RAF kinases regulate cell proliferation and survival and can be dysregulated in tumors1,2. A role for RAF in cell proliferation has been linked to its ability to activate MEK and ERK. Here, we identify a MEK-independent role for RAF in tumor growth. Specifically, in mitotic cells, CRAF becomes phosphorylated on serine 338 and localizes to the mitotic spindle of proliferating tumor cells in vitro and in murine tumor models or biopsies from cancer patients. Treatment of tumors with allosteric, but not ATP-competitive RAF inhibitors prevents CRAF phosphorylation on serine 338, localization to the mitotic spindle and causes cell cycle arrest at pro-metaphase. Furthermore, we identify phospho-S338 CRAF as a potential biomarker for tumor progression and a surrogate marker for allosteric RAF blockade. Mechanistically, CRAF, but not BRAF associates with Aurora-A and Polo-like kinase 1 at the centrosomes and spindle poles during G2/Mitosis. Indeed, allosteric or genetic inhibition of phospho-S338 CRAF impairs Plk1 activation and accumulation at kinetochores causing pro-metaphase arrest, while a phospho-mimetic S338D CRAF mutant potentiates Plk1 activation, mitosis and tumor progression in mice. These findings reveal a previously undefined role for RAF in tumor progression beyond the RAF-MEK-ERK paradigm, opening new avenues for targeting RAF in oncology.
ATP-competitive RAF inhibitors have shown clinical activity in cancers with activating RAF mutations3–8. However, in RAS driven tumors, these agents surprisingly promote CRAF serine 338 phosphorylation and tumor progression9–11. We described an allosteric inhibitor of RAF (compound 6, referred to here as KG5) that does not compete for ATP but inhibits phospho-S338 CRAF and tumor growth12.
KG5 exerted broad growth inhibitory activity against the NCI-60 panel (Supplementary Table 1) suggesting that KG5 influences a general mechanism that is independent of RAS or RAF activation status. Tumor cells exposed to KG5 appeared rounded and arrested in mitosis, whereas cells treated with ATP-competitive RAF or MEK inhibitors maintained adhesion and mitosis (Supplementary Figs. 1a and 2). KG5 inhibited phospho-S338 CRAF causing mitotic arrest at pro-metaphase followed by cell death in multiple tumor cell lines (Supplementary Figs. 1a, b, d, e, 2 and 3), mimicking the effects of paclitaxel. As for most kinase-targeted drugs, KG5 inhibits other targets (c-Kit, Flt-3 and PDGFR) that might contribute to the anti-proliferative effects observed. To address this, we evaluated KG1, a structural analogue of KG5 that inhibits the same targets except RAF12. KG1 did not inhibit cell proliferation, indicating the effects of KG5 are due to RAF inhibition (Supplementary Fig. 1a).
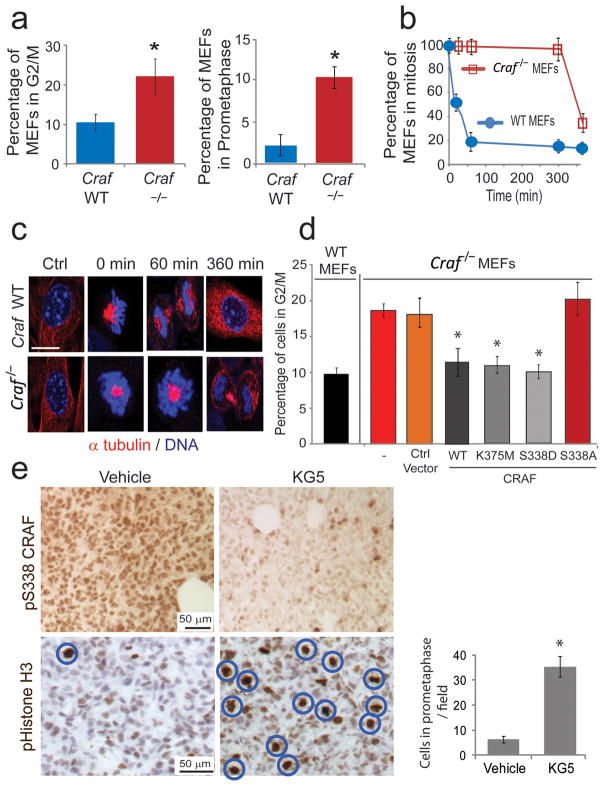
Phosphorylation of CRAF on serine 338 has been linked to cancer progression9–11,13–15. To determine whether KG5 could suppress this activity in vivo, we analyzed orthotopic breast and brain tumor tissues for phospho-S338 CRAF before or after systemic delivery of the drug. A single KG5 treatment led to a qualitative decrease in phospho-S338 CRAF and an accumulation of cells at pro-metaphase (Fig. 1e and Supplementary Fig. 4b). Importantly, while ectopic expression of active MEK failed to rescue the effects of KG5 on cell proliferation, cells expressing a phospho-mimetic S338D CRAF mutant showed resistance to KG5 (Supplementary Figs. 1c, e) suggesting CRAF S338 plays a MEK-independent role in cell proliferation that can be reversed by an allosteric inhibitor.
Figure 1. CRAF is required for mitotic progression.
(a) Cell cycle analysis of WT and Craf−/− MEFs. Left graph, Cells in G2/M were quantified by flow cytometry. Right graph, Cells at pro-metaphase were quantified using confocal microscopy. Error bars represent s.d. (n = 4); * P = 0.0079 (left graph) and P = 0.0055 (right graph) using a Mann Whitney U test. (b) WT and Craf−/− MEFs were synchronized at pro-metaphase with a thymidine-nocodazole block and subsequently released from the blockade and allowed to progress through mitosis. Quantification of cells in G2/M was performed by flow cytometry. Error bars represent s.d. (n = 3). (c) Confocal microscopy images of WT and Craf−/− cells progressing through mitosis at 0, 60 and 360 min after release from pro-metaphase blockade. Cells were stained for α-tubulin (in red) and DNA (TOPRO-3 in blue). Scale bar, 10 μm. (d) WT and Craf−/− MEFs were transfected with vector control, WT CRAF, kinase dead (K375M) CRAF, phospho-mimetic (S338D) CRAF or non-phosphorylatable (S338A) CRAF mutants. Cell cycle analysis and quantification of cells in G2/M was performed by flow cytometry. Error bars represent s.d. (n = 3); * P = 0.0084 using a Mann Whitney U test. (e) Immunohistochemical staining of phospho-S338 CRAF and phospho-histone H3 (mitotic marker) in orthotopic breast and tumor xenografts untreated or treated systemically with 50 mg kg−1 KG5 for 3 d. Scale bar, 50 μm. Circles indicate cells in pro-metaphase. Right, Quantification of cells in pro-metaphase. Error bars represent s.d. (n = 12); * P = 0.006 using Student’s t-test.
We next examined mouse embryonic fibroblasts (MEFs) derived from CRAF knockout embryos (Craf−/−)16. Asynchronized Craf−/− MEFs showed a significant increase in the number of cells in G2/M and pro-metaphase, compared to wildtype (WT) MEFs (Fig. 1a and Supplementary Fig. 5a). Following synchronization, Craf−/− MEFs accumulated in pro-metaphase with a dramatic delay in mitotic progression (Figs. 1b, c). Depletion of CRAF in tumor cells, similarly led to an accumulation of cells in G2/M (Supplementary Fig. 5e). Importantly, expression of kinase dead (K375M), phospho-mimetic S338D or WT CRAF rescued this mitotic defect, whereas a non-phosphorylatable (S338A) CRAF mutant, failed to do so (Fig. 1d and Supplementary Figs. 5b–d), even though this mutant maintains kinase activity and can dimerize with BRAF (data not shown). Accordingly, XPA-1 human pancreatic cancer cells expressing WT CRAF completed mitosis in twenty minutes whereas cells expressing CRAF S338A did so in 120 minutes (Supplementary Figs. 5f, g) providing genetic evidence to support a kinase-independent role for phospho-S338 CRAF in mitosis.
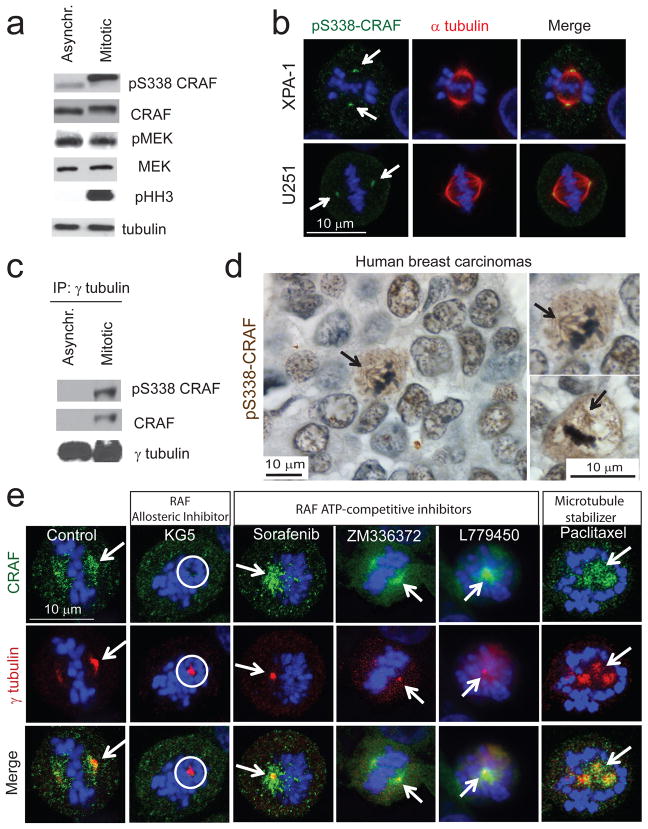
Furthermore, phospho-S338 CRAF was strongly increased in mitosis where it co-precipitated with γ-tubulin (Figs. 2a and 2c) and localized in G2/M to the centrosomes/mitotic spindle poles of tumor cells in vitro (Fig. 2b and Supplementary Fig. 6), or breast cancer tissues from mice or patients (Fig. 2d and Supplementary Fig. 7b). Importantly, KG5, but not ATP-competitive RAF inhibitors, prevented this localization (Fig. 2e). Moreover, in cells treated with the microtubule stabilizing agent paclitaxel, CRAF remained localized to the mitotic spindle suggesting that KG5 and paclitaxel arrest cells at pro-metaphase via distinct mechanisms (Fig. 2e).
Figure 2. Phospho-serine 338 CRAF is upregulated in mitosis and localizes to mitotic spindles in human cell lines and tumor biopsies.
(a) Immunoblot analysis of human colon carcinoma HCT-116 cells asynchronized and synchronized at pro-metaphase. pS338 refers to phospho-S338 CRAF, pMEK refers to phospho-MEK and pHH3 refers to phospho-histone H3. Data are representative of three independent experiments. (b) Confocal microscopy images of human pancreatic XPA-1 and glioblastoma U251 cells during mitosis, stained for phospho-S338 CRAF (in green), α-tubulin (in red) and DNA (TOPRO-3 in blue). Scale bar, 10 μm. White arrows indicate localization of phospho-S338 CRAF at the mitotic spindle. (c) Immunoblot analysis of γ-tubulin immunoprecipitates from human colon carcinoma HCT-116 cells asynchronized and synchronized at pro-metaphase. Data are representative of three independent experiments. (d) Immunohistochemical staining of phospho-S338 CRAF in tumor biopsies from breast cancer patients. Scale bar, 10 μm. (e) Confocal microscopy images of XPA-1 cells treated with KG5, sorafenib, ZM336372, L779450 or paclitaxel and stained for CRAF (in green), γ-tubulin (in red) and DNA (TOPRO-3 in blue). Scale bar, 10 μm. White arrows indicate localization of CRAF at the spindle pole. White circle indicates the absence of CRAF at the spindle pole.
Previous studies have revealed that CRAF and BRAF can form heterodimers17 and that BRAF can play a role in mitosis18–22. Interestingly, while BRAF/CRAF heterodimers could readily be detected in asynchronized cells we could not detect such heterodimers in mitotic cells (Supplementary Fig. 8c). In fact, phospho-S338 CRAF localized to the spindle pole in cells lacking BRAF (Supplementary Fig. 7a). These results suggest that the role that CRAF plays in mitosis is distinct from that of BRAF.
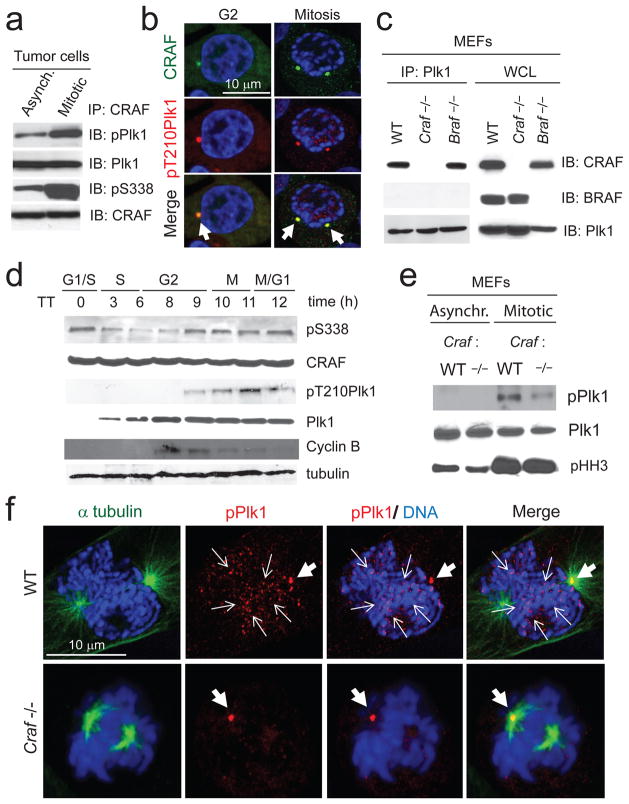
Mitosis is regulated by various mitotic kinases such as Aurora kinases, Plk1, and CDK123–25. Plk1 becomes activated by Aurora-A in G2, localizes to centrosomes, spindle poles and accumulates at kinetochores at pro-metaphase26–28 (Supplementary Fig. 10). Accordingly, tumor cells expressing oncogenic RAS are particularly dependent on Plk1 activity for mitotic progression and survival29,30, and inhibition or depletion of Plk1 causes cell cycle delay/arrest at pro-metaphase27,31–34. Interestingly, both Aurora-A and Plk1 co-precipitated with CRAF but not BRAF (Figs. 3a, c and Supplementary Fig. 8c and e) and CRAF co-localized with Aurora-A and Plk1 at the centrosomes in G2 and spindle poles in mitotic tumor cells (Fig. 3b and Supplementary Fig. 8b). Interestingly, CRAF, but not BRAF, co-precipitated with Plk1 (Fig. 3c) and this could be detected in cells expressing WT or kinase dead (K375M) CRAF (Supplementary Fig. 8d). However, this complex was minimally detected in cells expressing the S338A RAF mutant (Supplementary Fig. 8a) suggesting that the interaction between CRAF and Plk1 requires serine 338 phosphorylation but not kinase activity. Thus, CRAF may serve as a scaffold bringing Aurora-A and Plk1 into a functional mitotic complex.
Figure 3. CRAF interacts with Plk1 and promotes its activation and accumulation to the kinetochores at pro-metaphase.
(a) Immunoblot analysis of CRAF immunoprecipitates from HCT-116 asynchronized and synchronized at pro-metaphase cells. Data are representative of three independent experiments. (b) Confocal microscopy images of HCT-116 cells synchronized at G2 and pro-metaphase (as described in Methods) and stained for CRAF (in green), phospho-T210 Plk1 (in red) and DNA (TOPRO-3 in blue). White arrows indicate co-localization of CRAF with phospho-Plk1 at the centrosomes and mitotic spindle poles. (c) Immunoblot analysis of Plk1 immunoprecipitates from WT, Craf−/− and Braf−/− MEFs. Data are representative of three independent experiments. (d) Immunoblot analysis from G1-M, of phospho-S338 CRAF, total CRAF, phospho-T210 Plk1, total Plk1, cyclin B and tubulin of HCT-116 cells synchronized at the G1/S boundary. Cells were synchronized at the G1/S boundary by a double thymidine block as described in Methods. Data are representative of two independent experiments. (e) Immunoblot analysis of asynchronous and mitotic WT and Craf−/− MEFs. (f) Confocal microscopy images of WT and Craf−/− MEFs at pro-metaphase. Cells were stained for α-tubulin (in green), phospho-T210 Plk1 (in red) and DNA (TOPRO-3 in blue). Thick white arrows indicate localization of phospho-Plk1 at the mitotic spindle pole and narrow white arrows indicate localization of phospho-Plk1 at the kinetochores. Scale bar, 10 μm.
To determine whether the kinetics of CRAF serine 338 phosphorylation during cell cycle progression correlated with Plk1 activation, we analyzed CRAF (S338) and Plk1 (T210) phosphorylation throughout the cell cycle from G1-M. Interestingly, phospho-S338 CRAF levels were increased in G1, as expected, but showed a second wave of phosphorylation at G2/M beginning immediately prior to Plk1 phosphorylation (Fig. 3d), consistent with the notion that CRAF phosphorylation on serine 338 precedes Plk1 activation. To further explore a role for CRAF in Plk1 activation, lysates from WT and Craf−/− MEFs were subjected to immunoblotting for phospho-Plk1 or evaluated for Plk1 enzymatic activity (Fig. 3e and Supplementary Fig. 9a). Indeed, Craf−/− MEFs demonstrated minimal Plk1 activity compared to WT MEFs. Furthermore, tumor cells arrested in pro-metaphase with KG5 showed decreased Plk1 activity compared to cells arrested with nocodazole or paclitaxel (Supplementary Figs. 9b, c). While treatment of cells with KG5 had no effect on Plk1 localization to the spindle pole, it prevented the subsequent accumulation of active Plk1 at the kinetochores (Supplementary Figs. 9d, e) and similar findings were observed in Craf−/− MEFs (Fig. 3f). These findings were substantiated since an active form of Plk1 was able to rescue the G2/M delay caused by CRAF depletion (Supplementary Fig. 11). Together, these results indicate that CRAF potentiates Plk1 or Aurora-A activation leading to accumulation of active Plk1 at the kinetochores. This process facilitates mitotic progression through pro-metaphase and can be blocked by inhibition of phospho-S338 CRAF via allosteric RAF blockade.
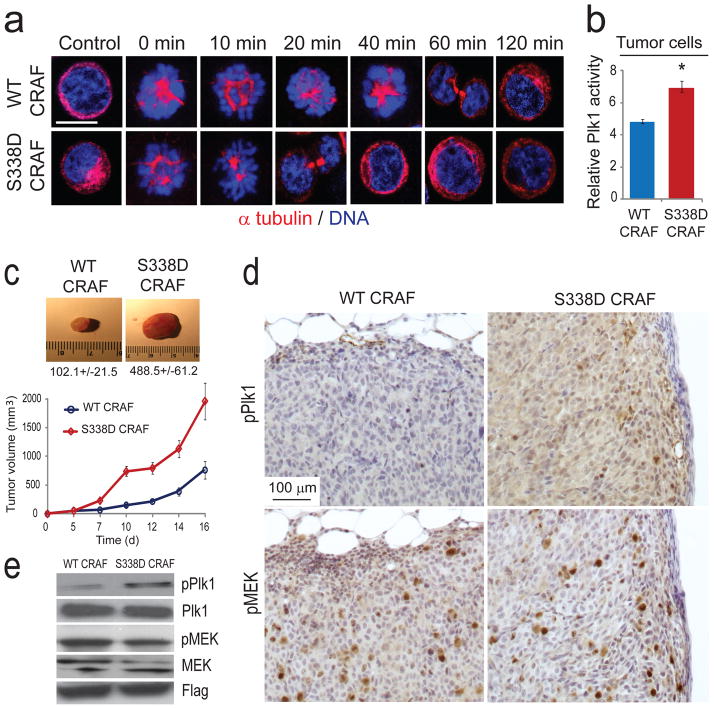
To investigate the impact of the CRAF/Plk1 signaling module in tumor progression, we evaluated the tumor growth capacity of human colon carcinoma and glioblastoma cells stably expressing the phospho-mimetic S338D CRAF mutant. HCT-116 colon cells expressing the S338D CRAF mutant showed accelerated mitosis and a significant increase in tumor growth relative to cells expressing WT CRAF and this was associated with increased phospho-Plk1 but not phospho-MEK expression in these tumors (Figs. 4a–e). Similarly, U-87 glioblastoma cells expressing S338D CRAF produced a significant increase in brain tumor growth relative to cells expressing WT CRAF (Supplementary Fig. 12). Furthermore, cells expressing a double mutant S338D kinase dead (S338D/K375M) also showed increase Plk1 activity and tumor growth in mice but failed to activate MEK (Supplementary Fig. 13). These results demonstrate that phospho-S338 CRAF is an important mediator of tumor progression based on its capacity to promote mitosis in a manner that is independent of active MEK.
Figure 4. Phospho-mimetic CRAF S338D mutation drives tumor growth and activates Plk1 in vivo.
(a) HCT-116 human colon carcinoma cells ectopically expressing either WT RAF or S338D mutant CRAF were arrested in pro-metaphase as described in Methods, and subsequently allowed to progress through mitosis. Cells were stained for α-tubulin (in red) and DNA (TOPRO-3 in blue) at 0, 10, 20, 40, 60 and 120 min after release from pro-metaphase blockade and mitotic progression was analyzed by confocal microscopy. Scale bar, 10 μm. Data are representative of three independent experiments. (b) Plk1 kinase activity assay performed in HCT-116 cells expressing WT CRAF or a phospho-mimetic S338D CRAF mutant. Error bars represent s.d. (n = 3); * P = 0.011 using a Mann Whitney U test. (c) HCT-116 cells expressing WT or S338D CRAF Flag tagged were injected subcutaneously in the flank of immune-compromised nude mice. Tumor images, average weights ± s.e. and tumor size measurements are shown (n = 20). (d) Immunohistochemical staining of phospho-Plk1 and phospho-MEK in mouse tissues from tumors expressing WT or S338D CRAF. Scale bar, 100 μm. (e) Immunoblot analysis of phospho-T210 Plk1, Plk1, phospho-MEK, MEK and FLAG in tumor lysates from HCT-116 cells expressing WT or S338D CRAF. Data are representative of five independent experiments.
While RAF is an essential component of the canonical MAPK signaling pathway various reports reveal that RAF exerts kinase independent functions16,35–37. We previously showed that CRAF, independent of its kinase activity, could translocate to the mitochondria and protect cells from apoptosis by inhibiting the pro-apoptotic protein ASK113,14. Here, we reveal a kinase independent function of CRAF in cell proliferation and demonstrate that phospho-S338 CRAF localizes to centrosomes/mitotic spindle poles in G2/M, where it interacts with Aurora-A and Plk1, promotes Plk1 activation and thereby mitotic and tumor progression (Supplementary Fig. 14). Whether CRAF interaction with Aurora-A and Plk1 is direct or indirect still needs to be determined. However, these data support the conclusion that CRAF may act as an adaptor protein to promote Aurora-A or Plk1 activation facilitating mitosis and tumor progression. While our findings may be relevant to all cells undergoing mitosis, targeting this pathway with allosteric RAF inhibitors, like KG5, might be particularly effective during angiogenesis12 and tumor growth, processes characterized by highly proliferative cells. Finally, our studies reveal that allosteric inhibitors designed to block phospho-S338 CRAF and its function in mitosis represent a new therapeutic approach to inhibit the growth of a wide range of cancers.
Methods
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturemedicine/.
Supplementary Material
Acknowledgments
We thank K. Elliott and J. Lesperance for their assistance with the mouse experiments and immunohistochemistry, and M. Schmid, M. Kaulich and J. Desgrosellier for discussions. We thank K. Lee (NIH-NCI) and R. Erikson (Harvard) for providing Plk1 constructs. D.A.C. was supported by CA78045, CA119335, CA95262, and CA104898 from the US National Institutes of Health.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
Author contributions
A.M., and D.A.C designed the studies; E.A.M. designed and provided KG5; A.M., L.S., M.H., MF.C., S.A., A.F., and S.J.A. performed experiments; A.M., L.S., M.H., MF.C., S.A., A.F., S.J.A. and S.M.W. analyzed data; A.M. and D.A.C. wrote the manuscript; D.A.C. supervised the project.
Competing financial interests
The authors declare no competing financial interests. Published online at http://www.nature.com/naturemedicine/.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 2.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 3.Bollag G, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty KT, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph EW, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci U S A. 2010;107:14903–14908. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King AJ, et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res. 2006;66:11100–11105. doi: 10.1158/0008-5472.CAN-06-2554. [DOI] [PubMed] [Google Scholar]
- 7.Tsai J, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010;70:5518–5527. doi: 10.1158/0008-5472.CAN-10-0646. [DOI] [PubMed] [Google Scholar]
- 9.Hatzivassiliou G, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 10.Heidorn SJ, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy EA, et al. Disruption of angiogenesis and tumor growth with an orally active drug that stabilizes the inactive state of PDGFRbeta/B-RAF. Proc Natl Acad Sci U S A. 2010;107:4299–4304. doi: 10.1073/pnas.0909299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301:94–96. doi: 10.1126/science.1082015. [DOI] [PubMed] [Google Scholar]
- 14.Alavi AS, Acevedo L, Min W, Cheresh DA. Chemoresistance of endothelial cells induced by basic fibroblast growth factor depends on Raf-1-mediated inhibition of the proapoptotic kinase, ASK1. Cancer Res. 2007;67:2766–2772. doi: 10.1158/0008-5472.CAN-06-3648. [DOI] [PubMed] [Google Scholar]
- 15.McGlynn LM, et al. Ras/Raf-1/MAPK pathway mediates response to tamoxifen but not chemotherapy in breast cancer patients. Clin Cancer Res. 2009;15:1487–1495. doi: 10.1158/1078-0432.CCR-07-4967. [DOI] [PubMed] [Google Scholar]
- 16.Mikula M, et al. Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf-1 gene. Embo J. 2001;20:1952–1962. doi: 10.1093/emboj/20.8.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber CK, Slupsky JR, Kalmes HA, Rapp UR. Active Ras induces heterodimerization of cRaf and BRaf. Cancer Res. 2001;61:3595–3598. [PubMed] [Google Scholar]
- 18.Borysov SI, Cheng AW, Guadagno TM. B-Raf is critical for MAPK activation during mitosis and is regulated in an M phase-dependent manner in Xenopus egg extracts. J Biol Chem. 2006;281:22586–22596. doi: 10.1074/jbc.M601432200. [DOI] [PubMed] [Google Scholar]
- 19.Borysov SI, Guadagno TM. A novel role for Cdk1/cyclin B in regulating B-raf activation at mitosis. Mol Biol Cell. 2008;19:2907–2915. doi: 10.1091/mbc.E07-07-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borysova MK, Cui Y, Snyder M, Guadagno TM. Knockdown of B-Raf impairs spindle formation and the mitotic checkpoint in human somatic cells. Cell Cycle. 2008;7:2894–2901. doi: 10.4161/cc.7.18.6678. [DOI] [PubMed] [Google Scholar]
- 21.Cui Y, Borysova MK, Johnson JO, Guadagno TM. Oncogenic B-Raf(V600E) induces spindle abnormalities, supernumerary centrosomes, and aneuploidy in human melanocytic cells. Cancer Res. 2010;70:675–684. doi: 10.1158/0008-5472.CAN-09-1491. [DOI] [PubMed] [Google Scholar]
- 22.Cui Y, Guadagno TM. B-Raf(V600E) signaling deregulates the mitotic spindle checkpoint through stabilizing Mps1 levels in melanoma cells. Oncogene. 2008;27:3122–3133. doi: 10.1038/sj.onc.1210972. [DOI] [PubMed] [Google Scholar]
- 23.Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007;17:60–65. doi: 10.1016/j.gde.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 25.Taylor S, Peters JM. Polo and Aurora kinases: lessons derived from chemical biology. Curr Opin Cell Biol. 2008;20:77–84. doi: 10.1016/j.ceb.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Macurek L, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–123. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 27.Petronczki M, Lenart P, Peters JM. Polo on the Rise-from Mitotic Entry to Cytokinesis with Plk1. Dev Cell. 2008;14:646–659. doi: 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knauf JA, et al. Oncogenic RAS induces accelerated transition through G2/M and promotes defects in the G2 DNA damage and mitotic spindle checkpoints. J Biol Chem. 2006;281:3800–3809. doi: 10.1074/jbc.M511690200. [DOI] [PubMed] [Google Scholar]
- 30.Luo J, et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137:835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McInnes C, et al. Inhibitors of Polo-like kinase reveal roles in spindle-pole maintenance. Nat Chem Biol. 2006;2:608–617. doi: 10.1038/nchembio825. [DOI] [PubMed] [Google Scholar]
- 32.Santamaria A, et al. Use of the novel Plk1 inhibitor ZK-thiazolidinone to elucidate functions of Plk1 in early and late stages of mitosis. Mol Biol Cell. 2007;18:4024–4036. doi: 10.1091/mbc.E07-05-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steegmaier M, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17:316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 34.Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov. 2010;9:643–660. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- 35.Huser M, et al. MEK kinase activity is not necessary for Raf-1 function. Embo J. 2001;20:1940–1951. doi: 10.1093/emboj/20.8.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hindley A, Kolch W. Extracellular signal regulated kinase (ERK)/mitogen activated protein kinase (MAPK)-independent functions of Raf kinases. J Cell Sci. 2002;115:1575–1581. doi: 10.1242/jcs.115.8.1575. [DOI] [PubMed] [Google Scholar]
- 37.Kamata T, et al. BRAF Inactivation Drives Aneuploidy by Deregulating CRAF. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-10-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.