-100% reporting a high degree of acceptability regarding the appeal and helpfulness of the group-based format of the intervention, amount of information covered, treatment session length, number of sessions, total time commitment, convenience of treatment, use of behavioral skills taught, and perceived impact on treatment adherence.
The results of this study should be taken within the context of its methodological strengths and limitations. Our use of a RCT design reduced selection bias and spurious causality inferences. The intervention was developed using empirically supported theory driven components as well as patient and family input on session content22. We also utilized a multi-method, multi-informant adherence assessment strategy to evaluate intervention effects, which is an uncommon approach in the adherence literature. However, the findings are limited by the small sample size used for this preliminary investigation. Second, although the sample was representative of the IBD population in general26,27, the lack of socioeconomic and ethnic diversity limits generalizability to economically disadvantaged or minority populations. Third, the TRAQ measure of adherence was developed for this study and does not have prior psychometric data. Fourth, high baseline levels of adherence across self-report and electronic monitor assessments may have prevented increases in adherence substantial enough to detect other statistically significant differences between groups. These high baseline levels likely occurred because we did not recruit a clinical sample (i.e., patients who currently demonstrate nonadherence) for this pilot study. Finally, as previously mentioned, the high percentage of patients using pill boxes interfered with electronic monitor data, resulting in unclear findings for that measure. Use of multiple alternative measures of adherence in patients who are prescribed multiple medications and supplements helps to account for these issues and may be preferable under these circumstances.
In conclusion, the findings of the present study are encouraging but clearly indicate that further investigation is required. Future research should involve replication with a more diverse sample in terms of socioeconomic status, ethnicity, and disease severity. Alternative adherence measurement approaches should also be considered, including electronic pill boxes and/or real-time self-report (e.g., via text messaging, electronic diaries, etc.). Additionally, larger scale testing of the intervention may be necessary to fully understand its efficacy. Continued examination of adherence promotion efforts is necessary to developing self-management programs and maximizing patient health outcomes in IBD.
Acknowledgments
Funding: Research supported in part by NIDDK K23 DK079037, PHS Grant P30 DK 078392, and USPHS Grant #UL1 RR026314 from the National Center for Research Resources, NIH
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin. Gastroenterol. 2007;5(12):1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Auvin S, Molinie F, Gower-Rousseau C, et al. Incidence, clinical presentation and location at diagnosis of pediatric inflammatory bowel disease: a prospective population-based study in northern France (1988-1999). J. Pediatr. Gastroenterol. Nutr. 2005 Jul;41(1):49–55. doi: 10.1097/01.mpg.0000162479.74277.86. [DOI] [PubMed] [Google Scholar]
- 3.Otley A, Smith C, Nicholas D, et al. The IMPACT questionnaire: A valid measure of health-related quality of life in pediatric inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2002 Oct;35(4):557–563. doi: 10.1097/00005176-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Hommel KA, Davis CM, Baldassano RN. Objective versus subjective assessment of oral medication adherence in pediatric inflammatory bowel disease. Inflammatory Bowel Disease. 2009;15(4):589–593. doi: 10.1002/ibd.20798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hommel KA, Denson LA, Crandall WV, Mackner LM. Behavioral functioning and treatment adherence in pediatric inflammatory bowel disease: Review and recommendations for practice. Gastroenterology & Hepatology. 2008 Nov 1;4(11):785. [PMC free article] [PubMed] [Google Scholar]
- 6.Hommel KA, Davis CM, Baldassano RN. Medication adherence and quality of life in pediatric inflammatory bowel disease. J. Pediatr. Psychol. 2008 Sep;33(8):867–874. doi: 10.1093/jpepsy/jsn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szigethy E, Levy-Warren A, Whitton S, et al. Depressive symptoms and inflammatory bowel disease in children and adolescents: a cross-sectional study. Journal of Pediatric Gastroeneterology and Nutrition. 2004;39(4):395–403. doi: 10.1097/00005176-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Mackner LM, Crandall WV. Psychological factors affecting pediatric inflammatory bowel disease. Curr. Opin. Pediatr. 2007;19(5):548–552. doi: 10.1097/MOP.0b013e3282ef4426. [DOI] [PubMed] [Google Scholar]
- 9.Mackner LM, Crandall WV. Brief report: Psychosocial adjustment in adolescents with inflammatory bowel disease. J. Pediatr. Psychol. 2006 Apr;31(3):281–285. doi: 10.1093/jpepsy/jsj023. [DOI] [PubMed] [Google Scholar]
- 10.Guilfoyle SM, Denson LA, Baldassano RN, Hommel KA. Paediatric parenting stress in inflammatory bowel disease: Application of the Pediatric Inventory for Parents. Child. Care. Health Dev. 2011 Feb 7; doi: 10.1111/j.1365-2214.2010.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. The American Journal of Medicine. 2003;114(4):39–43. doi: 10.1016/s0002-9343(02)01383-9. [DOI] [PubMed] [Google Scholar]
- 12.Higgins PDR, Rubin DT, Kaulback K, Schoenfeld PS, Kane SV. Systematic review: Impact of non-adherence to 5-aminosalicylic acid products on the frequency and cost of ulcerative colitis flares. Aliment. Pharmacol. Ther. 2009;29(3):247–257. doi: 10.1111/j.1365-2036.2008.03865.x. [DOI] [PubMed] [Google Scholar]
- 13.Hommel KA, Denson LA, Baldassano RN. Oral medication adherence and disease severity in pediatric inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2011 Mar;23(3):250–254. doi: 10.1097/MEG.0b013e328344019c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wysocki T, Harris MA, Buckloh LM, et al. Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. J. Pediatr. Psychol. 2006 Oct;31(9):928–938. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- 15.Stark LJ, Bowen AM, Tyc VL, Evans S, Passero MA. A behavioral approach to increasing calorie consumption in children with cystic fibrosis. J. Pediatr. Psychol. 1990 Jun;15(3):309–326. doi: 10.1093/jpepsy/15.3.309. [DOI] [PubMed] [Google Scholar]
- 16.Stark LJ, Mulvihill MM, Powers SW, et al. Behavioral intervention to improve calorie intake of children with cystic fibrosis: treatment versus wait list control. J. Pediatr. Gastroenterol. Nutr. 1996 Apr;22(3):240–253. doi: 10.1097/00005176-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Stark LJ, Knapp LG, Bowen AM, et al. Increasing calorie consumption in children with cystic fibrosis: replication with 2-year follow-up. J. Appl. Behav. Anal. Winter. 1993;26(4):435–450. doi: 10.1901/jaba.1993.26-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stark LJ, Opipari LC, Spieth L, et al. Contribution of behavior therapy to dietary treatment in cystic fibrosis: A randomized controlled study with two-year follow-up. Behavior Therapy. 2003;34:237–258. [Google Scholar]
- 19.Stark LJ, Janicke DM, McGrath AM, Mackner LM, Hommel KA, Lovell D. Prevention of osteoporosis: a randomized clinical trial to increase calcium intake in children with juvenile rheumatoid arthritis. J. Pediatr. Psychol. 2005 Jul-Aug;30(5):377–386. doi: 10.1093/jpepsy/jsi061. [DOI] [PubMed] [Google Scholar]
- 20.Stark LJ, Davis AM, Janicke DM, et al. A randomized clinical trial of dietary calcium to improve bone accretion in children with juvenile rheumatoid arthritis. J. Pediatr. 2006 Apr;148(4):501–507. doi: 10.1016/j.jpeds.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 21.Stark LJ, Hommel KA, Mackner LM, et al. Randomized trial comparing two methods of increasing dietary calcium intake in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2005 Apr;40(4):501–507. doi: 10.1097/01.mpg.0000157913.32465.45. [DOI] [PubMed] [Google Scholar]
- 22.Hommel KA, Odell S, Sander E, Baldassano RN, Barg FK. Treatment adherence in paediatric inflammatory bowel disease: Perceptions from adolescent patients and their families. Health and Social Care in the Community. 2011 Jan;19(1):80–88. doi: 10.1111/j.1365-2524.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hommel KA, Mackner LM, Denson LA, Crandall WV. Treatment regimen adherence in pediatric gastroenterology. Journal of Pediatric Gastroeneterology and Nutrition. 2008;47(5):526–543. doi: 10.1097/MPG.0b013e318175dda1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markowitz J, Grancher K, Kohn N, Lesser M, Daum F, Group TPMC A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn's disease. Gastroenterology. 2000;119:895–902. doi: 10.1053/gast.2000.18144. [DOI] [PubMed] [Google Scholar]
- 25.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007 Aug;133(2):423–432. doi: 10.1053/j.gastro.2007.05.029. PMID: 17681163. [DOI] [PubMed] [Google Scholar]
- 26.Mackner LM, Crandall WV. Oral medication adherence in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2005 Nov;11(11):1006–1012. doi: 10.1097/01.mib.0000186409.15392.54. [DOI] [PubMed] [Google Scholar]
- 27.Greenley RN, Stephens M, Doughty A, Raboin T, Kugathasan S. Barriers to adherence among adolescents with inflammatory bowel disease. Inflamm. Bowel Dis. 2010;16(1):36–41. doi: 10.1002/ibd.20988. [DOI] [PubMed] [Google Scholar]