Abstract
Background
Psychostimulants improve a variety of cognitive/behavioral processes in patients with attention deficit hyperactivity disorder (ADHD). Limited observations suggest a potentially different dose-sensitivity of prefrontal cortex (PFC)-dependent function (narrow inverted-U-shaped dose-response curves) vs. classroom/overt behavior (broad inverted-U) in children with ADHD. Recent work in rodents observed that methylphenidate (MPH; Ritalin®) elicits a narrow inverted-U shaped improvement in performance in PFC-dependent tests of working memory. The current studies first tested the hypothesis that PFC-dependent tasks, in general, display narrow dose sensitivity to the beneficial actions of MPH.
Methods
The effects of varying doses of MPH were examined on performance of rats in two tests of PFC-dependent cognition, sustained attention and attentional set shifting. Additionally, the effect of pretreatment with the α1-antagonist, prazosin (0.5 mg/kg), on MPH-induced improvement in sustained attention was examined.
Results
MPH produced a broad inverted-U-shaped facilitation of sustained attention and attentional set shifting. Prior research indicates α1-receptors impair, while α2-receptors improve, working memory. In contrast, attentional set shifting is improved with α1-receptor activation, while α2-receptors exert minimal effects in this task. Given the similar dose sensitivity of sustained attention and attentional set shifting tasks, additional studies examined whether α1-receptors promote sustained attention, similar to attentional set shifting. In these studies MPH-induced improvement in sustained attention was abolished by α1-receptor blockade.
Conclusions
PFC-dependent processes display differential sensitivity to the cognition-enhancing actions of psychostimulants that are linked to the differential involvement of α1- vs. α2-receptors in these processes. These observations have significant preclinical and clinical implications.
Keywords: ADHD, Prefrontal Cortex, Cognition, Methylphenidate, Norepinephrine, Dopamine
Psychostimulants exert behavioral-calming and cognition-enhancing actions in the treatment of attention deficit hyperactivity disorder (ADHD). In laboratory testing, clinical doses of psychostimulants improve a variety of behavioral and cognitive processes dependent on the prefrontal cortex (PFC) in ADHD subjects (1). Moreover, these effects are not unique to ADHD, with similar actions observed in normal human and animal subjects, provided low and clinically-relevant doses are used (2–5).
Early studies by Sprague and Sleator reported that in children with ADHD, methylphenidate (MPH, Ritalin®) produced a narrow inverted-U shaped facilitation of performance in a laboratory-based test of ‘cognition/learning’, while classroom behavior was improved across a wider range of doses (6). However, subsequent studies generally failed to observe differential dose sensitivity across a variety of cognitive and behavioral tests in ADHD patients (for review, 7–9). A potentially important factor in this work is that the cognitive task utilized by Sprague and Sleator involved short-term memory testing across short delays (seconds), typical of tests used to assess PFC-dependent working memory (6). Moreover, MPH also produced a narrow inverted-U shaped improvement in a PFC-dependent test of response inhibition in ADHD patients which differed from a generally linear dose-dependent improvement in overt behavior (9). Combined, these observations suggest the hypothesis that psychostimulants exert differential dose-dependent effects on PFC-dependent vs. PFC-independent processes.
In rats and monkeys, MPH elicits a narrow inverted-U-shaped facilitation of performance in PFC-dependent delayed-response tests of working memory (4, 5, 10–13). Sustained attention is also dependent on the PFC (14) and in an earlier study, MPH improved performance of rats in a signal detection test of sustained attention at a dose that maximally improved working memory performance (0.5 mg/kg; 4). The current studies tested the hypothesis that differing PFC-dependent processes display similar restricted dose sensitivity to psychostimulants. These studies first examined whether the dose-dependent actions of MPH on sustained attention are comparable to those seen in tests of working memory. Surprisingly, MPH produced a broader and right-shifted inverted-U shaped facilitation of sustained attention relative to that seen with working memory testing. To assess whether this broader inverted-U curve is limited to focused, relatively inflexible attention, additional studies examined the dose-dependent effects of MPH in a PFC-dependent attentional set shifting task, that requires attentional shifts to previously irrelevant stimuli (15, 16). In this task, MPH also produced a broad inverted-U shaped facilitation of performance, virtually identical to that seen in sustained attention testing.
Noradrenergic α1-receptors are known to impair performance in tests of working memory while α2-receptors promote performance and contribute to the beneficial effects of MPH in these tests (17). In contrast, α1-receptors improve attentional set shifting while α2-receptors have little impact in this test (18). These observations suggest that the narrow vs. broad inverted-U shaped actions of psychostimulants may involve differential actions of α2- vs. α1-receptors, respectively. Currently, the role of α1-receptors in sustained attention is not known. Thus, additional studies examined the effects of pretreatment with the α1-antagonist, prazosin (0.5 mg/kg), on MPH-induced improvements in sustained attention. These studies demonstrate that α1-receptor blockade completely prevents the beneficial effects of MPH on sustained attention.
Combined with earlier observations, these studies demonstrate that distinct PFC-dependent cognitive processes differ in their dose sensitivity to the cognition-enhancing actions of psychostimulants. This difference in sensitivity to psychostimulants is associated with differential actions of noradrenergic α2- vs. α1-receptors across these processes.
METHODS
MPH Dose-Response Curve in the Signal Detection Test of Sustained Attention (Madison, WI)
Subjects
7 male Sprague-Dawley rats (290–370 g, Harlan, Madison, WI) were housed 2–3/cage on a 12-hr light/dark cycle. Care and testing of animals was in accordance with federal and university animal care guidelines.
Testing Procedures
Body weight was reduced to 85% of ad libitum weight while water was provided ad libitum. Animals were trained and tested in an operant-based signal detection test of sustained attention previously described (19; see also 20). Briefly, on one half of the trials (selected at random, p = 0.5) an LED was illuminated and two levers were projected into the chamber (“Signal Trials”). The signal length was variable, randomly selected from the list: 0.125, 0.25, 0.5, 0.75, 1.0, 1.5, and 2.0-sec with replacement. On the other half of the trials, the LED remained dark, after which both levers were inserted (“No Signal Trials”). The levers remained in the chamber until a response was made, at which time both levers were retracted. On signal trials, a right lever press was scored as a ‘Hit’ and reinforced with two sucrose pellets (45 mg pellets, Dustless Precision Pellets, Bio-Serv, Frenchtown, NJ) while a left lever press was scored as a “miss”. On a no signal trial, a right lever press was scored as a “false alarm”. A left lever press on a no-signal trial was reinforced with 2 pellets (“correct rejection”). For all correct response trials, the house lights were illuminated for 5-sec with reward presentation. For all incorrect trials, the levers were retracted and a 5-sec time-out period ensued (houselights off). Failure to respond within 5-sec of lever insertion, the levers were retracted, followed by a 5-sec blackout. A variable inter-trial interval of 13-sec (minimum 5-sec), on average, elapsed before the start of new trial. Trials with no response occurred infrequently and were excluded from analyses. Single trials lasted about 20-sec and all sessions lasted for 100 trials. Animals were trained until proportion of correct trials reached 75%–85%.
MPH testing occurred in a three-arm design. Each arm consisted of either “low”, “medium”, or “high” doses of MPH with vehicle (normal saline) treatment included in each arm and each dose replicated 3 times per arm. Treatments were randomized within an arm. The first arm consisted of 0.5 mg/kg and 2.0 mg/kg MPH, doses that define the inverted-U shaped dose response curve for working memory testing. Based on unexpected results obtained in the first arm, the second arm consisted of 2.0 mg/kg (to replicate the first arm) and 4.0 mg/kg MPH. Finally, the third arm utilized 8.0 mg/kg MPH. All treatments were delivered IP 30-min prior to testing. Dependent measures include the proportion of trials with a correct response (proportion of hits + proportion of correct rejections), the probability of a hit (number of right responses/number of signal trials), probability of a false alarm (number of right responses/number of no signal trials), c (response bias), and d′, a relative measure of stimulus detectability (d′ = Z(N) − Z(SN). Z(N) = Z-score of the Noise Distribution = Z-score of (1−probability of false alarms). Z(SN) = Z-score of the Signal + Noise distribution = Z-score of 1−probability of a hit). d′ takes into account both the probability of a hit and the probability of a false alarm (see 21).
MPH Dose-Response Curve in Attentional Set Shifting (Durham, NH)
Subjects
12 male Sprague Dawley rats (275–300 g, Charles River, Wilmington, MA) were subjected to food restriction (18 gms) and allowed ad libitum access to water and housed on a 12-hr light cycle.
Testing Procedures
All training and testing was as described previously (22). Briefly, rats were trained to dig in pots for food reinforcers (45 mg food pellet, Dustless Precision Pellets, Bio-serv, Frenchtown, NJ) until they successfully retrieved 10 buried reinforcers. On the following day, rats began exemplar training on three simple discriminations of texture, odor or digging media. Rats were then tested on a simple discrimination, compound discrimination and reversal to facilitate formation of an attentional set (e.g. focus attention on odor). Animals were then tested on an intradimensional shift (IDS), where a novel set of stimuli is introduced but the attribute that predicts reward remains the same (e.g. odor). After the successful completion of six consecutive IDS trials, a reinforcement reversal was performed (IDR), where the alternate odor from the testing pair was reinforced. We next tested for extradimensional attentional set shifts (EDS), which involved reinforcing a previously unattended dimension, (e.g. if odor had previously been reinforced, digging material is then reinforced). After successful completion of the EDS, a final reversal (EDR) of the session occurred (e.g. the alternate digging media in the pair predicts reward).
Animals were treated IP with either vehicle or varying doses of 0.5 mg/kg, 2.0 mg/kg, and 4.0 mg/kg MPH 15-minutes prior to IDS testing. The number of trials to reach criterion performance level of six consecutive correct trials was recorded for IDS, EDS, IDR and EDR.
α1-Receptor Action in a Signal Detection Test of Sustained Attention Task (Philadelphia, PA)
Subjects
Six male Sprague Dawley Rats (150–176g, Taconic, Germantown, PA) were trained in an operant-based signal detection task of sustained attention (12-hr light cycle). Animals were water restricted, receiving 10-min free drink in their home cage after testing (~20 ml).
Testing Procedures
Testing and apparatus were similar to that described above, with the following exceptions. Hits and Correct Rejections resulted in the delivery of 40 μl water. On signal trials, rats were trained to respond to a short duration (15-msec) light. Testing occurred daily over 45-min and rats received equal numbers of randomly presented signal and non-signal trials. Criterion performance required a rat to achieve greater than 59% signal and non-signal trials and less than 25% omissions per day for three consecutive days. Rats were treated IP with vehicle (saline), MPH (0.5 mg/kg, 2.0 mg/kg, 3.5 mg/kg), 0.5 mg/kg of the α1-antagonist, prazosin, or 0.5 mg/kg prazosin + 2.0 mg/kg MPH. Prazosin was administered IP 15-min before MPH and 35-min prior to testing. For the prazosin studies, animals were first prescreened for sensitivity to MPH. The ~85% of animals that showed improvement with MPH were used for these studies.
Statistical Analysis
Sustained Attention MPH Dose-Response
Repeated-measures ANOVA with planned comparisons were used to determine the degree to which MPH treatment differed from vehicle treatment.
Attentional set shifting
ANOVA was used to assess treatment effects on attentional set shifting and reversal trials. The number of trials needed to reach criterion performance in the ID was compared to the ED using a within-subjects ANOVA with Dose (4 levels) and Test (ID vs. ED; 2 levels) as factors. In a series of planned comparisons, the effects of MPH on ID and ED were compared to those of vehicle. A separate repeated-measures ANOVA with Test (2 levels, IDR and EDR) and Dose (4 levels) was used to determine the effects of MPH on the number of trials to reach criterion on reversal learning. One rat failed to engage in testing following drug administration and was excluded from the analysis.
α1-Antagonist + MPH in Sustained Attention
Repeated-measures ANOVA with planned comparisons was used to assess treatment effects on sustained attention performance relative to baseline performance (4 levels: baseline, MPH, prazosin, MPH + prazosin) and to compare the effects of MPH to those of prazosin and MPH + prazosin.
RESULTS
MPH Exerts a Broad Inverted-U Shaped Facilitation of Sustained Attention
We first examined whether sustained attention is improved by MPH across the same dose range observed previously for working memory in our laboratory (4). Animals (n=7) were treated with vehicle or varying doses of MPH (0.5 mg/kg, 2.0 mg/kg, 4.0 mg/kg, 8.0 mg/kg) 30-min prior to sustained attention testing as described above.
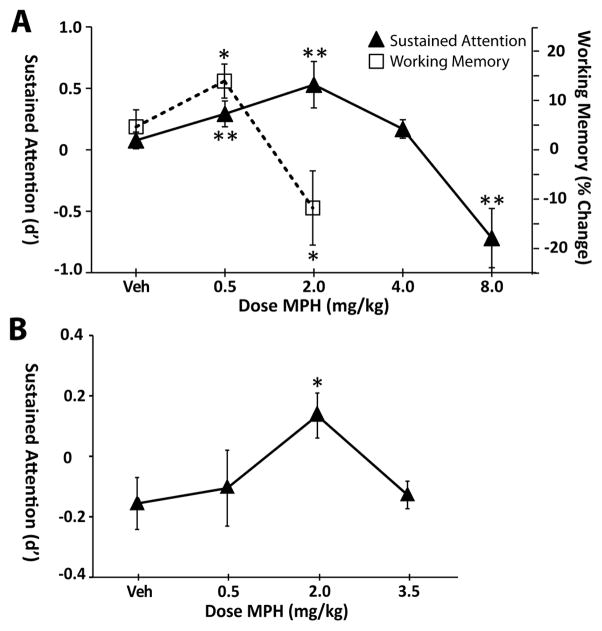
These studies demonstrate that MPH facilitates sustained attention, as measured by the change in d′ from baseline, in an inverted-U shaped manner (see Figure 1A and Table 1; arm 1, F2,12=6.12, p=0.015; arm 2, F2,12=6.02, p=0.015; arm 3, F1,6=20.01, p=0.004). Planned comparisons indicated that, as observed previously (4), 0.5 mg/kg MPH increased d′ relative to vehicle treatment (t6=3.40, p<0.007). In contrast to that seen in working memory testing, 2.0 mg/kg MPH further improved sustained attention as measured by d′ (arm 1, t6=3.07, p = 0.01). This effect of 2.0 mg/kg MPH was replicated in the second testing arm (F2, 12=6.02, p=0.015; vehicle vs. 2.0 mg/kg, t6=5.53, p<0.001). This beneficial effect of MPH decreased with increasing dose such that 4.0 mg/kg did not significantly alter (t6=1.20, p=0.14) while 8.0 mg/kg MPH significantly reduced d′ (t6=−4.48, p=0.002).
Figure 1.
MPH exerts a broad inverted-U dose-dependent improvement in performance in a sustained attention test. Panel A: Shown are the effects of varying doses of MPH on performance in a signal detection test of sustained attention as measured by change in d′ from baseline performance (Madison, WI testing; Triangles, left axis). Relative to vehicle treatment (Veh) 0.5 mg/kg MPH significantly improved performance, consistent with our previous observations (4). 2.0 mg/kg MPH produced an even larger improvement in performance in this task while 4.0 mg/kg MPH did not improve and 8.0 mg/kg MPH significantly impaired performance. The breadth of this dose response curve differs from that seen in a delayed response task of spatial working memory (Squares, right axis; data from 10). For working memory testing, animals were required to alternate arm entries following a delay (10–120 seconds) that yielded 70–80% accurate performance. MPH produces a substantially narrower inverted-U shaped facilitation of working memory performance than seen in the sustained attention task, with maximal improvement occurring at 0.5 mg/kg and impairment at 2.0 mg/kg. Panel B: Effects of varying doses of MPH on sustained attention as tested in Philadelphia, PA. Despite significant differences in testing procedures and baseline levels of performance (see Methods and Results) nearly identical dose-response effects of MPH on sustained attention were obtained to those shown in Panel A, with maximal improvement in performance occurring at 2.0 mg/kg. In particular, the magnitude of the increase in d′ seen with this dose of MPH was nearly identical across the two laboratories. *P < 0.05; **P < 0.01 relative to vehicle treatment.
Table I.
Effects of varying doses of MPH on differing aspects of performance in the sustained attention task.
| MPH Dose (mg/kg) | |||||
|---|---|---|---|---|---|
| Veh | 0.5 | 2.0 | 4.0 | 8.0 | |
| d′ | 0.08 ± 0.07 | 0.29 ± 0.11** | 0.53 ± 0.19** | 0.17 ± 0.08 | −0.72 ± 0.24** |
| Correct | 0.01 ± 0.01 | 0.04 ± 0.01* | 0.05 ± 0.02* | 0.01 ± 0.01 | −0.12 ± 0.05** |
| Pr. Hit | 0.01 ± 0.02 | −0.01 ± 0.01 | 0.07 ± 0.02* | 0.02 ± 0.03 | −0.21 ± 0.10* |
| Pr. FA | 0.01 ± 0.02 | −0.09 ± 0.02 | 0.03 ± 0.02ç | −0.01 ± 0.02 | 0.040 ± 0.05 |
Shown are the effects of MPH on different aspects of sustained attention performance. expressed as a change from baseline performance levels. Shown are the mean ± SEM for: d′, proportion correct (Correct), probability of a hit (Pr. Hit) and probability of false alarms (Pr. FA). Vehicle data are an average from the 3 arms of testing (see Methods/Results). 2.0 mg/kg data are the average from the 2nd arm of testing in which this dose was used.
P < 0.05,
P < 0.01 vs. vehicle-treatment.
As shown in Table 1, a virtually identical pattern was observed for most other measures of performance. Thus, probability of a hit was increased with 2.0 mg/kg MPH (F2, 12=7.57, p< 0.01; t6=3.71, p=0.004), with a strong trend for improvement at 0.5 mg/kg (t6=1.77, p=0.06). This effect of 2.0 mg/kg MPH was generally replicated in arm 2 testing (F2,12=1.40, p=0.29; t6=1.93, p=0.051). As with d′, 4.0 mg/kg did not alter while 8.0 mg/kg reduced the probability of a hit (t6=−2.34, p=0.03). MPH significantly affected the proportion of correct trials (arm 1, F2,12=5.51, p=0.02; arm 2, F2,12=3.15, p=0.08; arm 3, F1,6=14.81, p=0.008) with improvement seen at 0.5 mg/kg (t6 = 2.96, p = 0.012) and 2.0 mg/kg (arm 1, t6=2.84, p=0.015; arm 2, t6=3.35, p=0.007), no effect at 4.0 mg/kg (t6=−0.10, p=0.46) and a decrease in this measure at 8.0 mg/kg (t6=−3.85, p=0.004). MPH had less consistent effects on probability of false alarms (arm 1, F2,12=1.40, p=0.29; arm 2, F2,12=3.16, p= 0.08; arm 3; F1,6=0.67, p=0.44).
The above-described results were obtained in Madison, WI. Similar studies were conducted in Philadelphia, PA, where the effects of 0.5 mg/kg, 2.0 mg/kg and 3.5 mg/kg MPH (n = 6) were examined. These studies obtained nearly identical inverted-U dose-dependent effects of MPH on sustained attention, with maximal improvement in performance at 2.0 mg/kg MPH (see Table II; F1,5=1.55, p=0.27; t5=−3.37, p=0.02). The one difference observed across the two laboratories is that the 0.5 mg/kg dose of MPH did not significantly improve performance in these latter studies. This could be related to the fact that the shorter signal duration used in the Philadelphia laboratory appears to make the task more perceptually-challenging (d′ of saline-treated animals, Madison, WI = 2.1 ± 0.1; d′ of saline-treated animals, Philadelphia, PA = 0.6 ± 0.2) and thus possibly more dependent on the perceptual actions of methylphenidate, which largely occur at higher doses, including 2.0 mg/kg (see 23, 24). Additionally, Madison testing utilized 3 replications of each treatment, likely reducing variability in treatment effects.
MPH Exerts a Broad Inverted-U Shaped Facilitation of Extradimensional Attentional Set shifting
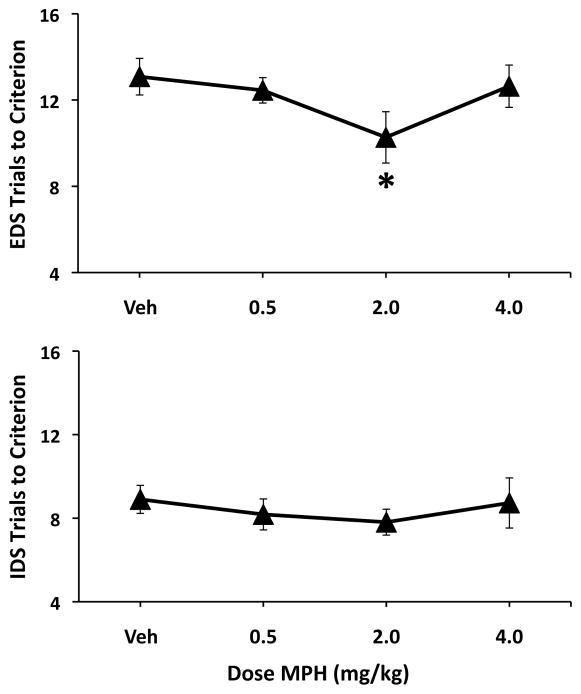
To determine whether the broader inverted-U dose response curve is unique to sustained attention, we examined the effects of MPH on attentional set shifting, a task that requires shifting of attention across intra- and extradimensional cues (Figure 2). The number of trials to reach criterion performance in the intradimensional shift was significantly lower than on the extradimensional shift (F1,30=26.18, p<0.001), indicating that shifting attention set is more difficult than maintaining attention set. All subjects required more trials to reach criterion performance on extradimensional reversals than intradimensional reversals (see Figure 2; F1,10=7.20, p=0.02). Planned comparisons demonstrated that 2.0 mg/kg MPH facilitated extradimensional shifts relative to vehicle treatment (t10=3.21, p=0.009). No other dose of MPH produced a significant change in extradimensional shifts (all p>0.32). MPH did not significantly affect intradimensional shifts (all p>0.19) or reversals (Dose: F3,30=0.37, p=0.78; Test × Dose F3,30=1.25, p=0.31).
Figure 2.
Dose-dependent improvement in attentional set shifting by MPH is similar to that seen in sustained attention testing. Shown are the effects of varying doses of MPH (0.5 mg/kg, 2.0 mg/kg and 4.0 mg/kg) on intradimensional (IDS) and extradimensional (EDS) tests. As shown in the top panel, MPH significantly improved EDS as indicated by the reduction in the number of trials needed to reach criterion performance on this test of attentional set shifting. Similar to that seen in the sustained attention task, maximal facilitation (as indicated by the number of trials to reach criterion) was observed at 2.0 mg/kg (IP), but not 4.0 mg/kg. There was no effect of the drug on the formation of the attentional set (bottom graph). *P < 0.05; **P < 0.01 relative to vehicle treatment.
α1-Receptor Activation is Necessary for the Facilitatory Effects of MPH on Sustained Attention
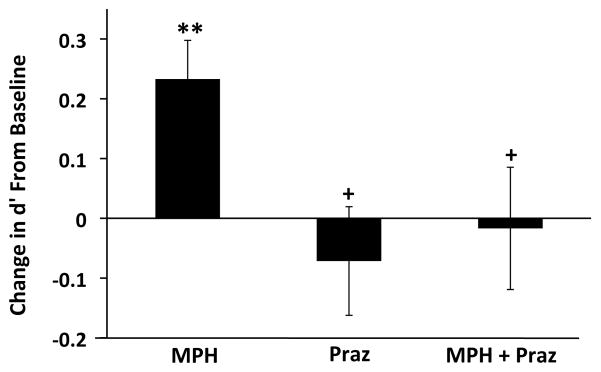
Prior studies demonstrate that attentional set shifting is facilitated by α1-receptor stimulation while α2-receptors facilitate working memory (18). Given similar actions of MPH on attentional set shifting and sustained attention, we hypothesized that α1-receptors may facilitate sustained attention, similar to attentional set shifting, and that this action of α1-receptors may contribute to the beneficial effects of MPH on sustained attention. Thus, the effects of pretreatment with the α1-antagonist, prazosin (0.5 mg/kg), on MPH (2.0 mg/kg)-induced improvement in sustained attention were examined (n=14). Animals were treated with either MPH, prazosin, or combined treatment with prazosin + MPH. ANOVA indicated a significant effect of treatment on performance relative to baseline (F3,39=4.01, p=0.01). As shown in Figure 3, 2.0 mg/kg MPH once again significantly improved sustained attention relative to baseline (t13=3.60, p<0.01) while prazosin alone did not affect sustained attention (t13=0.79, p=0.45). However, prazosin pretreatment completely blocked the beneficial effects of 2.0 mg/kg MPH, leaving performance indistinguishable from baseline (t13=0.16, p=0.87).
Figure 3.
α1-receptor blockade prevents MPH-induced improvement in sustained attention. Animals were treated with either: 1) 2.0 mg/kg MPH; 2) 0.5 mg/kg of the α1-receptor antagonist, prazosin; 3) 0.5 mg/kg prazosin + 2.0 mg/kg MPH. Shown are values for d′, expressed as a change from baseline. 2.0 mg/kg MPH improved performance in this task, similar to that seen in Figure 1. Treatment with prazosin had no effect on sustained attention performance. In contrast, this dose of prazosin completely blocked the facilitating effects of MPH on sustained attention. **P < 0.01 vs. baseline; +P < 0.05 vs. vehicle.
DISCUSSION
At clinically-relevant doses, psychostimulants improve a variety of PFC-dependent processes in ADHD patients as well as normal human and animal subjects (1, 9, 25). In humans, monkeys and rats, MPH elicits a narrow inverted-U-shaped improvement in PFC-dependent working memory and response inhibition (4, 5, 6, 9, 12, 25). However, the current studies demonstrate that the narrow inverted-U-shaped action of MPH is not universally observed across all PFC-dependent tasks. Specifically, in two additional tests of PFC-dependent cognition, sustained attention and attentional set shifting, MPH produced beneficial actions across a broader and/or higher dose range than seen in tests of working memory. Importantly, the differential dose sensitivity across tasks does not reflect a differential dependency on the PFC as inactivation/lesion of this area dramatically impairs performance in all of these tasks (15, 16). In particular, we recently observed that temporary inactivation of the medial PFC of rats decreases performance to chance levels in both working memory and sustained attention tasks (unpublished observations, RC Spencer and CW Berridge).
The narrow vs. broad/right-shifted dose-response curves observed in our studies are strikingly similar to those described in children with ADHD across PFC-dependent working memory/response inhibition tests (narrow) vs. overt behavior (broad; 6, 9). Moreover, a similar pattern in dose sensitivity to psychostimulants has been observed in preliminary studies in monkeys tested in a delayed-response task of working memory (narrow) vs. the ability of the animal to stay focused and engaged in the task (broad; 11). These observations indicate distinct PFC-dependent cognitive/behavioral processes vary in sensitivity to psychostimulant dose. The fact that these differing actions of MPH across tasks have been documented within and across laboratories as well as across species adds significant strength to this conclusion (see above, 4, 5, 10–13, 6).
Receptor and circuit mechanisms underlying differential dose sensitivity to psychostimulants
These studies further provide the first evidence that differences in sensitivity to psychostimulants across PFC-dependent tasks involve differential actions of noradrenergic α2- and α1-receptors. For example, an extensive body of work demonstrates that noradrenergic α1-receptor activation within the PFC impairs, while activation of postsynaptic α2-receptors improves working memory performance. Consistent with this, α2-receptor blockade prevents MPH-induced improvement in tests of working memory (5). However, a very different pattern of α1- vs. α2-receptor action is observed in attentional tasks that display broad/right-shifted dose-dependent actions of MPH. Specifically, prior work demonstrates that α1-receptors within the PFC facilitate attentional set shifting (in the absence of MPH), while α2-receptor activation fails to alter performance in this task (18). The current observations indicate a similar beneficial action of α1-receptors in sustained attention. Moreover, these observations demonstrate that α1-receptor activation is necessary for MPH-induced improvement in sustained attention. Although it remains unknown whether α1-receptors are necessary for MPH-induced improvement in attentional set-shifting remains, prior observations provide strong support for this hypothesis (18). Moreover, regardless of whether α1-receptors are necessary for MPH-induced improvement in attentional set shifting, it is clear that differing modulatory actions of α1- vs. α2-receptors on performance across these tasks are associated with differing sensitivity to psychostimulants.
Clinically-relevant doses of MPH that maximally improve working memory performance produce a relatively selective elevation of extracellular catecholamines within the PFC, while modestly higher doses affect extracellular catecholamine levels more broadly within the brain (19). Thus, the broader/right-shifted inverted-U curves seen in the sustained attention and attentional set shifting tasks could also reflect actions of MPH on catecholamine signaling in regions outside the PFC. In this regard, it is of interest that higher doses of MPH were observed to increase responsiveness of weakly responding neurons in somatosensory cortex (24), similar to that seen with α1-agonists (26). Therefore, the beneficial effect of higher doses of MPH on attentional processes may involve drug-induced alterations in α1-receptor signaling in posterior cortical regions. Clinically, the simultaneous targeting of α1-receptors in posterior cortices and PFC-promoting α2-receptors may explain why simultaneous treatment with α2A agonists and psychostimulants can be more efficacious than either treatment alone in some patients with ADHD (27). Moreover, attention- and/or sensory-related actions of α1-receptors may have implications for disorders outside ADHD, including post-traumatic stress disorder, which is associated with hypervigilance and treated pharmacologically with α1-antagonists (28, 29).
What Cognitive Processes Are Involved in the Differential Dose Sensitivity to Psychostimulants?
The cognitive processes that underlie the differential dose sensitivity across tasks are not clear. All of the tests used to study the cognitive/behavioral effects of low-dose psychostimulants require a variety of perceptual, cognitive and motivational processes. The current results indicate that the divergence of dose-response curves across PFC-dependent tasks cannot simply be ascribed to ‘focused’ vs. ‘flexible’ cognition given higher doses improved performance in both sustained attention (focused) and the attentional set shifting tasks (flexible). However, recent studies in monkeys demonstrate that at a dose of that improves focused attention, MPH impaired both working memory performance and a form of task switching involving suppression of prepotent responses, similar to tests of response inhibition (9, 11, 30). These observations suggest that tasks associated with narrow inverted-U shaped dose response curves may involve specific form(s) of cognitive flexibility and/or response inhibition. Further research is needed to identify the cognitive/behavioral processes that contribute to differing dose sensitivity of these tasks to low-dose psychostimulants.
Preclinical and Clinical Implications
Preclinically, the current observations raise the question of whether a subset of PFC-dependent cognitive tasks is more relevant for drug discovery programs focused on ADHD. Extensive evidence demonstrates that the pharmacology of delayed-response tests of working memory is in close alignment with the pharmacology of ADHD. Specifically, performance in these tasks is improved by all approved ADHD-related drugs, including α2A agonists (31), low-dose psychostimulants (25) and selective NE reuptake blockers (e.g. atomoxetine; 12). Moreover, at least in the case of psychostimulants, beneficial effects on working memory performance only occur across a narrow dose range that produce clinically-relevant plasma concentrations (11, 19, 31–34). Lastly, working memory performance of rats and monkeys is impaired by stressors known to impair PFC-dependent cognition in humans (e.g. Stroop test; 13, 35). These observations indicate that delayed response tests of working memory are a suitable preclinical tool for identifying potentially efficacious compounds for use in ADHD and other clinical conditions associated with PFC dysfunction (e.g. stress).
In contrast, in the sustained attention and attentional set shifting tasks, MPH facilitates performance at doses that produce plasma concentrations in rats estimated to exceed the clinically-relevant range (19, 33). Additionally, limited observations indicate that α2-agonists lack beneficial effects on attentional set shifting (18) as well as sustained attention (unpublished observations, JL Berkowitz, BD Waterhouse, JS Shumsky). Thus, the potential utility of these tests in a preclinical program focused on the pharmacology of ADHD is less clear. Further pharmacological research is needed to definitively address this issue.
Clinically, and as suggested by Sprague and Sleator (6) and Tannock and colleagues (9), the current results raise the question of whether doses that are optimal for controlling classroom behavior impair, or no longer improve, cognitive/behavioral processes important for other domains of academic and/or social functioning. For example, largely anecdotal evidence has suggested that psychostimulant treatment of ADHD can result in cognitive constriction or over-focusing (for review, 9). Such an action may be related to the ability of higher doses of MPH to improve attention while impairing certain forms of cognitive flexibility (9, 30). If so, the current results could suggest the use of α1-antagonists as a potential adjunct treatment for ADHD. Additional clinical studies are needed to better understand how the actions of differing doses of psychostimulants across differing tests of PFC-dependent cognition relate to clinical, social, and academic outcomes and the degree to which α1-receptors participate in adverse or beneficial clinical actions of psychostimulants and other drugs used in the treatment of ADHD.
Acknowledgments
This work was supported by PHS grants, MH081843, DA000389, DA017960, MH087921, the National Science Foundation (NSF 0918555), the Wisconsin Institutes of Discovery and the University of Wisconsin Graduate School and PA Tobacco Formula Funds.
Footnotes
Financial Disclosures: Dr. Berridge has received consulting fees from Shire Pharmaceuticals and Phase 2 Discovery, and expert witness fees from Teva Pharmaceutical, Activis, Aurobindo Pharmaceuticals, Mylan Pharmaceuticals, and Apotex. Dr. Devilbiss is the founder of NexStep Biomarkers, LLC. NexStep Biomarkers had no role in the preparation of this article or in the studies described in this article. Drs. Andrzejewski, McGaughy, Shumsky and Waterhouse and Mr. Spencer have no financial disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mehta MA, Sahakian BJ, Robbins TW. Comparative psycholpharmacology of methylphenidate and related drugs in human volunteers, patients with ADHD, and experimental animals. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. New York: Oxford University Press; 2001. pp. 303–331. [Google Scholar]
- 2.Rapoport JL, Buchsbaum MS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ. Dextroamphetamine - Its Cognitive and Behavioral-Effects in Normal and Hyperactive Boys and Normal Men. Archives of General Psychiatry. 1980;37:933–943. doi: 10.1001/archpsyc.1980.01780210091010. [DOI] [PubMed] [Google Scholar]
- 3.Rapoport JL, Inoff-Germain G. Responses to methylphenidate in Attention-Deficit/Hyperactivity Disorder and normal children: update 2002. J Atten Disord. 2002;6:S57–S60. doi: 10.1177/070674370200601s07. [DOI] [PubMed] [Google Scholar]
- 4.Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sprague RL, Sleator EK. Methylphenidate in hyperkinetic children: differences in dose effects on learning and social behavior. Science. 1977;198:1274–1276. doi: 10.1126/science.337493. [DOI] [PubMed] [Google Scholar]
- 7.Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behavioural Brain Research. 1998;94:127–152. doi: 10.1016/s0166-4328(97)00175-7. [DOI] [PubMed] [Google Scholar]
- 8.Rapport MD, Kelly KL. Psychostimulant effects on learning and cognitive function: findings and implications for children with attention deficit hyperactivity disorder. Clin Psych Rev. 1991;11:61–92. [Google Scholar]
- 9.Tannock R, Schachar R, Logan G. Methylphenidate and cognitive flexibility: dissociated dose effects in hyperactive children. J Abnorm Child Psychol. 1995;23:235–266. doi: 10.1007/BF01447091. [DOI] [PubMed] [Google Scholar]
- 10.Devilbiss DM, Berridge CW. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol Psychiatry. 2008;64:626–635. doi: 10.1016/j.biopsych.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zdrale A, Meier TB, Berridge CW, Populin LC. Effect of methylphenidate on monkey prefrontal cortex-mediated behavior. Soc Neurosci Abst. 2008:388.18. [Google Scholar]
- 12.Gamo NJ, Wang M, Arnsten AF. Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49:1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartley LR, Adams RG. Effect of noise on the Stroop Test. J Exp Psychol. 1974;102:62–66. doi: 10.1037/h0035695. [DOI] [PubMed] [Google Scholar]
- 14.Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- 17.Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 19.Berridge CW. Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology. 2006;31:2332–2340. doi: 10.1038/sj.npp.1301159. [DOI] [PubMed] [Google Scholar]
- 20.McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- 21.Geshcheider GA. Psychophysics: Method, Theory and Application. 2. Hillsdale, NJ: Lawerence Erlbaum Associates; 1985. [Google Scholar]
- 22.McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drouin C, Page M, Waterhouse B. Methylphenidate enhances noradrenergic transmission and suppresses mid- and long-latency sensory responses in the primary somatosensory cortex of awake rats. J Neurophysiol. 2006;96:622–632. doi: 10.1152/jn.01310.2005. [DOI] [PubMed] [Google Scholar]
- 24.Drouin C, Wang D, Waterhouse BD. Neurophysiological actions of methylphenidate in the primary somatosensory cortex. Synapse. 2007;61:985–990. doi: 10.1002/syn.20454. [DOI] [PubMed] [Google Scholar]
- 25.Berridge CW, Devilbiss DM. Psychostimulants as Cognitive Enhancers: The Prefrontal Cortex, Catecholamines, and Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devilbiss DM, Waterhouse BD. Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse. 2000;37:273–282. doi: 10.1002/1098-2396(20000915)37:4<273::AID-SYN4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Spencer TJ, Greenbaum M, Ginsberg LD, Murphy WR. Safety and effectiveness of coadministration of guanfacine extended release and psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:501–510. doi: 10.1089/cap.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berridge CW. The Locus Coeruleus-Noradrenergic System and Stress: Implications for Post-Traumatic Stress Disorder (PTSD) In: Shiromani PJ, Keane TM, Ledoux JE, editors. Posttraumatic Stress Disorder: Basic Science and Clinical Practice. New York, NY: Humana/Springer; 2008. [Google Scholar]
- 29.Southwick SM, Bremner JD, Rasmusson A, Morgan CA, III, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 30.Rajala A, Reininger LC, Populin LC. Effect of methylphenidate on PFC-mediated task-switching behavior in monkeys. Soc Neurosci Abst. 2010:805.18. [Google Scholar]
- 31.Arnsten AF. The use of α-2A adrenergic agonists for the treatment of attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2010;10:1595–1605. doi: 10.1586/ern.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther. 2001;296:876–883. [PubMed] [Google Scholar]
- 33.Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doerge DR, Fogle CM, Paule MG, McCullagh M, Bajic S. Analysis of methylphenidate and its metabolite ritalinic acid in monkey plasma by liquid chromatography/electrospray ionization mass spectrometry. Rapid Communications in Mass Spectrometry. 2000;14:619–623. doi: 10.1002/(SICI)1097-0231(20000430)14:8<619::AID-RCM916>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Arnsten AF. The biology of being frazzled. Science. 1998;280:1711–1712. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]