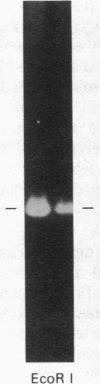
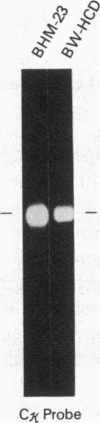
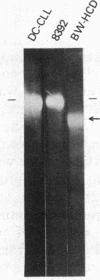
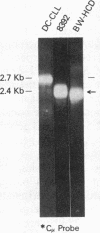
Abstract
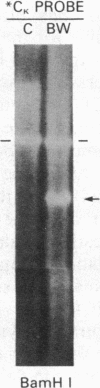
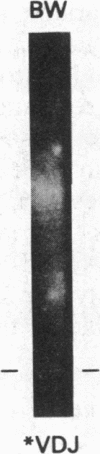
The human heavy chain disease protein BW is an immunoglobulin mu-chain variant whose amino terminus is initiated at the fifth amino acid of the first constant region domain. We cloned and analyzed both rearranged heavy chain alleles from BW leukemic cells to determine the molecular basis for this deleted protein. The phenotypically excluded heavy-chain allele possessed two intermediate recombinations of separate variable-diversity (V-D) and diversity-joining (D-J) junctions, neither of which were expressed. The productive allele, responsible for the mu chain, had a complete V-D-J4 recombination but as a result of a single-base deletion possessed stop codons within the variable region. More important, a small DNA insertion/deletion eliminated the J4 donor splice site. This necessitated an aberrant RNA splice between the leader region and the first constant region domain creating a shortened 2.35-kilobase muRNA. A recognition sequence for signal peptidase predicted a cleavage at the fifth amino acid of the first constant region domain. These molecular events are responsible for the truncated mu chain that lacks a variable region and fails to assemble light chains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander A., Steinmetz M., Barritault D., Frangione B., Franklin E. C., Hood L., Buxbaum J. N. gamma Heavy chain disease in man: cDNA sequence supports partial gene deletion model. Proc Natl Acad Sci U S A. 1982 May;79(10):3260–3264. doi: 10.1073/pnas.79.10.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshi A., Minowada J., Arnold A., Cossman J., Jensen J. P., Whang-Peng J., Waldmann T. A., Korsmeyer S. J. Lymphoid blast crises of chronic myelogenous leukemia represent stages in the development of B-cell precursors. N Engl J Med. 1983 Oct 6;309(14):826–831. doi: 10.1056/NEJM198310063091404. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Brandt C. R., Morrison S. L., Birshtein B. K., Milcarek C. Loss of a consensus splice signal in a mutant immunoglobulin gene eliminates the CH1 domain exon from the mRNA. Mol Cell Biol. 1984 Jul;4(7):1270–1277. doi: 10.1128/mcb.4.7.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Canaani E., Dreazen O., Klar A., Rechavi G., Ram D., Cohen J. B., Givol D. Activation of the c-mos oncogene in a mouse plasmacytoma by insertion of an endogenous intracisternal A-particle genome. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7118–7122. doi: 10.1073/pnas.80.23.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choi E., Kuehl M., Wall R. RNA splicing generates a variant light chain from an aberrantly rearranged kappa gene. Nature. 1980 Aug 21;286(5775):776–779. doi: 10.1038/286776a0. [DOI] [PubMed] [Google Scholar]
- Cossman J., Neckers L. M., Braziel R. M., Trepel J. B., Korsmeyer S. J., Bakhshi A. In vitro enhancement of immunoglobulin gene expression in chronic lymphocytic leukemia. J Clin Invest. 1984 Feb;73(2):587–592. doi: 10.1172/JCI111247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackowski W., Morrison S. L. Two alpha heavy chain disease proteins with different genomic deletions demonstrate that nonexpressed alpha heavy chain genes contain methylated bases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7091–7095. doi: 10.1073/pnas.78.11.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick W., Rabbitts T. H., Milstein C. An immunoglobulin deletion mutant with implications for the heavy-chain switch and RNA splicing. Nature. 1980 Aug 14;286(5774):669–675. doi: 10.1038/286669a0. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Franklin E. C., Frangione B., Prelli F. The defect in mu heavy chain disease protein GLI. J Immunol. 1976 Apr;116(4):1194–1195. [PubMed] [Google Scholar]
- Jelinek W. R., Schmid C. W. Repetitive sequences in eukaryotic DNA and their expression. Annu Rev Biochem. 1982;51:813–844. doi: 10.1146/annurev.bi.51.070182.004121. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J., Arnold A., Bakhshi A., Ravetch J. V., Siebenlist U., Hieter P. A., Sharrow S. O., LeBien T. W., Kersey J. H., Poplack D. G. Immunoglobulin gene rearrangement and cell surface antigen expression in acute lymphocytic leukemias of T cell and B cell precursor origins. J Clin Invest. 1983 Feb;71(2):301–313. doi: 10.1172/JCI110770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Smith L., Hawley R., Hozumi N., Shulman M. Intracisternal A-particle genes as movable elements in the mouse genome. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1992–1996. doi: 10.1073/pnas.80.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Potash M. J., Lehrach H., Shulman M. J. Deletions in immunoglobulin mu chains. EMBO J. 1982;1(5):555–563. doi: 10.1002/j.1460-2075.1982.tb01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. A mutant immunoglobulin light chain is formed by aberrant DNA- and RNA-splicing events. Nature. 1980 Aug 21;286(5775):779–783. doi: 10.1038/286779a0. [DOI] [PubMed] [Google Scholar]
- Seligmann M., Mihaesco E., Preud'homme J. L., Danon F., Brouet J. C. Heavy chain diseases: current findings and concepts. Immunol Rev. 1979;48:145–167. doi: 10.1111/j.1600-065x.1979.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Ravetch J. V., Korsmeyer S., Waldmann T., Leder P. Human immunoglobulin D segments encoded in tandem multigenic families. Nature. 1981 Dec 17;294(5842):631–635. doi: 10.1038/294631a0. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Strauss A. W., Boime I. Compartmentation of newly synthesized proteins. CRC Crit Rev Biochem. 1982 Mar;12(3):205–235. doi: 10.3109/10409238209108707. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Weinert T. A., Schaus N. A., Grindley N. D. Insertion sequence duplication in transpositional recombination. Science. 1983 Nov 18;222(4625):755–765. doi: 10.1126/science.6314502. [DOI] [PubMed] [Google Scholar]