Abstract
Background
Previous research suggests that technology-enabled healthcare delivery may improve access to dermatologic specialty care. Outcomes research utilizing validated outcomes measures is necessary for evaluation of novel healthcare delivery models.
Objective
To compare the clinical equivalence of a novel patient-centered online healthcare delivery model with standard in-office care for follow-up management of psoriasis patients.
Methods
Sixty-four participants with psoriasis were randomized to receive follow-up care either in-office or online over a 24-week period. Patients randomized to the online group underwent standardized training on capturing high-quality digital images of their psoriatic skin and transmitting these images and clinical history to a dermatologist securely. The dermatologist then performed asynchronous, online evaluation and provided recommendations directly to patients. We used clinically validated disease severity and quality of life measures to assess effectiveness between the models.
Results
Both online and in-office groups showed improvement in psoriasis disease severity as measured by mean improvement in Psoriasis Area and Severity Index (PASI) (online group: μ = −3.4,, in-office: μ = −3.4,). Patient-centered online care resulted in similar improvement in psoriasis severity compared to in-person follow-up care (mean difference in PASI change 0.1, 95% CI: −2.2 to 2.3, a priori equivalence margin of 2.5). Investigator’s Global Assessment (IGA) and Dermatology Life Quality Index (DLQI) scores also improved during the study period; no significant differences existed between the two groups.
Limitations
The follow-up period was limited to 24 weeks.
Conclusion
A patient-centered online model may be an effective alternative to in-office care for follow-up management of psoriasis.
Keywords: Psoriasis, comparative effectiveness, equivalency trial, randomized controlled clinical trial (RCT), telemedicine, e-medicine, e-health, teledermatology, store-and-forward teledermatology, asynchronous teledermatology
INTRODUCTION
Patient access to dermatologists is limited in many rural and medically underserved urban communities, which results in long waiting periods for new and urgent visits.1–4 Telemedicine has been utilized as a mechanism to improve access as well as cost-effectiveness of health care services. Studies suggest that asynchronous teledermatology may improve patient satisfaction and increase access to dermatologic specialty care.5–11
Traditionally, store-and-forward telemedicine has been used in a consultative fashion with the primary care provider sending the necessary skin images and patient history to the dermatologist; this requires investment of time and resources by the primary care physician. The model used in this study leverages secure, online communication to provide patient-centered care that requires no intermediary healthcare provider.
In this innovative model of care delivery, patients submit skin images and clinical history directly to their dermatologist via a secure online site. This model allows for direct provision of care between the patient and dermatologist that is time and location-independent, and it eliminates patient travel and potential lost work from medical visits. This type of patient-centered online healthcare delivery method is most likely beneficial for follow-up management of patients with chronic dermatoses such as acne,12 eczema, and psoriasis.
Psoriasis is a chronic, inflammatory skin disease affecting approximately 2% of the American population.13 In addition to coping with medical aspects of this disease, patients face significant psychosocial burden.14 Affected individuals often take time away from work or school activities at regular intervals to seek dermatological care. A thoughtfully developed telehealth program has the potential to improve access, clinical outcomes, and quality of life for patients with psoriasis.
The purpose of this comparative effectiveness study was to assess the clinical equivalence of conventional, in-person dermatology with online, patient-centered care for follow-up management of psoriasis. We hypothesized that, compared to in-office care, an asynchronous patient-centered online model would provide similar improvements in psoriasis disease severity and quality of life.
METHODS
Study Design and Subjects
We conducted a randomized controlled equivalency trial comparing the clinical outcomes of subjects randomized to an online healthcare delivery modality with those followed in a traditional office-based setting. The study protocol was approved by the institutional review board of the University of California Davis School of Medicine, and all subjects provided written informed consent. Participants were recruited between August 2009 and January 2010 in Sacramento, California. Participants were eligible for the study if they were eighteen years or older with a diagnosis of psoriasis, spoke and read English, had access to a computer with Internet connection and a digital camera, and were able to have their skin imaged by themselves or someone else. Subjects were not excluded based on severity of disease, insurance status, or baseline treatment regimen. This trial was registered on clinicaltrials.gov NCT00971477.
Study Schedule and Interventions
Following confirmation of diagnosis and informed consent, all subjects participated in an initial in-clinic visit to obtain baseline images and study assessments. At this visit, the dermatologist completed a baseline Psoriasis Area Severity Index (PASI)15 and Investigator Global Assessment (IGA), and each participant completed a baseline Dermatology Life Quality Index (DLQI). Study staff then took a series of 8 standardized photographs of each subject which included four full body poses (full front, full back, right side, left side) as well as four additional images of typical psoriatic lesions (or of healthy skin if no disease could be found) in each of the four regions of the body represented in the Psoriasis Area Severity Index (PASI) score (head, arms, trunk and legs).
Participants randomized to the online group received additional training at this baseline visit on how to take a standardized set of high quality digital photographs of their skin as well as how to complete a secure online visit in the e-medicine platform, RelayHealth®. Patients in the online group were asked to submit: (1) a completed psoriasis specific skin questionnaire, (2) a quality of life survey (DLQI), and (3) eight digital photographs (four full body images & four close-up images of representative lesions from each region of the body) to their personal RelayHealth® account. Participants without disease in a specific body region were instructed to include photographs of healthy skin in that region. Completed online visits were instantaneously sent to the dermatologist who responded within three business days with recommendations and prescribed prescriptions electronically.
All participants had two follow-up visits either in person or online spaced eight weeks apart. During the follow-up visits, standardized photographs, PASI and IGA assessments, and quality-of-life assessments were obtained from both groups. At the conclusion of the study, all subjects returned for a final in-clinic visit, where the dermatologist completed PASI and IGA assessments, and each subject completed an outcome quality of life survey (DLQI).
Outcome measures
The primary end point of the study was the extent and severity of psoriasis as measured by PASI between the first and final study visits. PASI is a validated psoriasis severity instrument that includes dimensions of erythema, plaque thickness, scale, and the extent of surface area involvement in each of four regions of the body--head, trunk, arms and legs.15 Secondary outcome measures included (1) IGA and (2) DLQI. The IGA is a physician’s global assessment of a patient’s psoriasis disease severity based on a 6-point scale. The DLQI is a ten-question dermatology specific instrument that is used to assess disease-related impact on quality of life; the scores range from 0 to 30, with higher scores indicating more severe impact on quality of life.16 All of these measures have been well validated and are commonly used in the evaluation of psoriasis in clinical trials.15
Sample Size and Randomization
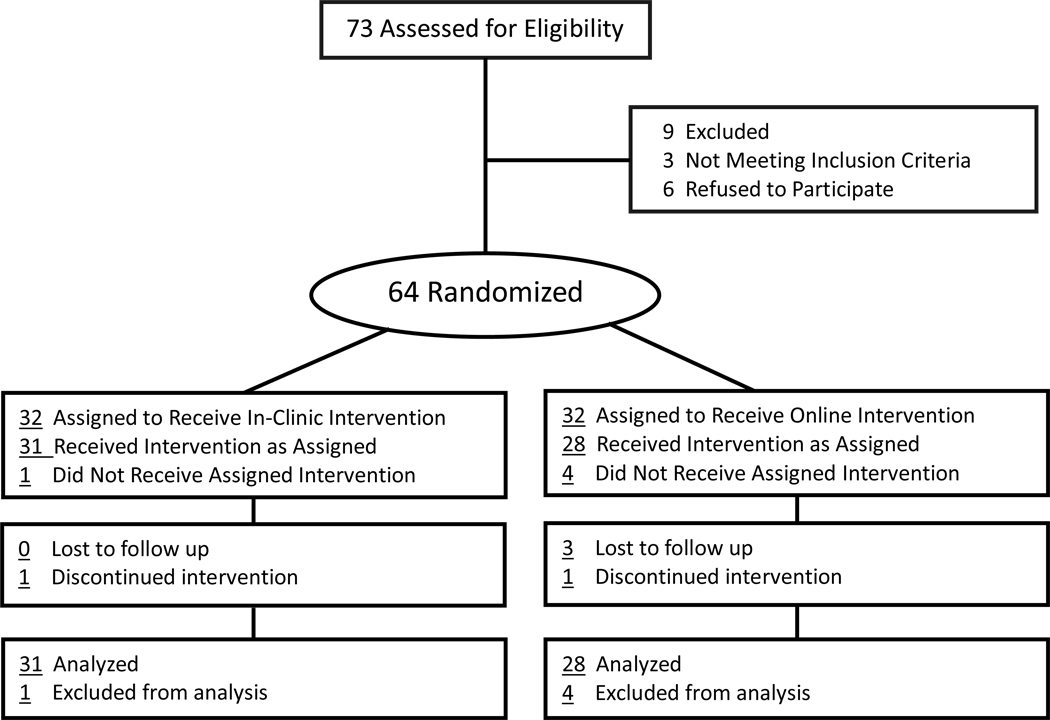
A simple 1:1, non-stratified randomization scheme was generated using equivalence criterion in STATA 11 (College Station, Texas). Randomization sequences were concealed within numbered envelopes until interventions were assigned. A total of 64 patients were randomly assigned to either the in-clinic or online treatment groups (Figure 1). With a sample size of 32 subjects in each arm, the study had a 90% power to detect a minimal clinically relevant mean difference (Δ) of 2.5 points in the PASI score, assuming a standard deviation of 3.0 and a two-tailed α of 0.05.
Figure 1.
Randomization Scheme
Statistical Analysis
Data were examined based on per-protocol analysis because, unlike superiority trials, per-protocol is the more conservative statistical approach for equivalency trials. Independent two-sample t-tests were used to compare treatment groups’ demographic variables and outcome measures at baseline and each of the follow-up visits, as well as the change in measure from baseline to final visit. Categorical measures were compared between groups using chi-square (Χ2) or Fisher exact tests as appropriate. All results achieving a two-tailed P-value less than 0.05 were considered statistically significant. Calculations were performed with SAS software, version 9.1 (SAS Institute Inc, Cary, North Carolina). Figures were created using R Statistical Package, version 2.10.1 (The R Foundation for Statistical Computing).
RESULTS
Study Sample
Recruitment began in August 2009 and continued until January 2010 at the UC Davis Department of Dermatology in Sacramento, California. A total of 73 subjects were assessed for eligibility, and 64 were randomly assigned to the two treatment groups. Of the nine patients that were excluded, three did not meet inclusion criteria (two did not have access to a digital camera), and six declined to participate (Figure 1). Baseline characteristics of the two treatment groups were well matched with the exception of age, where the online group was on average seven years older than the in-office group (Table I). Approximately 58% of the participants were male, and the average duration of psoriasis was approximately 15.5 years. The baseline mean PASI scores were not significantly different between the groups. Numbers analyzed in the per protocol sample were 31 and 28 for the in-office and online groups respectively.
Table I.
Baseline Demographics
| Characteristic | In-Clinic | Online | P-Value |
|---|---|---|---|
| Age | 43 | 51 | 0.01 |
| Sex | 0.20 | ||
| Male | 21 | 16 | |
| Female | 11 | 16 | |
| Race/Ethnicity (White) | 39% | 35% | 0.44 |
| PASI | 7.88 | 7.73 | 0.93 |
| IGA | 2.41 | 2.44 | 0.91 |
| DLQI | 8.75 | 8.62 | 0.91 |
Primary outcome measure
The changes in PASI scores from baseline to final visit were compared between groups (Table II). Psoriasis patients randomized to the online group had a significant decrease in disease severity, as assessed by mean change in PASI scores (μ = −3.4, 95% CI: −4.5, −2.4). Similarly, patients in the in-office group experienced a significant decrease in disease severity based on the mean change in PASI (μ = −3.4, 95% CI: −5.3, −1.4). The changes in PASI scores between the two intervention groups were within the pre-specified equivalency margin (mean difference in PASI change 0.1, 95% CI: −2.2 to 2.3), which indicated clinical equivalence between the study arms (Figure 2).
Table II.
Comparison of PASI and DLQI Changes between Treatment Groups (per protocol)
| In-Office | Online | Group Comparisons | ||||||
|---|---|---|---|---|---|---|---|---|
| Measure | n | μ1* | 95% CI | n | μ2** | 95% CI | μ1 – μ2 | 95% CI |
| PASI | 31 | −3.4 | (−5.3, −1.4) | 28 | −3.4 | (−4.5, −2.4) | 0.1 | (−2.2, 2.3) |
| DLQI | 31 | −4.9 | (−7.3, −2.4) | 28 | −3.8 | (−5.5, −2.1) | −1.1 | (−4.1, 2.0) |
Indicates change in PASI or DLQI score between the first and last visit in the in-office group.
Indicates change in PASI or DLQI scores between the first and last visit in the online group.
Figure 2.
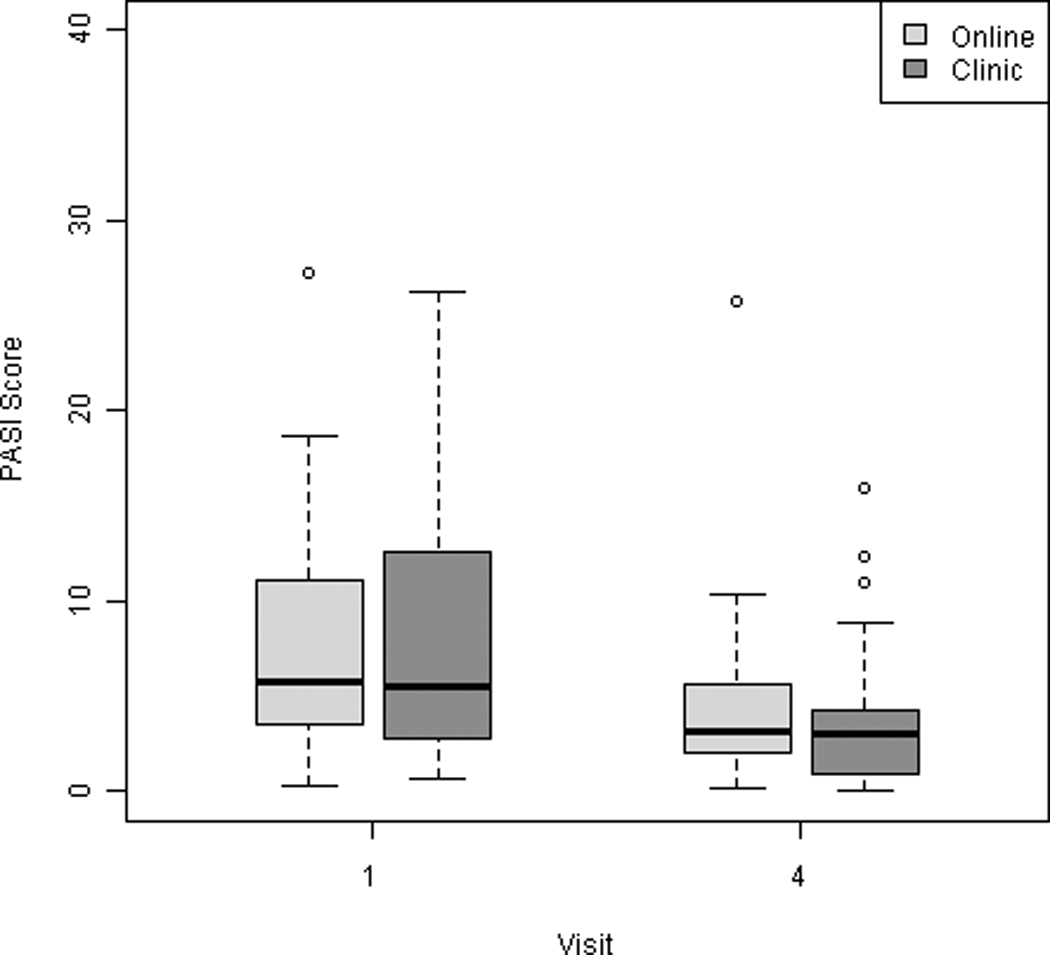
Changes in PASI score between the first and last study visits.
Secondary outcome measures
The IGA was analyzed between groups at each of the four visits using a chi-square test. Chi-square analysis of the frequencies found that the groups were not significantly different in their categorical distribution during any of the visits (baseline: p= 0.74, follow-up 1: p= 0.80, follow-up 2: p= 0.16, outcome visit: p= 0.70). Additionally, the proportion of patients who had cleared disease based on the physician’s global assessment score at final visit was not significantly different between the control and intervention group.
Finally, the DLQI was analyzed at each of the four visits, and group comparisons were made using independent two-sample t-tests. In addition, the change in DLQI score from baseline to final visit was calculated and compared between groups. DLQI values had a steady decline in average score over time, and both groups showed a significant within-group improvement in quality of life over the course of the study via repeated measures analysis (online group: μ = −3.8, 95% CI: −5.5, −2.1; in-office group: μ = −4.9, 95% CI: −7.3, −2.4) (Figure 3). Between-group comparisons showed that no significant differences existed in the degree of improvement between online versus in-person care (mean difference in DLQI change 1.1, 95% CI −4.1 to 2.0)
Figure 3.
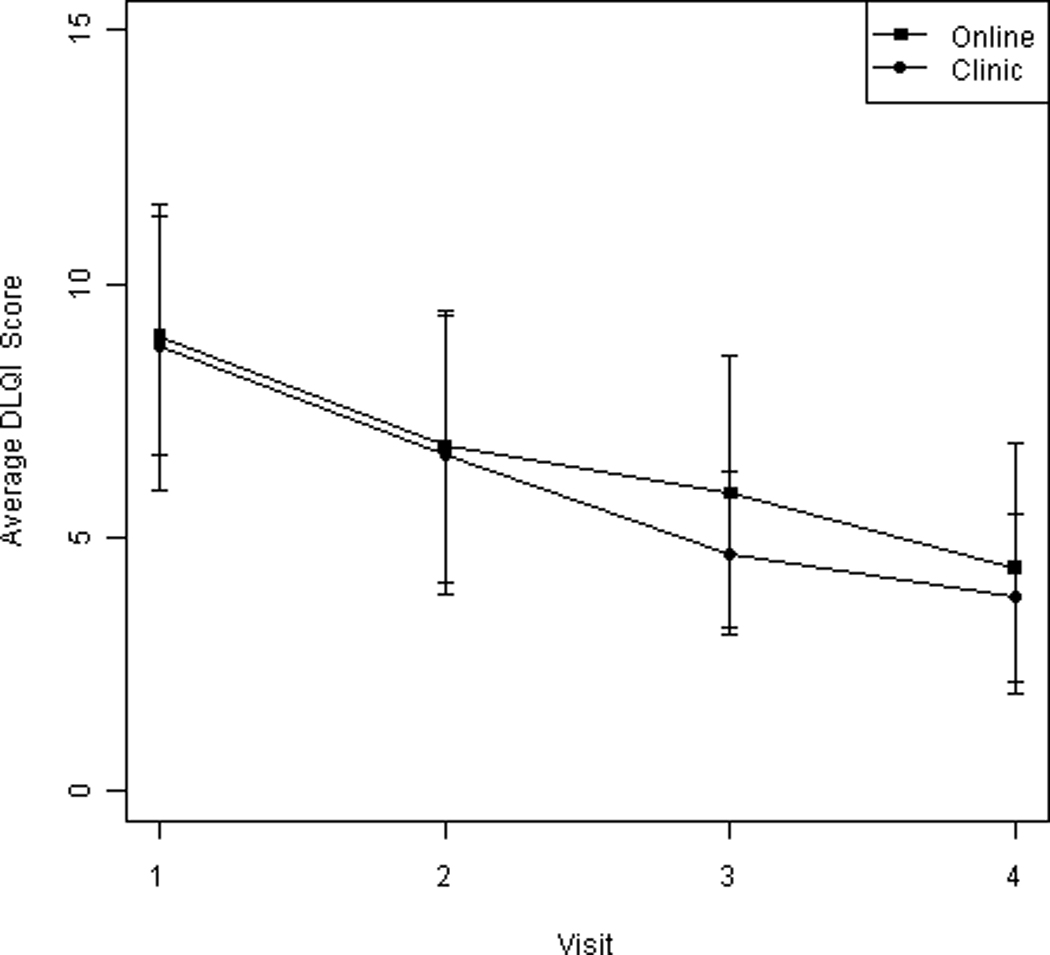
Average change in DLQI scores over the course of the study.
DISCUSSION
With the advancement in healthcare delivery technology, prudent and appropriate use of health information technology may present a feasible solution to improve access to quality specialist care and contain costs. The legitimate value of technology-enabled healthcare delivery will depend on selected and relevant applications based on the nature of the disease as well as technological capabilities. Follow-up management of chronic medical conditions is likely one of the areas in healthcare delivery where technology-enabled models will serve the greatest benefit.
The purpose of this study was to determine if regular follow-up via online, patient-centered teledermatology could produce equivalent clinical and quality of life outcomes compared to standard in-clinic follow-up care for the management of psoriasis. Patients in both the online and in-clinic groups experienced clinically equivalent reduction in psoriasis disease severity, and we found no significant differences in mean changes in their quality of life.
Outcomes-based controlled trials examining technology-enabled models of healthcare delivery in dermatology are scarce.17,12 Pak et al. examined a traditional store-and-forward teledermatology model, where primary care providers sent skin images and patient history to the dermatologist, and found that clinical outcomes were equivalent to that of in-office care.17 Watson and Bergman et al. evaluated clinical outcomes from acne patients who conducted electronic visits online and found that outcomes were similar compared to those seen in-office.12 The findings from this study corroborate the previous studies and extend our knowledge on patient-centered online healthcare delivery models for management of chronic dermatological conditions.
The unique strengths of the present study are the use of validated, disease-specific clinical outcomes and generalizability of results to psoriasis patients with varying disease severity. While the total sample size is relatively conservative, the demographic characteristics of the study population are diverse and varied in age, race and economic status. While younger individuals have traditionally been perceived to be better able to navigate the internet and use a digital camera, our study found that older psoriasis patients were able to actively participate in the online care model. Specifically, 53% of online participants were over 50 years old with a range of 27 to 78 years old. Several participants of varying ages reported recruiting family members or close friends to assist in the photo taking and navigation of the online interface.
There were several limitations to this study. Although most patient-transmitted photographs were appropriate for PASI assessment, we found that scalp lesions are often difficult to assess through the photographs due to the presence of hair. Furthermore, long-term effectiveness of patient-centered online care beyond 24 weeks in psoriasis patients will need to be evaluated in future studies with longer follow-up periods.
Patient-centered care considers patient preference, volition, and engagement in one’s own healthcare; a philosophy of care that may be ideally integrated into emerging online healthcare delivery applications. The results of this study suggest that patient-centered, online care for the follow-up management of psoriasis patients is an effective alternative to standard in-office visits. While this innovative patient-centered model has now been studied in the short term management of both acne and psoriasis, it may also be especially well suited for other chronic, low-risk dermatologic diseases as well as chronic non-dermatologic diseases with physiologic or visual parameters amenable to remote monitoring. In an ongoing randomized controlled trial, our group is additionally investigating the effectiveness of this patient-centered online model in patients with atopic dermatitis.
While not all patients may be initially suitable to participate in patient-centered online care, this novel health care delivery model appears to be effective in providing quality care to patients with selected chronic dermatologic conditions who are trained in using online transmission of health information. Future studies with longer follow-up periods will be valuable in understanding long-term effectiveness of this novel healthcare delivery model.
ACKNOWLEDGEMENTS
This publication was made possible by Grant Number UL1 RR024146 from the National Center for Research Resources. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
The authors gratefully acknowledge the contributions of all the study site personnel including Sandy Fleener, Jamie Chapman, Lynda Ledo and Caitlin Harskamp as well as Drs. Fu-Tong Liu, Nicholas Kenyon, Stephen McCurdy, Saul Schafer and Fredrick Meyers for their invaluable mentorship, support, and critical evaluation in the development and implementation of this research project. We also thank Professor Andrew Y. Finlay for permission to use the Dermatology Life Quality Index (DLQI).
Abbreviation and Acronym List
- PASI
Psoriasis Area and Severity Index
- IGA
Investigator Global Assessment
- DLQI
Dermatology Life Quality Index
- RCT
randomized controlled trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: None reported
Disclosure statement of potential conflicts of interest: Dr. Armstrong is an investigator and consultant to Abbott and Centocor.
Prior Presentation: This research has been presented in oral and poster format at the 2010 Association for Clinical Research Training/Society for Clinical and Translational Science (ACRT/SCTS) in Washington DC, and the 2010 National Predoctoral Clinical Research Training Program Meeting, in St. Louis, MO.
References
- 1.Bystryn JC. Dermatology manpower needs. Dermatol Clin. 2000;18:303–312. doi: 10.1016/s0733-8635(05)70176-5. x. [DOI] [PubMed] [Google Scholar]
- 2.Resneck J, Jr., Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50–54. doi: 10.1016/j.jaad.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Suneja T, Smith ED, Chen GJ, Zipperstein KJ, Fleischer AB, Jr., Feldman SR. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303–1307. doi: 10.1001/archderm.137.10.1303. [DOI] [PubMed] [Google Scholar]
- 4.Tsang MW, Resneck JS., Jr. Even patients with changing moles face long dermatology appointment wait-times: a study of simulated patient calls to dermatologists. J Am Acad Dermatol. 2006;55:54–58. doi: 10.1016/j.jaad.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Collins K, Walters S, Bowns I. Patient satisfaction with teledermatology: quantitative and qualitative results from a randomized controlled trial. J Telemed Telecare. 2004;10:29–33. doi: 10.1258/135763304322764167. [DOI] [PubMed] [Google Scholar]
- 6.Eminovic N, Witkamp L, de Keizer NF, Wyatt JC. Patient perceptions about a novel form of patient-assisted teledermatology. Arch Dermatol. 2006;142:648–649. doi: 10.1001/archderm.142.5.648. [DOI] [PubMed] [Google Scholar]
- 7.Krupinski E, Barker G, Rodriguez G, Engstrom M, Levine N, Lopez AM, et al. Telemedicine versus in-person dermatology referrals: an analysis of case complexity. Telemed J E Health. 2002;8:143–147. doi: 10.1089/15305620260008075. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi AA, Brandling-Bennett HA, Giberti S, McClure D, Halpern EF, Kvedar JC. Evaluation of digital skin images submitted by patients who received practical training or an online tutorial. J Telemed Telecare. 2006;12:79–82. doi: 10.1258/135763306776084392. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi AA, Brandling-Bennett HA, Wittenberg E, Chen SC, Sober AJ, Kvedar JC. Willingness-to-pay stated preferences for telemedicine versus in-person visits in patients with a history of psoriasis or melanoma. Telemed J E Health. 2006;12:639–643. doi: 10.1089/tmj.2006.12.639. [DOI] [PubMed] [Google Scholar]
- 10.Weinstock MA, Nguyen FQ, Risica PM. Patient and referring provider satisfaction with teledermatology. J Am Acad Dermatol. 2002;47:68–72. doi: 10.1067/mjd.2002.119666. [DOI] [PubMed] [Google Scholar]
- 11.Whited JD, Hall RP, Foy ME, Marbrey LE, Grambow SC, Dudley TK, et al. Patient and clinician satisfaction with a store-and-forward teledermatology consult system. Telemed J E Health. 2004;10:422–431. doi: 10.1089/tmj.2004.10.422. [DOI] [PubMed] [Google Scholar]
- 12.Watson AJ, Bergman H, Williams CM, Kvedar JC. A randomized trial to evaluate the efficacy of online follow-up visits in the management of acne. Arch Dermatol. 2010;146:406–411. doi: 10.1001/archdermatol.2010.29. [DOI] [PubMed] [Google Scholar]
- 13.Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136–139. doi: 10.1046/j.1087-0024.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- 14.Hayes J, Koo J. Psoriasis: depression, anxiety, smoking, and drinking habits. Dermatol Ther. 2010;23:174–180. doi: 10.1111/j.1529-8019.2010.01312.x. [DOI] [PubMed] [Google Scholar]
- 15.Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64(Suppl 2):ii65–ii68. doi: 10.1136/ard.2004.031237. discussion ii9–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159:997–1035. doi: 10.1111/j.1365-2133.2008.08832.x. [DOI] [PubMed] [Google Scholar]
- 17.Pak H, Triplett CA, Lindquist JH, Grambow SC, Whited JD. Store-and-forward teledermatology results in similar clinical outcomes to conventional clinic-based care. J Telemed Telecare. 2007;13:26–30. doi: 10.1258/135763307779701185. [DOI] [PubMed] [Google Scholar]