SUMMARY
Abdominal wall plication is known to cause increased intra-abdominal pressure (IAP). Whether plication-associated increased IAP causes lower extremity venous stasis, a recognized risk factor for DVT, remains unknown. A 55 year old woman had a unilateral pedicled TRAM procedure for mastectomy reconstruction. Prior to plication, duplex ultrasound measured proximal femoral vein (PFV) cross-sectional diameter and volume-flow. PFV measurements were repeated immediately after plication and on post-operative days (POD) 1, 2, and 4. Bladder pressure was measured at similar timepoints. PFV volume-flow decreased from 0.22 L/min to 0.16 L/min (73% of baseline) immediately post-plication and reached a nadir of 0.08 L/min (36% of baseline) on POD 2. Bladder pressure increased from 13mm Hg to 19mm Hg after plication, and peaked at 31mmHg after intra-operative trunk flexion to 30°. Thus, abdominal wall placation was associated with increased intra-abdominal pressure and ultrasound-documented lower extremity venous stasis that persisted for 48 hours after surgery.
Keywords: Deep venous thrombosis, pulmonary embolism, venous thromboembolism, intraabdominal pressure, abdominal compartment syndrome, plication, venous stasis, abdominoplasty, body contouring
INTRODUCTION
Patients who have transverse rectus abdominus myocutaneous (TRAM) flap breast reconstruction after mastectomy are at high risk for post-operative venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE). Symptomatic VTE occurs in 0.8% to 2.2% of patients after autologous tissue breast reconstruction 1,2. However, many VTE remain asymptomatic and recent studies indicate that the true rate (symptomatic plus asymptomatic) of post-operative VTE in TRAM patients ranges from 3.4% to 16.7% 3,4.
Abdominal wall plication is known to cause increased intra-abdominal pressure (IAP) in cosmetic abdominoplasty and TRAM patients 5–8. Previous work has hypothesized that plication-associated increased IAP may contribute to increased DVT risk by creating venous stasis. Here, we present a case report which examines the effect of abdominal wall plication on both IAP and lower extremity venous flow.
METHODS
A 55 year old woman with body mass index of 28.8 presented for a delayed unilateral TRAM procedure after a previous left-sided mastectomy for breast cancer. The patient had a duplex ultrasound to evaluate for DVT pre-operatively and on post-operative day (POD) 1, 2, and 4.
A unilateral pedicled TRAM flap was elevated that included a cuff of anterior rectus fascia (4cm maximum horizontal distance). Immediately prior to fascial closure and contralateral abdominal wall plication, real-time intraoperative ultrasound was performed by a registered vascular technologist (RVT) using the Antares Ultrasound System (Siemens, Mountain View, CA). Measurements included bilateral proximal femoral vein (PFV) cross-sectional diameter and volume-flow. The PFV was identified just cranial to the junction between the femoral and profunda veins and measurements were taken within 1 cm of this location. The PFV was chosen as a measure of flow in the deep venous system (e.g. without contribution from the great saphenous vein, as would be seen in the common femoral vein). Subsequently, the urinary catheter was clamped and the bladder filled with 50mL of sterile saline. Bladder pressure, known to be highly correlated with IAP, was then measured via the catheter using the Stryker Intra-Compartmental Pressure Monitor (Stryker Corporation, Kalamazoo, MI).
A running, permanent suture closed the 4cm horizontal fascial defect. The contralateral anterior rectus sheath was plicated at a maximum horizontal distance of 5cm to centralize the umbilicus and contour the abdomen. Plication was performed from costal margin to pubis. Immediately after plication, PFV diameter, PFV volume-flow, and bladder pressure were re-measured. The TRAM flap was tunneled and inset, the trunk flexed to 30°, and the donor site closed primarily. The patient was extubated without difficulty.
Bilateral PFV diameter and volume-flow measurements were repeated POD 1, 2, and 4. Bladder pressure was re-measured intra-operatively after abdominal closure with the trunk flexed to 30° and on POD 1 and 2. The urinary catheter was removed on POD 2 per protocol.
Ultrasound studies were interpreted by an RVT and a board-certified vascular surgeon. Data from left and right PFV were pooled at each timepoint and descriptive statistics were generated.
This study was approved by the University of Michigan Institutional Review Board.
RESULTS
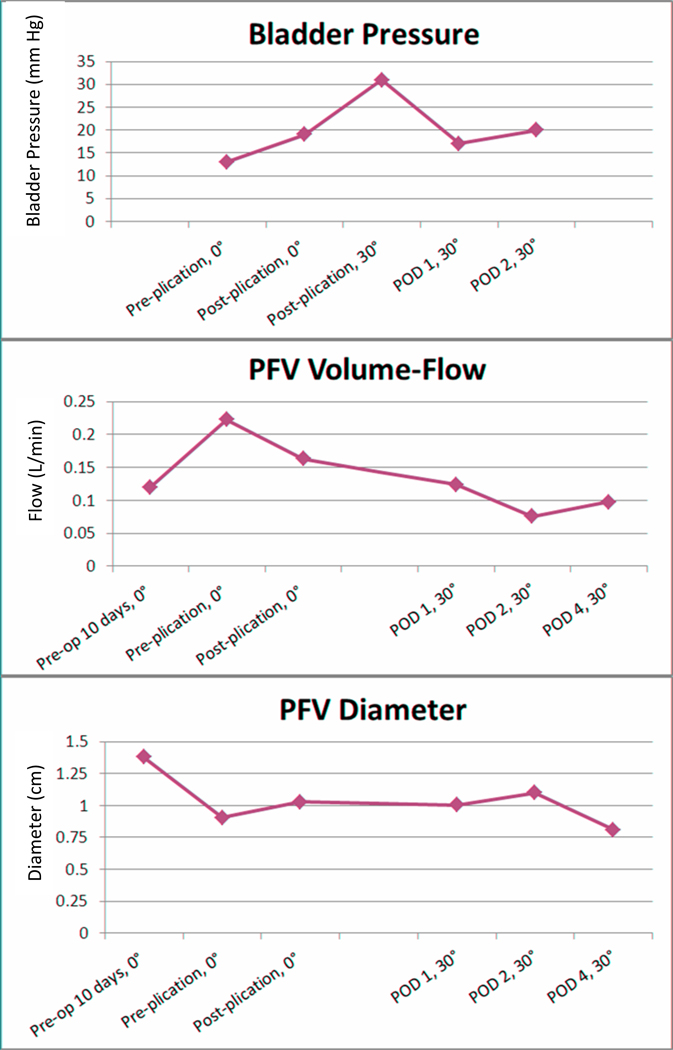
Abdominal wall plication caused an immediate increase in bladder pressure from 13mm Hg to 19mm Hg. The combination of intra-operative trunk flexion to 30° prior to TRAM inset and abdominal wall closure increased bladder pressure to 31mm Hg. Bladder pressure was measured at 17mm Hg on POD 1 and 20mm Hg on POD 2 (Figure 1).
Figure 1.
Change in bladder pressure, PFV volume-flow, and PFV diameter over time. X axis indicates when measurement was performed and degree of trunk flexion.
PFV volume-flow decreased from a pre-plication baseline of 0.22 L/min to 0.16 L/min (73% of baseline) immediately post-plication and reached a nadir of 0.08 L/min (36% of baseline) on POD 2. POD 4 PFV volume-flow was slightly increased (0.10 L/min) when compared to POD 2. Plication was associated with an immediate 14% increase in PFV diameter from 0.91cm to 1.03cm. PFV diameter peaked on POD 2 at 1.10cm and decreased on POD 4 (Figure 1).
No asymptomatic DVT was diagnosed. The patient had adequate urine output (>30mL/hour) for the duration of hospitalization. She showed no signs of symptoms of volume overload or of abdominal compartment syndrome. She tolerated a regular diet on POD 2 and was discharged home on POD 4.
DISCUSSION
This case study suggests that abdominal wall plication may cause alterations in drainage of the lower extremity deep venous system. In the proximal femoral vein, which is the most cranial portion of the deep venous system before its confluence with the great saphenous vein, plication resulted in decreased flow that persisted for 48 hours after surgery and began to normalize by POD 4. Deep venous stasis, as indicated by decreased PFV flow and increased PFV diameter, occurred after plication. Operative venodilation is a known predictor of post-operative DVT, possibly due to vein distension causing intimal microtears.
Breast cancer reconstruction patients have multiple risk factors for VTE. Cancer patients are known to have twice the risk of DVT and three times the risk of PE when compared to patients without cancer undergoing similar operative interventions 9. VTE is the second most common cause of death in breast cancer patients after cancer itself. Additionally, TRAM flap harvest involves manipulation and direct injury to major branches of the deep venous system (superior and inferior epigastric veins) and abdominal wall plication, known to cause increased IAP 5–8.
In general surgery patients who have laparoscopic surgery, increased IAP impairs venous return via direct pressure on the inferior vena cava (IVC) and retroperitoneal veins and by functional narrowing of the IVC at the diaphragmatic hiatus 10; venous flow improves when abdominal insufflation is lost. In contrast, this case report indicates that plication-associated venous stasis may persist for at least two days after surgery. Further research is necessary to examine 1) the rate and time period over which IAP and lower extremity venous drainage return to normal after abdominal wall plication and 2) how these factors may contribute to risk for DVT after TRAM procedures.
CONCLUSION
In this case report, abdominal wall plication performed during TRAM breast reconstruction was associated with increased intra-abdominal pressure and ultrasound-documented lower extremity venous stasis that persisted for 48 hours after surgery.
Acknowledgments
Dr. Pannucci receives salary support from the NIH T32 grant program (T32 GM-08616).
This study was funded by a research grant from the Frederick A. Coller Surgical Society (to CJP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Meeting Disclosures
This work has not previously been presented at a regional or national meeting.
Conflict of Interest: None
Contributor Information
Christopher J. Pannucci, Section of Plastic Surgery, University of Michigan, Ann Arbor, Michigan.
Amy K. Alderman, The Swan Center for Plastic Surgery, Atlanta, Georgia.
Sandra L. Brown, Diagnostic Vascular Unit, University of Michigan, Ann Arbor, Michigan.
Thomas W. Wakefield, Section of Vascular Surgery, University of Michigan, Ann Arbor, Michigan.
Edwin G. Wilkins, Section of Plastic Surgery, University of Michigan, Ann Arbor, Michigan.
REFERENCES
- 1.Mehrara BJ, Santoro TD, Arcilla E, Watson JP, Shaw WW, Da Lio AL. Complications after microvascular breast reconstruction: experience with 1195 flaps. Plast Reconstr Surg. 2006;118:1100–1109. doi: 10.1097/01.prs.0000236898.87398.d6. discussion 1110–1. [DOI] [PubMed] [Google Scholar]
- 2.Pannucci CJ, Chang EY, Wilkins EG. Venous thromboembolic disease in autogenous breast reconstruction. Ann Plast Surg. 2009;63:34–38. doi: 10.1097/SAP.0b013e318188bedf. [DOI] [PubMed] [Google Scholar]
- 3.Lemaine V, McCarthy C, Kaplan K, et al. Venous thromboembolism following microsurgical breast reconstruction: an objective analysis in 225 consecutive patients using low-molecular-weight heparin prophylaxis. Plast Reconstr Surg. 2011;127:1399–1406. doi: 10.1097/PRS.0b013e318208d025. [DOI] [PubMed] [Google Scholar]
- 4.Kim EK, Eom JS, Ahn SH, Son BH, Lee TJ. The efficacy of prophylactic low-molecular-weight heparin to prevent pulmonary thromboembolism in immediate breast reconstruction using the TRAM flap. Plast Reconstr Surg. 2009;123:9–12. doi: 10.1097/PRS.0b013e3181904be7. [DOI] [PubMed] [Google Scholar]
- 5.Losken A, Carlson GW, Tyrone JW, et al. The significance of intraabdominal compartment pressure after free versus pedicled TRAM flap breast reconstruction. Plast Reconstr Surg. 2005;115:261–263. [PubMed] [Google Scholar]
- 6.Losken A, Carlson GW, Jones GE, Hultman CS, Culbertson JH, Bostwick J., 3rd Significance of intraabdominal compartment pressures following TRAM flap breast reconstruction and the correlation of results. Plast Reconstr Surg. 2002;109:2257–2264. doi: 10.1097/00006534-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Huang GJ, Bajaj AK, Gupta S, Petersen F, Miles DA. Increased intraabdominal pressure in abdominoplasty: delineation of risk factors. Plast Reconstr Surg. 2007;119:1319–1325. doi: 10.1097/01.prs.0000254529.51696.43. [DOI] [PubMed] [Google Scholar]
- 8.Al-Basti HB, El-Khatib HA, Taha A, Sattar HA, Bener A. Intraabdominal pressure after full abdominoplasty in obese multiparous patients. Plast Reconstr Surg. 2004;113:2145–2150. doi: 10.1097/01.prs.0000122543.44977.46. discussion 2151–5. [DOI] [PubMed] [Google Scholar]
- 9.Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg. 2006;243:89–95. doi: 10.1097/01.sla.0000193959.44677.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schein M, Wittmann DH, Aprahamian CC, Condon RE. The abdominal compartment syndrome: the physiological and clinical consequences of elevated intra-abdominal pressure. J Am Coll Surg. 1995;180:745–753. [PubMed] [Google Scholar]