Abstract
Objective
We evaluated the expression of human trophoblast cell-surface marker (Trop-2) in endometrial endometrioid carcinoma (EEC) and the potential application of hRS7, a humanized monoclonal anti-Trop-2 antibody, as a therapeutic agent against poorly-differentiated EEC.
Methods
Trop-2 expression was evaluated by immunohistochemistry in 131 EEC with different degrees of differentiation and 32 normal endometrial controls (NEC). Trop-2 expression was also evaluated by real-time polymerase-chain-reaction (qRT-PCR) and flow cytometry in 3 primary EEC cell lines derived from patients harboring poorly-differentiated EEC. Finally, sensitivity of G3 EEC cell lines to hRS7 antibody-dependent cellular-cytotoxicity (ADCC) was tested in standard 5-hours 51Cr-release assays.
Results
Trop-2 expression was detected in 126 of 131 (96.2%) EEC samples. Tumor tissues showed markedly increased Trop-2 positivity as compared to NEC (p=0.001). Trop-2 expression was significantly higher in all grades of EEC vs. NEC. G3 tumors displayed significantly stronger Trop-2 immunostaining compared to G1 EEC (p=0.01). High Trop-2 expression by qRT-PCR and flow cytometry was found in one G3 EEC primary cell line (EEC-ARK-1). Unlike Trop-2-negative EEC cell lines, EEC-ARK-1 was found highly sensitive to hRS7-mediated ADCC in vitro (range of killing: 33.9% to 50.6%) (p=0.004). Human serum did not significantly inhibit hRS7-mediated-cytotoxicity against EEC-ARK-1 (p= 0.773).
Conclusions
Trop-2 is highly expressed in EEC and its expression is significantly higher in poorly-differentiated EEC when compared to well-differentiated EEC. Primary G3 EEC overexpressing Trop-2 are highly sensitive to hRS7-mediated cytotoxicity in vitro. hRS7 may represent a novel therapeutic agent for the treatment of high-grade EEC refractory to standard treatment modalities.
Keywords: Endometrial cancer, Endometrioid adenocarcinoma, Immunotherapy, Natural Killer Cells, Trop-2 protein
INTRODUCTION
Endometrial adenocarcinoma is the most common gynecologic malignancy in the United States with an estimated 43,470 cases and 7,950 deaths in 2010.1 On the basis of clinical as well as histopathological characteristics, two pathogenetic types of endometrial carcinoma have been described by Bokhman.2 Type I endometrial cancers, which account for the majority of cases, are usually well- or moderately-differentiated and endometrioid in histology. These neoplasms are associated with a history of unopposed estrogen exposure or other hyperestrogenic risk factors, such as obesity. Typically these patients have a favorable prognosis with appropriate therapy. In contrast, Type II endometrial cancers include poorly-differentiated endometrioid tumors (G3 tumors), serous papillary, and clear cell carcinomas. These tumors are not associated with hyperestrogenic factors and they are more likely to be deeply invasive in the myometrium and/or metastatic at presentation and often recur despite aggressive clinical interventions. G3 endometrioid endometrial carcinoma (EEC) accounts for the majority of Type II endometrial carcinomas and a significant number of relapses and deaths occur in these patients.2–3 Thus, a better understanding of the molecular basis of the aggressive biologic behavior of these tumors as well as the development of novel, target-specific and effective treatment modalities against this subgroup of poorly-differentiated endometrial cancers remains a high priority.
Large-scale gene expression analysis, using techniques such as high-density oligonucleotide and cDNA microarrays, represents a powerful new tool to identify genes involved in carcinogenesis. Using this technology, our group has recently investigated the genetic fingerprint of G3 EEC separately from those of G1/G2 EEC and the other histologic variants of Type II endometrial cancer. Of interest, in these studies, the intronless gene encoding for trophoblast cell-surface marker (Trop-2, also termed TACSTD2, GA733-1, M1S1, EGP-1) was identified as one of the top differentially expressed genes in G3 EEC when compared to normal human endometrial cells (NEC).4 Trop-2 is a surface glycoprotein originally identified in human placental trophoblast5 and subsequently reported to be highly expressed by various human carcinomas, but rarely in normal adult tissues.5–9 Although the biological role of Trop-2 is still unclear, its overexpression has been found to correlate with invasive behavior and poor prognosis in multiple human carcinomas.10–13 Consistent with this view, our group has recently reported Trop-2 as an independent marker for poor overall survival in ovarian carcinoma patients.14 Importantly, its overexpression by epithelial tumor cells and its transmembrane localization render Trop-2 an attractive target for cancer immunotherapy.
hRS7 is a humanized IgG1 monoclonal antibody (mAb) developed against Trop-2 using complementary-determining-region (CDR) and transfection techniques of the murine RS7-3G11 antibody (Immunomedics, Inc., Morris Plains, NJ, USA).15–17 hRS7 was initially tested labeled with 131I-IMP-R4 to evaluate its effectiveness in preclinical radioimmunotherapy (RAIT) studies on breast cancer xenograft models.17 Complete remission was reported in 5 out of 11 mice treated with 131I-IMP-R4-hRS7.17 Although these results suggest a promising use of hRS7 as a carrier for radiometabolic therapy after labeling with suitable radionuclides, to our knowledge, the ability of hRS7 in inducing antibody dependent cellular cytotoxicity (ADCC) against primary high grade human EEC cell lines has not been previously studied.
To fill this gap in knowledge, we carefully investigated the expression of Trop-2 in a large number of endometrial carcinoma specimens characterized by different degrees of differentiation. More importantly, we evaluated for the first time the in vitro potential of hRS7 as a novel immunotherapeutic agent against biologically aggressive G3 EEC cell lines showing different levels of Trop-2 expression.
MATERIALS AND METHODS
Tissue microarrays and immunohistochemistry (IHC)
Tissue microarray blocks (TMAs) were created from 131 formalin-fixed, paraffin-embedded EECs and 32 NECs collected from the Department of Surgical Pathology, University of Brescia, Italy. TMAs were constructed using an automated tissue microarrayer (TMA Master, 3DHistech, Budapest, Hungary). Representative areas were chosen for sampling from hematoxylin and eosin (H&E) stained sections of selected NEC and EEC cases. Four 0.6-mm cores have been collected from different areas of each tumor block, in order to overcome tumor heterogeneity and the possible loss of tissue due to cutting. Briefly, TMA sections were subjected to antigen retrieval before application of the purified goat polyclonal antibody against the recombinant human Trop-2 extracellular domain (R&D Systems,Inc., Minneapolis, MN), diluted 1:100. The antibody was revealed with a biotinylated rabbit anti-goat antibody (Vector Labs, Burlingame, CA), diluted 1:250, followed by HRP-streptavidin (Dako, Glostrup, Denmark) and diaminobenzidine (DAB) as chromogen. Immunoreactivity was evaluated as previously described by four independent observers14. Briefly, slides were analyzed at medium/high power view (×20 and ×40 magnification) and a scoring method based on the intensity of the staining and on the percentage of positive tumor cells was applied, as follows: intensity was scored 0 (negative), 1 (weak), 2 (moderate), 3 (strong), while the percentage of positive cells was scored as 0 (0%); 1 (1–10%), 2 (11–50%), 3 (51–100%). A single scale with scores 0–9 was obtained multiplying the intensity and the percentage staining score and a total score was calculated grouping score 0 in total score 0, 1–3 in total score 1, 4 and 6 in total score 2 and 9 in total score 3.
Establishment of G3 EEC Cell Lines
Primary EEC cell lines from 3 patients harboring poorly-differentiated tumors (i.e., G3 EEC) were obtained from fresh tumor biopsies collected at the time of surgery under approval of the Institutional Review Board and established after sterile processing of the tumor samples, as described previously for uterine serous tumors.18 Tumors were staged according to the International Federation of Gynecologists and Obstetricians 2010 operative staging system. Source-patient characteristics of these 3 poorly differentiated EEC cell lines (EEC-ARK-1, EEC-ARK-2, and EEC-ARK-3) are described in Table 1.
Table 1.
| Sample | Age years |
Racea | Flow | RT-PCR | FIGOb | |
|---|---|---|---|---|---|---|
| (%) | MFIc | mRNA RQd(mean) | Grade | |||
| NEC 1 | 1 | |||||
| NEC 2 | 0.5 | |||||
| EEC-ARK-1 | 58 | C | 100% | 704.8 | 8525.5 | G3 |
| EEC-ARK-2 | 66 | AA | 4.0% | 15.8 | 1.8 | G3 |
| EEC-ARK-3 | 80 | C | 6.4% | 21.2 | 1.3 | G3 |
AA, African American; C, Caucasian;
FIGO, International Federation of Gynecology and Obstetrics
Mean Fluorescence Intensity
RQ, Relative quantification
Quantitative real-time polymerase chain reaction
RNA isolation and Quantitative real-time polymerase chain reaction (qRT-PCR) for all 3 primary EEC cell lines used in the cytotoxicity experiments were performed as previously described.18 Briefly, since Trop-2 is an intronless gene, the TaqMan Gene Expression Assay was designed within the exonic region. All RNA samples were then treated with TURBO DNase enzyme (TURBO DNA-free Kit; Ambion, Inc. Applied Biosystem Business, CA) to remove contaminating DNA eventually present. Four µg of total RNA were digested with 2U of TURBO DNase enzyme in a 25 µl-reaction for 30 minutes at 37°C. The digestion was stopped by adding 2,5 µl of DNase Inactivation Reagent, followed by centrifugation. One µg of DNase-treated RNA was reverse transcribed using random hexamers in a final volume of 20 µl according to the SuperScript TM II RT RnaseH-reverse transcriptase protocol (Invitrogen life Technologies, Carlsbad, CA, USA). QRT-PCR was performed with a 7500 Real Time PCR System using the manufacturer's recommended protocol (Applied Biosystem, Applera UK, Cheshire, UK) using the TaqMan Universal PCR master mix and the following Assay on Demand (Applied Biosystem): Hs00242741_s1 (TACSTD2). Negative controls consisting in reactions without reverse transcriptase were included to identify eventual genomic DNA contamination. Data were normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as internal control.
Flow cytometry
The humanized anti-Trop-2 mAb hRS7 (Immunomedics, Inc., Morris Plains, NJ) was used for flow cytometry studies. Briefly, 3 primary EEC cell lines obtained from the above described patients were stained with 2 µg/ml of hRS7. 2.5 µg/ml of the chimeric anti-CD20 mAb rituximab (Rituxan, Genentech, San Francisco, CA) was used as a negative control. A goat anti-human F(ab’)2 immunoglobulin (BioSource International, Camarillo, CA) was used as a secondary reagent. Analysis was conducted with a FACScan using Cell Quest software (Beckton Dickinson, Franklin Lakes, NJ).
Tests for ADCC
A standard five-hours chromium(51Cr)-release assay was performed to measure the cytotoxic reactivity of Ficoll-Paque™ PLUS (GE Healthcare, Uppsala, Sweden) separated peripheral blood lymphocytes (PBL) obtained from several healthy donors against all 3 EEC cell lines. The release of 51Cr from the target cells was measured as evidence of tumor cell lysis after exposure of tumor cells to 2 µg/ml of hRS7. Controls included the incubation of target cells alone or with PBL or mAb separately. The chimeric anti-CD20 mAb rituximab was used as a negative control for hRS7 in all bioassays. ADCC was calculated as the percentage of killing of target cells observed with hRS7 plus effector cells compared with 51Cr release from target cells incubated alone.
Test for complement-mediated target cell lysis and γ-globulin inhibition
A standard 5-hours chromium (51Cr) release assay identical to those performed for ADCC assays was used, except that human serum in a dilution of 1:2 was added in place of the effector cells. This human serum was used as a source of complement to test for complement-mediated target cell lysis. To evaluate the potential inhibition of ADCC against EEC cell lines by physiological human serum concentrations of γ-globulin, human serum diluted 1:2 was added in the presence or absence of effector PBL. In some experiments, heat-inactivated human serum (56°C for 60 minutes) was added in the presence of effector PBL. Controls included the incubation of target cells alone or with either lymphocytes or mAb separately. Rituximab was used as a control mAb.
Statistical analysis
Differences in Trop-2 expression by flow cytometry were analyzed by the unpaired Student’s t-test while the Wilcoxon rank-sum test was used to compare EEC subtypes to normal endometrium for differences in IHC staining intensities. Sample-type differences were expressed as odds ratios accompanied by 95% confidence limits. Kruskal-Wallis test and chi-square analysis were used to evaluate differences in hRS7-induced ADCC levels in primary tumor cell lines. Statistical analysis was performed using PASW version 18 (SPSS, Chicago, IL).
RESULTS
Trop-2 protein expression by immunohistochemical staining in EEC
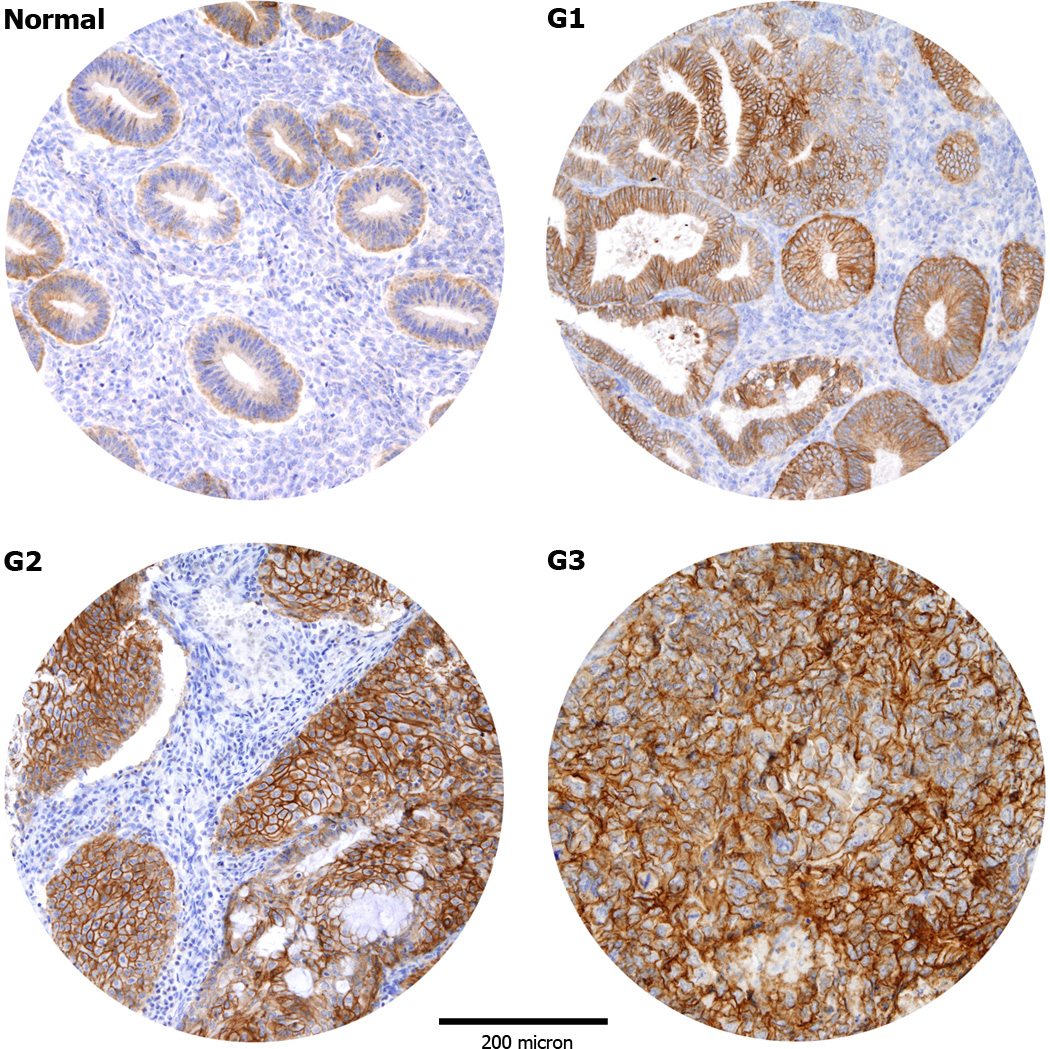
To analyze Trop-2 expression results at the protein level, immunohistochemistry was carried out on 131 EECs (40 G1, 60 G2 and 31 G3) and 32 NECs (15 during proliferative phase and 17 during secretory phase) TMAs. As shown in Table 2, NEC tissues showed predominantly weak immunoreactivity for Trop-2, with presence of score 1 in 50% of cases. In contrast, a positive staining for Trop-2 was detected in 126 out of 131 (96.2%) EEC samples, with a moderate to strong staining (score 2/3) detected in 71.8% of the samples. The difference in Trop-2 expression between tumor tissues and NEC was significant (p=0.001). As displayed in Table 2, almost all G3 cases (30 out of 31, 96.8%) were positive for Trop-2 and most of them (25 out of 31, 80.7%) showed a membrane staining with a moderate/strong score. Similarly, most of the G2 cases (57 out of 60, 95.0%) were also Trop-2 positive and showed a moderate/strong score in 70% of cases. In contrast, while almost all G1 tumors showed expression for Trop-2 by IHC (39 out of 40, 97.5%), the striking majority of them (35 out of 40, 87.5%) demonstrated a weak/moderate score. When EEC with different degrees of differentiation were compared for Trop-2 expression to NEC, we found the staining to be significantly higher in G1 EECs vs NECs (p=0.0408), in G2 EECs vs NECs (p=0.0030) and in G3 EECs vs NECs (p=0.0004). Of interest, G3 tumors displayed a significantly stronger Trop-2 immunostaining when compared to well differentiated G1 tumors (p=0.0186). The comparisons between G1 EECs and G2 EECs and between G2 EECs and G3 EECs were not significant. In all samples, Trop-2 immunoreactivity was localized exclusively to the membrane of neoplastic epithelium, with no positivity in adjacent stromal cells (Figure 1).
Table 2.
Trop-2 immunoreactivity in tissue microarrays of endometrial carcinomas (EECs) and normal endometrium (NECs).
| n | Score=0 | Score=1 | Score=2 | Score=3 | |
|---|---|---|---|---|---|
| n(%) | n(%) | n(%) | n(%) | ||
| EECs | 131 | 5 (3.8) | 32 (24.4) | 58 (44.3) | 36 (27.5) |
| Histological Grade | |||||
| G1 | 40 | 1 (2.5) | 12 (30.0) | 23 (57.5) | 4 (10.0) |
| G2 | 60 | 3 (5.0) | 15 (25.0) | 23 (38.3) | 19 (31.7) |
| G3 | 31 | 1 (3.2) | 5 (16.1) | 12 (38.7) | 13 (42.0) |
| FIGO Stage | |||||
| I | 81 | 3 (3.7) | 20 (24.7) | 40 (49.4) | 18 (22.2) |
| II | 27 | 1 (3.7) | 6 (22.2) | 12 (44.4) | 8 (29.7) |
| III | 20 | 1 (5) | 4 (20) | 6 (30) | 9 (45) |
| IV | 3 | 0 | 2 (66.7) | 0 | 1 (33.3) |
| NECs | 32 | 3 (9.4) | 16 (50.0) | 11 (34.4) | 2 (6.2) |
Figure 1.
Representative immunohistochemical staining for Trop-2 in tissue microarrays of normal endometrial controls (NEC) and endometrial carcinomas (EC). NEC tissues show predominantly a weak immunoreactivity for Trop-2 (A). Positive Trop-2 membrane staining (B–D): grade 1 (B), grade 2 (C), grade 3 (D) endometrioid ECs. (Original magnification X100). The figure were taken at the same magnification and the periphery of the core was resized using Adobe photoshop, with similar reduction of the total core area (>5%).
Trop-2 transcript levels in EEC
Three primary tumor cell lines established from patients harboring poorly-differentiated G3 EEC were tested for Trop-2 expression by qRT-PCR. Table 1 shows the histopathological characteristics of the EEC patients. Among the 3 primary tumor cell lines tested, 1 carcinoma (EEC-ARK-1) showed a high mRNA expression, (mean RQ ± SD: 8525.5 ± 140.3) (Table 1). The difference in Trop-2 expression between this primary EEC cell line and NEC was statistically significant (p<0.001). In contrast, low Trop-2 expression by qRT-PCR was detected in the other 2 cell lines established from poorly-differentiated EEC, ranging from 1.28 to 1.82 (Table 2). The difference between Trop-2 expression in NEC versus EEC cell lines with low Trop-2 expression was not statistically significant (p=0.152).
Trop-2 surface expression by flow cytometry in EEC primary cell lines
To determine whether the high or low expression of Trop-2 mRNA detected by qRT-PCR in the three EEC cell lines available to this study also resulted in a similarly high or low expression of the protein on the surface of tumor cells, we performed flow cytometry using hRS7 on all primary tumors. EEC-ARK-1 once again showed high Trop-2 surface expression by flow cytometry (mean fluorescence intensity ± SD: 704.8 ± 6.1), while low to negligible expression was detected on the surface of EEC-ARK-2 and EEC-ARK-3 cell lines in multiple experiments (Figure 2; Table 1). Thus, Trop-2 surface expression results from flow cytometric analysis were found to be in good agreement with Trop-2 expression results found by qRT-PCR in all three available primary EEC cell lines. In multiple experiments, the difference in Trop-2 surface expression between the cell lines with low and high Trop-2 expression was statistically significant (p<0.001).
Figure 2.
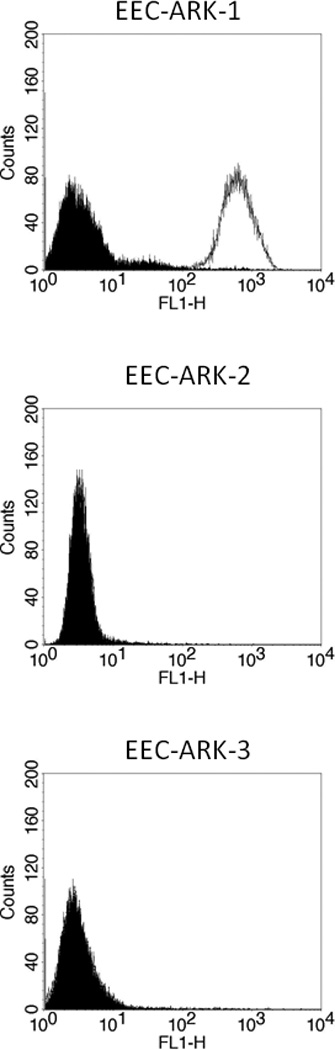
Flow cytometry histograms of primary endometrioid endometrial carcinoma (EEC) G3 cell lines showing high (EEC-ARK-1), and low/negligible (EEC-ARK-2 and EEC-ARK-3) expression of Trop-2. Isotype (black line); hRS7 (dashed line). Note that in EEC-ARK-2 and EEC-ARK-3 cell lines, due to the lack of surface expression for Trop-2, the isotype histograms (black line) completely overlap with the hRS7 (dashed line) histograms.
EEC cell lines are highly resistant to NK cell activity but sensitive to hRS7-mediated ADCC
All three primary EEC cell lines were evaluated for their sensitivity to natural killer cells (NK). These cell lines were exposed to PBL collected from multiple healthy donors. Using dose titration experiments with different doses of hRS7, killing of the EEC cells was found to plateau at a hRS7 concentration of 2 μg/ml (data not shown). Thus, this dose was used in all following experiments. As representatively shown in Figure 3 for EEC-ARK-1, EEC-ARK-2 and EEC-ARK-3 cell lines, we found these poorly differentiated tumors to be highly resistant to NK-mediated killing with exposure to PBL at an effector to target ratio (E/T) of 50:1 (mean killing ± SD: 6.2% ± 0.6). In contrast, significant killing was demonstrated against the Trop-2-positive cell line after incubation with hRS7 to mediate ADCC (range of killing 33.9-50.6%, mean ± SD: 47.4% ± 5.3; p=0.004) (Figure 3, upper panel), while EEC-ARK-2 and EEC-ARK-3 Trop-2-negative cell lines were resistant to hRS7 (Figure 3, middle and lower panel). The results are consistent with the Trop-2 expression data by qRT-PCR and flow cytometry (Figure 2; Table 1). All cell lines were resistant to incubation with rituximab control antibody in the presence of PBL (Figure 3).
Figure 3.
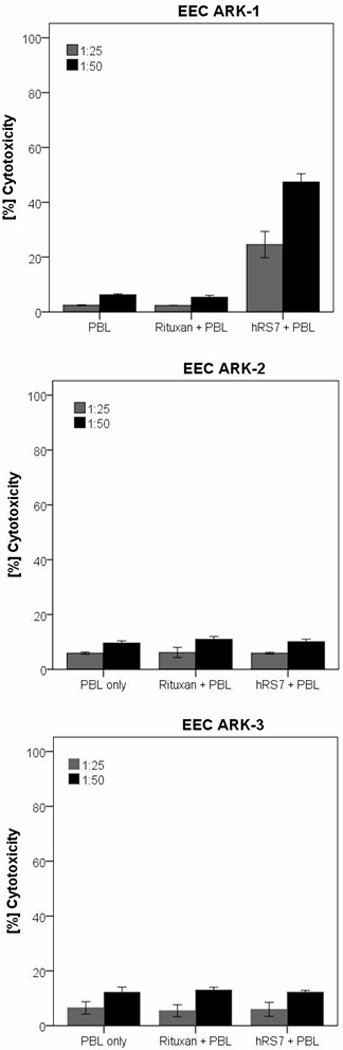
Representative cytotoxicity experiments (Effector to target: E/T; 25:1 and 50:1) using hRS7 against the Trop-2-positive cell line (i.e., EEC-ARK-1, upper panel) versus the Trop-2-negative cancer cell lines (i.e., EEC-ARK-2, middle panel and EEC-ARK-3, lower panel). High levels of hRS7 induced cytotoxicity were evident against EEC-ARK-1 primary cell line expressing high levels of Trop-2. In contrast, negligible cytotoxicity was detectable against EEC-ARK-2 and EEC-ARK-3 (i.e., low Trop-2 expressor cell lines). In all cell lines tested, no significant cytotoxicity was detected in the absence of hRS7 or in the presence of rituximab control mAb.
Effect of complement and physiological concentrations of IgG on hRS7-mediated ADCC
In order to evaluate the effect of complement on the hRS7-mediated ADCC as well as the potential inhibition of hRS7 activity by physiological IgG serum concentrations, human serum diluted 1:2 (with and without heat inactivation) was added to EEC-ARK-1 during 51Cr release assays in the presence or absence of PBL. The addition of untreated serum in the absence of PBL with or without hRS7 was not able to induce significant cytotoxicity against EEC-ARK-1 (data not shown). These data illustrate the lack of significant cytotoxicity mediated by complement proteins in the absence of effector cells. Importantly, as representatively demonstrated in Figure 4, the addition of endogenous IgG (i.e., heat-inactivated plasma (diluted 1:2) to PBLs or untreated serum (diluted 1:2) in the presence of hRS7) did not significantly alter the degree of ADCC (figure 4) (hRS7 + PBL vs. hRS7 + PBL + Serum: p= 0.773).
Figure 4.
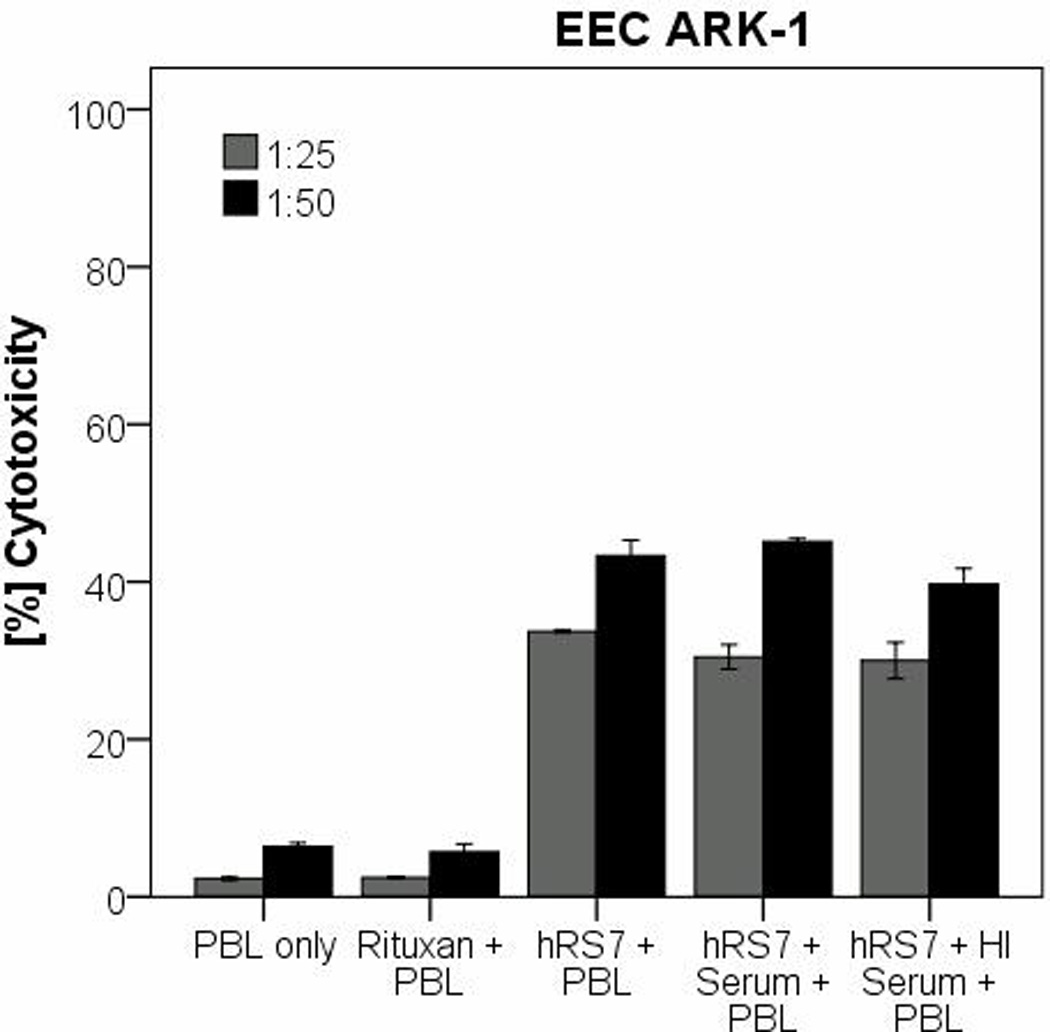
Representative cytotoxicity experiments adding human serum (diluted 1:2) to hRS7 against EEC-ARK-1 cell line. The tumor cell line was treated with serum (with or without heat inactivation) in the presence or absence of the effector cells and hRS7 in a standard 5-hours 51Cr-release assays. Incubation with physiological concentrations of IgG (i.e., heat-inactivated (HI) serum diluted 1:2) to PBL in the presence of hRS7 did not significantly reduce the degree of ADCC achieved in the presence of hRS7 against EEC-ARK-1 (p= 0.773).
DISCUSSION
Endometrial cancer is the most common gynecologic malignancy in developed countries and it is generally considered a neoplasm with good prognosis. Nevertheless, up to 35% of uterine cancer patients may be diagnosed with biologically aggressive Type II tumors, with G3 EEC accounting for the majority of the cases.2–3 For several of these patients the prognosis remains poor, regardless of their treatment with gold standard therapies including surgery, adjuvant radiation and/or chemotherapy. Using a genome wide examination of the genetic fingerprints of poorly-differentiated EEC (i.e., HG-U133 plus 2.0 chip covering 47,000 human transcripts and variants by Affymetrics), our group has recently reported evidence that G3 EEC genetic fingerprints can clearly be distinguished from those of normal endometrial cells.4 More importantly, amongst the multiple potential targets identified in the genomic analysis, Trop-2 surface glycoprotein was found as one of the top differentially expressed genes in EECs when compared to normal endometrial cells. Because of its limited expression in normal human tissues and its localization on the cell surface of poorly differentiated EEC, Trop-2 may represent an attractive target for immunotherapy of these biologically aggressive tumors.
In this study, we have investigated Trop-2 expression and localization by immunohistochemistry in normal endometrium, and compared such expression to a large number of EEC showing different degrees of differentiation. In addition, we have evaluated the in vitro potential of hRS7 mAb as a new form of therapy against biologically aggressive cell lines established from patients harboring poorly-differentiated EEC. Our findings demonstrate that (i) Trop-2 is highly overexpressed in tumor tissues when compared to NEC tissue by IHC (p=0.001), (ii) Trop-2 immunostaining is significantly higher in poorly-differentiated endometrial tumors (i.e., G3) when compared to well-differentiated (i.e., G1) EEC (p=0.01); and (iii) primary poorly-differentiated EEC cell lines overexpressing Trop-2 are highly susceptible to ADCC mediated by hRS7, a humanized anti-Trop-2 mAb. Our results may therefore have important implications for the treatment of patients harboring biologically aggressive EEC resistant to standard treatment modalities.
Of interest, in a recent study in uterine papillary serous tumors, another biologically aggressive endometrial tumor resembling high grade ovarian cancer, our research group reported high expression of Trop-2 in the majority of tumors and primary cell lines tested.18 Although the relationship between high Trop-2 expression and aggressiveness of epithelial neoplasms remains unclear, it has been suggested that Trop-2, possessing cytoplasmic serine and tyrosine phosphorylation sites, might function as a cell signal transducer and regulator of tumor cell growth and increase tumor cell resistance to apoptosis.19 Consistent with this view, Guerra et al. have shown that a large fraction of human cancers express a bicistronic CYCLIN D1-TROP2 mRNA chimera, acting as an oncogene able to induce aggressive tumor growth.20 Another study has reported that Trop-2 is necessary for tumorigenesis in colon carcinoma cell lines, showing that Trop-2 targeting with specific antibodies may result in inhibition of tumor cell migration and invasion.21 Taken together, these observations combined with the results of our IHC studies, showing a significantly higher expression of Trop-2 in poorly-differentiated EEC when compared to well-differentiated EEC, support the possibility that aberrant expression of Trop-2 may account for the enhanced invasive behavior and increased biologic aggressiveness of multiple human cancers including Type II endometrial tumors.
Our experimental results suggest that targeting cancer cells with high surface expression of Trop-2 may be a novel, potentially effective option to treat residual/resistant poorly-differentiated EEC after standard adjuvant chemotherapy. Therefore, in this study we have tested the ability of hRS7, a humanized anti-Trop-2 antibody,15–17 to kill in vitro multiple primary EEC cell lines expressing or not expressing Trop-2. In this regard, one of the primary G3 EEC cell lines available to this study expressed significant levels of Trop-2 by qRT-PCR and flow cytometry. In agreement with its high Trop-2 expression, and unlike the two EEC cell lines showing negligible Trop-2 expression, EEC-ARK-1 was found to be highly susceptible to ADCC when incubated with effector cells in the presence of hRS7. These data, therefore, demonstrate that although these poorly-differentiated tumors are per se resistant to NK activity in the absence of tumor specific antibodies, they remain highly sensitive to lysis by NK cells when these are engaged by the Trop-2 specific antibody hRS7.
In vivo, ADCC activity is known to be dependent upon the availability of the effector cells to interact with the antibody at the target site in the presence of high concentrations of irrelevant human IgG. In this study, we show that ADCC against EEC cell lines was not significantly decreased by high concentrations (up to 50%) of human serum. These results indicate that the binding of hRS7 to the Fc receptor on mononuclear effector cells is likely enabled in the in vivo situation.
In conclusion, this is the first report on Trop-2 protein expression and hRS7 therapeutic activity in poorly-differentiated EEC, the most commonly diagnosed type II endometrial cancer. Our study has demonstrated that Trop-2 expression is highly and consistently expressed in a large number of poorly-differentiated endometrial tumors. Importantly, the high density and the membranous localization of Trop-2 on G3 EEC cells, combined with its negative expression in mesothelial type cells in the abdominal cavity (data not shown), suggests that this protein could represent an accessible tumor target antigen for both intravenous and intraperitoneal antibody-based therapies. The future design and implementation of clinical trials in this regard will ultimately determine the validity of this novel therapeutic approach.
ACKNOWLEDGEMENTS
The Authors thank Immunomedics, Inc., Morris Plains, NJ, USA for providing hRS7 free of charge for our studies.
Supported in part by NIH R01 CA122728-01A2 to ADS, and grants 501/A3/3,0027557 to ADS and 527/B4/4 to SP from the Italian Institute of Health (ISS). This investigation was also supported by NIH Research Grant CA-16359 from the National Cancer Institute and from Berlucchi Foundation, Brescia, ItalySL is supported by “Borsa di studio Prof. Roberto Tosoni” (Garda Vita, BCCdel Garda).
Abbreviations
- EEC
endometrial endometrioid adenocarcinoma
- Trop-2
human trophoblast-cell-surface marker
- qRT-PCR
quantitative real-time polymerase chain reaction
- SEM
standard error of the mean
- NEC
normal endometrial controls
- ADCC
antibody-dependent cellular-cytotoxicity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 3.Deligdisch L, Cohen CJ. Histologic correlates and virulence implication of endometrial carcinoma associated with adenomatous hyperplasia. Cancer. 1985;56:1452–1455. doi: 10.1002/1097-0142(19850915)56:6<1452::aid-cncr2820560637>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Bignotti E, Ravaggi A, Tassi RA, et al. Trefoil factor 3: a novel serum marker identified by gene expression profiling in high-grade endometrial carcinomas. Br J Cancer. 2008;99:768–773. doi: 10.1038/sj.bjc.6604546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipinski M, Parks DR, Rouse RV, Herzenberg LA. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci USA. 1981;78:5147–5150. doi: 10.1073/pnas.78.8.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miotti S, Canevari S, Menard S, et al. Characterization of human ovarian carcinoma-associated antigens defined by novel monoclonal antibodies with tumor-restricted specificity. Int J Cancer. 1987;39:297–303. doi: 10.1002/ijc.2910390306. [DOI] [PubMed] [Google Scholar]
- 7.Alberti S, Miotti S, Stella M, et al. Biochemical characterization of Trop-2, a cell surface molecule expressed by human carcinomas: formal proof that the monoclonal antibodies T16 and MOv-16 recognize Trop-2. Hybridoma. 1992;11:539–545. doi: 10.1089/hyb.1992.11.539. [DOI] [PubMed] [Google Scholar]
- 8.Nakashima K, Shimada H, Ochiai T, et al. Serological identification of TROP2 by recombinant cDNA expression cloning using sera of patients with esophageal squamous cell carcinoma. Int J Cancer. 2004;112:1029–1035. doi: 10.1002/ijc.20517. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Zhou W, Velculescu VE, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 10.Muhlmann G, Spizzo G, Gostner J, et al. TROP2 expression as prognostic marker for gastric carcinoma. J Clin Pathol. 2009;62:152–158. doi: 10.1136/jcp.2008.060590. [DOI] [PubMed] [Google Scholar]
- 11.Fong D, Moser P, Krammel C, et al. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008;99:1290–1295. doi: 10.1038/sj.bjc.6604677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fong D, Spizzo G, Gostner JM, et al. TROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol. 2008;21:186–191. doi: 10.1038/modpathol.3801001. [DOI] [PubMed] [Google Scholar]
- 13.Fang YJ, Lu ZH, Wang GQ, et al. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int J Colorectal Dis. 2009;24:875–884. doi: 10.1007/s00384-009-0725-z. [DOI] [PubMed] [Google Scholar]
- 14.Bignotti E, Todeschini P, Calza S, et al. Trop-2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patients. Eur J Cancer. 2010;46:944–953. doi: 10.1016/j.ejca.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Stein R, Basu A, Chen S, Shih LB, Goldenberg DM. Specificity and properties of MAb RS7-3G11 and the antigen defined by this pancarcinoma monoclonal antibody. Int J Cancer. 1993;55:938–946. doi: 10.1002/ijc.2910550611. [DOI] [PubMed] [Google Scholar]
- 16.Stein R, Govindan SV, Mattes MJ, et al. Targeting human cancer xenografts with monoclonal antibodies labeled using radioiodinated, diethylenetriaminepentaacetic acid-appended peptides. Clin Cancer Res. 1999;5:3079s–3087s. [PubMed] [Google Scholar]
- 17.Govindan SV, Stein R, Qu Z, et al. Preclinical therapy of breast cancer with a radioiodinated humanized anti-EGP-1 monoclonal antibody: advantage of a residualizing iodine radiolabel. Breast Cancer Res Treat. 2004;84:173–182. doi: 10.1023/B:BREA.0000018417.02580.ef. [DOI] [PubMed] [Google Scholar]
- 18.Varughese J, Cocco E, Bellone S, et al. Uterine serous papillary carcinomas overexpress human trophoblast-cell-surface marker (Trop-2) and are highly sensitive to immunotherapy with hRS7, a humanized anti-Trop-2 monoclonal antibody. Cancer. 2011 doi: 10.1002/cncr.25891. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cubas R, Li M, Chen C, Yao Q. Trop2: a possible therapeutic target for late stage epithelial carcinomas. Biochimica et Biophysica Acta. 2009;1796:309–314. doi: 10.1016/j.bbcan.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Guerra E, Trerotola M, Dell' Arciprete R, et al. A bicistronic CYCLIN D1-TROP2 mRNA chimera demonstrates a novel oncogenic mechanism in human cancer. Cancer Research. 2008;68:8113–8121. doi: 10.1158/0008-5472.CAN-07-6135. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Day R, Dong Y, Weintraub SJ, Michel L. Identification of Trop-2 as an oncogene and an attractive therapeutic target in colon cancers. Mol Cancer Ther. 2008;7:280–285. doi: 10.1158/1535-7163.MCT-07-2003. [DOI] [PubMed] [Google Scholar]