Abstract
Motoneurons (MN) as well as most neuronal populations undergo a temporally and spatially specific period of programmed cell death (PCD). Several factors have been considered to regulate the survival of MNs during this period, including availability of muscle-derived trophic support and activity. The possibility that target-derived factors may also negatively regulate MN survival has been considered, but not pursued. Neurotrophin precursors, through their interaction with p75NTR and sortilin receptors have been shown to induce cell death during development and following injury in the CNS. In this study, we find that muscle cells produce and secrete proBDNF. ProBDNF through its interaction with p75NTR and sortilin, promotes a caspase-dependent death of MNs in culture. We also provide data to suggest that proBDNF regulates MN PCD during development in vivo.
Keywords: programmed cell death, apoptosis, muscle, p75NTR, sortilin, motor neurons
Introduction
Lumbar spinal motoneurons die during development in a spatially and temporally specific manner, and seminal experiments involving experimental manipulation of target size have demonstrated that the death of these cells is regulated by interaction with the target. For example, in the chick embryo, between E6 and E10, 40–50% of generated motoneurons die presumably because they have failed to acquire sufficient target-derived trophic support (Chu-Wang and Oppenheim, 1978 a, b). In fact, it has been proposed that unless otherwise rescued by survival signals (e.g., by trophic factors), neurons follow a default death program (Raff, 1992). The concept that targets actively regulate both survival and death of MNs has been proposed, but specific death promoting factors have not been identified (Cunningham, 1982; Oppenheim, 1991; Ricart et al., 2006). For sympathetic neurons target innervation is thought to initiate a series of feedback loops that mediate both the survival and death of these cells (Deppmann et al., 2008). In an earlier study, we found that although muscle derived factors support significant MN survival, a subpopulation of MNs die in a caspase 3/7 dependent manner in response to these factors (Taylor et al., 2007a).
Though targets may be the source of both pro and anti-apoptotic signals during the period of naturally occurring programmed cell death, with age MNs lose their reliance on targets, suggesting that over time, their susceptibility to these pro- and anti-apoptotic factors may be altered by context-dependent changes in trophic factor receptor expression, activation or utilization (Escandon et al., 1994; Funakoshi et al., 1995; Griesbeck et al., 1995; Fox et al., 2007) or as in the case of superior cervical ganglia cells the ability to fully activate the cell death program (Easton et al., 1997; Putcha et al., 2002). For example, the neurotrophin receptor p75NTR is highly expressed during MN development and throughout the period of PCD, but is down regulated postnatally and into adulthood (Ernfors et al., 1989; Johnson et al., 1999). The role of p75NTR during these developmental stages is unclear, however, due largely to the diversity of activating ligands. In various contexts, p75NTR has been implicated in neuronal survival, death and regeneration, through its association with the Trk, Sortilin, LINGO and Nogo receptors. We have previously shown that embryonic MNs die in response to target-derived factors via p75NTR; however maturation of these neurons in vitro prevents this from occurring (Taylor et al., 2007a).
Neurotrophin precursors have been shown to contribute to neuronal death by signaling through the p75NTR and the sortilin receptors (Lee et al., 2001; Beattie et al., 2002; Nykjaer et al., 2004; Teng et al., 2005; Massa et al., 2006; Domeniconi et al., 2007; Koshimizu et al., 2010; Tauris et al., 2011). These proneurotrophins (e.g. proBDNF and proNGF) can be secreted from cells, or can be cleaved intracellularly leading to the formation of mature neurotrophin dimers. In this study we find that proBDNF derived from the target muscle leads to the death of MNs in a sortilin and p75NTR –dependent manner in vitro. Moreover, functional blockade of sortilin and proBDNF significantly reduces caspase 3/7 activity that is observed in MNs following treatment with muscle-derived factors. Lastly, we provide data that suggest a similar mechanism of MN death during PCD in vivo.
Materials and Methods
MN cultures
MNs were obtained and cultured from E5.5 chick embryos (Poulet Rouge Fermier; Joyce Foods; Winston-Salem, NC) as previously described (Bloch-Gallego et al., 1991; Milligan et al., 1994; Taylor et al., 2007b). Lumbar spinal cords were dissected into Ca2+ free phosphate buffered saline (PBS), digested in 0.05% Trypsin, dissociated, layered on a 5% Iodixanol (Sigma; St. Louis, MO) gradient, and centrifuged at 900g. The interface layer, which is highly enriched with MNs was collected, resuspended in complete media, and centrifuged at 700g over a 4% bovine serum albumin (BSA) cushion. The pellet was collected, MNs were resuspended and plated in complete media on poly-DL-ornithine hydrobromide (Sigma; 10 µg/ml) and laminin (Sigma; 2 µg/ml) coated dishes at a density of 100 cells/mm2. Cultures that received conditioned media and/or function blocking antibodies were treated one hour after plating. Antibodies, trophic factors, and inhibitors used to treat cultures were anti-p75NTR (AB1554; Chemicon; Temecula, CA), anti-NGF 2.5S (N-6655; Sigma), anti-BDNF (AB1513P; Chemicon), anti-proBDNF (AB9042; Chemicon), anti-NT3 (AB1780SP; Chemicon), anti-Sortilin (612101; BD Biosciences; San Jose, CA), mouse NGF 2.5S and human NT3 (Alomone Labs; Jerusalem, Israel), human BDNF (a kind gift of Amgen; Thousand Oaks, CA), Neurotensin 8–13 (Bachem; King of Prussia, PA), and z-DEVD-FMK (R & D Systems; Minneapolis, MN).
MN viability was assessed at 3 days in vitro (DIV) utilizing 1 µM of the cell-permeable, vital dye, Calcein-AM (Invitrogen; San Diego, CA). MNs were counted and their purity determined at 20× magnification under epifluoresence using previously established criteria (Taylor et al., 2007b). Each condition was typically performed in triplicate and was replicated in at least four different cultures.
The MN cultures used here have been extensively characterized in terms of survival and death in our lab and by others (see for example, Milligan et al., 1994, 1995; Barnes et al., 1997; Li et al., 1998, 2001; Newbern et al., 2005, 2007; Taylor et al., 2007 a,b; Robinson et al., 2005). Cells are plated at a density of 104/1.1 cm2. Two hours after plating, the number of cells present in conditions with or without muscle extract is the same. Within 48 hours of cultures, the number of cells supplied with muscle extract is similar to the number observed at 2 hours; however, the number of cells present in cultures without muscle extract at 48 hours is significantly reduced (Milligan et al., 1994). For this reason, cells with muscle extract have been considered control conditions. Results are expressed as % control where control represents cells cultured with muscle extract, muscle conditioned media, or astrocyte conditioned media.
Astrocyte cultures
Astrocytes were isolated from the lumbar spinal cord of E10 chick embryos using a modification of methods previously described (Levison and McCarthy, 1991). Following isolation, spinal cords were digested in 0.05% Trypsin, dissociated, layered on a 4% BSA cushion, and centrifuged at 500g. The resulting cell pellet was resuspended in DMEM-F12 supplemented with 10% Fetal Bovine Serum (Invitrogen), Glutamax, penicillin/streptomycin, and fungizone, and plated in a T-75 flask. Cultures were grown to confluencey, at which time contaminating cells were removed by shaking flasks overnight at 260 rpm. Remaining astrocytes were then lifted off with 0.25% Trypsin, pelleted, rinsed, and differentiated in DMEM-F12 containing G5 (Invitrogen), a supplement that supports the growth of astrocytes (Michler-Stuke and Bottenstein, 1982). Cultures were routinely monitored for purity and were ≥ 99% immunoreactive for GFAP (Dako; High Wycombe, UK). Few, if any, dead cells were present in the cultures as determined by the Live/Dead kit (Invitrogen).
Muscle cell cultures
Muscle cells were isolated from E11 chick hindlimb as described previously, with minor modifications (Neville et al., 1998; Robinson et al., 2005). Muscle cells were grown to confluence in serum containing growth medium where single myocytes fuse into myotubes. Myotubes are then maintained in serum-free differentiation medium for the duration of the culture. After differentiation and fusion of myocytes, spontaneously contracting skeletal muscles were observed. Few, if any, dead cells were present in the cultures as determined by the Live/Dead kit (Invitrogen).
Preparation of astrocyte and muscle conditioned media
Astrocytes and muscles were cultured as described above. Upon reaching a desired density, cultures were maintained in basal media (DMEM-F12 for astrocytes, DMEM for muscles) for 48 hours. Media was collected, centrifuged at 200g, and passed through a 40 µm nylon cell strainer. Conditioned media proteins were precipitated with a 25–75% fraction of (NH4)2SO4 and were collected by centrifugation at 20,000g. Pellets were resuspended in MilliQ water, and dialyzed against PBS, pH 7.4. Following dialysis, proteins were passed through a 0.2 µm filter, the protein concentration determined, and stored at −80 C until further use.
In ovo treatments
Fertilized chick eggs were incubated at 37°C with 60% humidity until embryonic day (E) 5 (Stage 26; Hamburger and Hamilton, 1951). On E5, a window was created in the shell over the embryo. The window was sealed with Parafilm and the eggs were returned to the incubator. From E6 to E8 embryos were treated daily with the following agents: anti-BDNF (40 µg/dose; AB1513P; Chemicon), anti-proBDNF (10 µl/dose; AB9042 Chemicon), anti-Sortilin (20 µg/dose; 6121101 BD Biosciences) or anti-p75NTR (10 µl/dose; 9651 kind gift from Moses Chao, Huber and Chao, 1995). Treatment agents were diluted in PBS + 0.1% BSA to a final volume of 200 µl and applied to the highly vascularized chorioallantoic membrane. On E9, embryos were decapitated, and placed in Carnoy’s fixative overnight and 70% ethanol the following day. After 24 hours in ethanol, the lumbar spinal cord was dissected and embedded in paraffin. Embedded tissue was cut into 10 µm sections, stained with thionin, and MNs counted in every 20th section as previously described (Chu-Wang and Oppenheim, 1978a,b; Clarke and Oppenheim, 1995).
Developmental analysis of proBDNF, BDNF, p75NTR and sortilin receptor expression
Thigh muscles and lumbar spinal cords were collected from chick embryos daily between E5 and E12. Tissue was flash frozen in liquid nitrogen, weighed, and the protein extracted with 10 volumes of lysis buffer (Muscle: 0.1 M PIPES, pH 7.0 containing 0.5 M NaCl, 0.1% Triton X-100, 1 µg/ml leupeptin, 1 µg/ml aprotinin, and 1 mM PMSF; Spinal Cord: 50 mM Tris, pH 7.5 containing 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 µg/ml leupeptin, 1 µg/ml aprotinin, and 1 mM PMSF). Samples were sonicated, cleared by centrifugation, and assayed for protein content. Equivalent amounts of protein were resolved on 10% SDS-polyacrylamide gels that were subsequently transferred to Immobilon-P membrane (Millipore; Billerica, MA). Membranes were blocked with 5% milk prepared in Tris Buffered Saline containing 0.1% Tween-20 (TBS-T), followed by overnight incubation with the following primary antibodies diluted according to the manufacturer’s specifications: proBDNF (AB5613P; Chemicon), BDNF (AB1513P; Chemicon) p75NTR (N3908; Sigma), Sortilin (612101; BD Biosciences) actin (MAB1501; Millipore). Membranes were rinsed, incubated in the appropriate HRP-conjugated secondary antibody (Jackson ImmunoResearch; West Grove, PA), and proteins were detected by electrochemiluminescence (Pierce; Rockford, IL). As the expression of housekeeping proteins fluctuates during development, equal loading was confirmed by incubation of membranes in 0.1% India ink diluted in TBS-T (Havercroft and Cleveland, 1984; Kulwichit et al., 1998).
The antibody to BDNF did not recognize the epitope on Western blots using the standard lysis buffer to isolate protein from muscle. For these samples, we used the lysis buffer described in the antibody product sheet (100mM phosphate buffer containing 1 mM EDTA, 2 M guanidine hydrochloride (pH 7.2), 10 mM N-ethylmaleimide, 0.36 mM pepstatin, 1mM PMSF). The levels of BDNF were measured by densitometry and standardized to actin on the same blot. Unfortunately, the antibody to proBDNF did not detect the epitope in these protein samples. ProBDNF was therefore detected in protein samples collected in the standard lysis buffer described above. The levels of proBDNF were measured and standardized to actin in the same blot as was done for the BDNF blots. The standardized levels were used to determine the ratio of proBDNF to BDNF in developing muscle.
p75NTR immunoprecipitaion
Lumbar spinal cords were collected between E6 and E12, flash frozen in liquid nitrogen and the protein extracted by sonication in 50 mM Tris, pH 7.5 containing 150 mM NaCl, 1% NP-40, 1 mM EDTA and protease inhibitors. Following protein extraction, samples were cleared by centrifugation, and assayed for protein content. 200 µg of spinal cord protein was incubated with 0.2 µg of p75NTR antibody (ab3125; Abcam) for 2 hours at 4°C. Immune complex was collected on Protein G agarose (P4619; Sigma) for 4 hours at 4°C, centrifuged, and rinsed with 10 mM Tris, pH 7.0 containing 1M NaCl, 0.5% TX-100, and 1 mM EDTA. Complex was eluted off agarose by boiling in 2x Laemmli sample buffer for 5 minutes. Immunoprecipitations were verified with multiple p75NTR antibodies (N3908; Sigma, AB1554; Chemicon).
Determination of caspase 3/7 activity
MNs were scraped into cell lysis buffer (50 mM Tris, pH 7.5 containing 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 µg/ml leupeptin, 1 µg/ml aprotinin, and 1 mM PMSF), sonicated, and cleared by centrifugation. After analyzing its protein concentration, caspase 3/7 activity was determined using the Apo-One Homogenous Caspase 3/7 Assay (Promega; Madison, WI). One microgram of cell extract that was diluted into 20 µl of lysis buffer was mixed with an equal volume of substrate in the supplied buffer. The fluorescent signal, which resulted after 1 hour of incubation, was read using a spectrofluorometer at 488 λ/535 λ.
proBDNF
The cleavage-resistant proBDNF protein was used in these experiments. The purified histidine-tagged, cleavage-resistant proBDNF was prepared as previously described (Woo et al., 2005; Pang et al., 2004). Point mutations were introduced into the proconvertase cleavage site of proBDNF to convert the Arg-Arg (amino acid positions 129,130) to Ala-Ala. In vitro experiments demonstrated that this proBDNF is resistant to cleavage by plasmin. The protein was dialyzed against ACSF, and stored in small aliquots at −80°C until use.
BDNF Protein Measurement using ECLIA
Chick lower limb muscle was rapidly dissected in cold PBS (4°C), pH 7.4, and snap frozen in isopentane cooled by dry ice. Tissue samples were stored at −80°C before homogenization. BDNF was extracted according to previously published protocols, and ECLIA was used to determine BDNF protein concentrations, and was performed as previously described (Ma et al., 1998; Pollock et al., 2001).
Results
Inhibition of muscle-derived BDNF, but not other neurotrophins increases MN survival
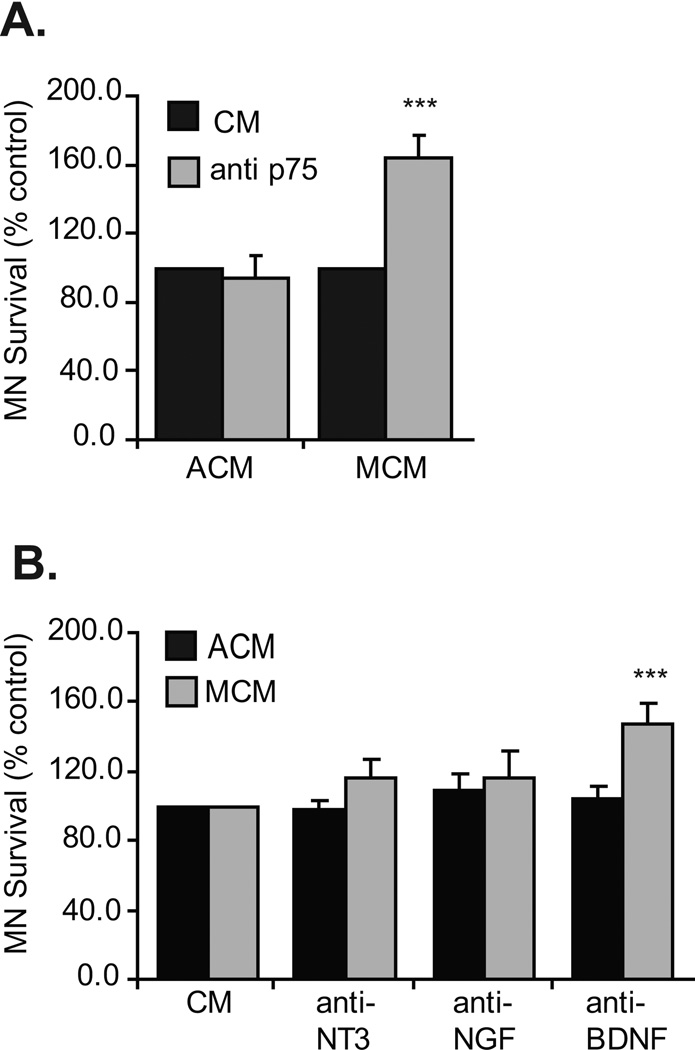
Target derived trophic support has been extensively demonstrated to promote MN survival both in vivo and in vitro (see Oppenheim, 1996; Henderson et al., 1998 for reviews). For MNs in culture, 20–30% of cells, as compared to those supplied with muscle extract, a potent source of target derived trophic support, survive if they are cultured without muscle extract (Milligan et al., 1994). We previously observed that if cells are cultured with astrocyte conditioned media (ACM), there was a 3–8-fold increase in survival as compared to muscle extract or muscle conditioned media (MCM) (Taylor et al., 2007a). Furthermore, MNs treated with MCM died in a p75NTR and caspase 3/7 dependent manner (Taylor et al., 2007a; Figure 1A). In an attempt to identify the p75NTR ligand responsible for MN death, MCM-treated cultures were exposed to function-blocking antibodies targeted to individual neurotrophins. Although blockade of NGF and NT3 from MCM did not enhance MN survival, inhibition of BDNF significantly increased MN survival (Figure 1). To confirm that this result was specific to MCM, MNs were also cultured with ACM and treated with the functional blocking antibodies. As expected, blockade of these neurotrophins did not alter MN survival (Figure 1).
Figure 1. p75NTR and BDNF blockade increases MN survival in response to muscle-derived factors in vitro.
A. MNs were cultured from the time of plating with either ACM or MCM in the presence or absence of p75NTR neutralizing antibodies and viability determined after three days. Though neutralization of p75NTR function did not affect MNs treated with ACM, a significant subpopulation of MNs treated with MCM was rescued (***p<0.001; ANOVA with Tukey-Kramer post hoc test; n=6 cultures of 3 wells / condition / culture). B. MNs were cultured from plating with either ACM or MCM in the presence or absence of NT3, NGF or BDNF neutralizing antibodies and viability determined after three days. Neutralization of these factors did not alter ACM-induced MN survival; however blockade of BDNF increased the survival of MCM-treated MNs (***p<0.001; ANOVA with Tukey-Kramer post hoc test; n=5 cultures of 3 wells / condition / culture). For all in vitro experiments, results are expressed as mean ± SEM of % control where control represents cultures supplied with appropriate conditioned media.
Inhibition of the sortilin receptor increases MN survival in response to MCM
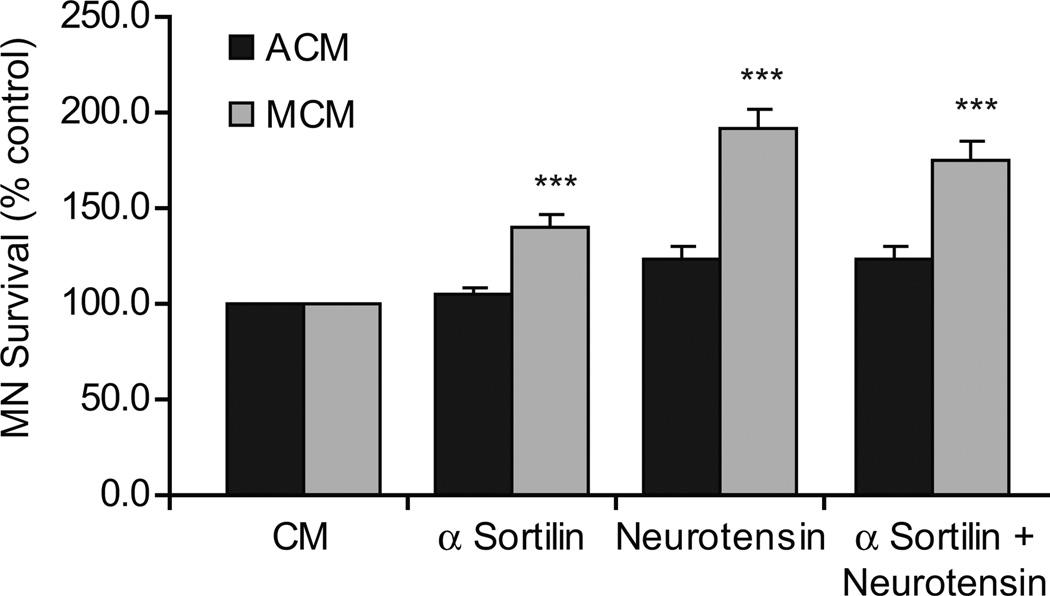
Neuronal death in response to neurotrophin signaling is thought to be regulated through a protein complex consisting of the p75NTR and sortilin receptors. To evaluate the role of the sortilin receptor in MCM-induced death, we blocked its function in MNs exposed to MCM. In the presence of sortilin receptor blockade, a significant number of MNs were rescued from MCM-induced death (Figure 2). Furthermore, saturating amounts of neurotensin (80 µM), an endogenous ligand of the sortilin receptor, inhibited the death of MNs in response to MCM (Figure 2). We confirmed in dose-response experiments that neurotensin treatment alone does not confer any inherent survival benefits to MNs, as treatment with this peptide in the absence of additional trophic factor did not augment (or reduce) their survival (data not shown). When MCM-treated MNs were supplemented with both the sortilin receptor blocking antibody and the neurotensin peptide, no additive survival was observed, suggesting that neurotensin was in fact blocking the pro-apoptotic effects of MCM through the sortilin receptor, in accordance with the findings of others (Teng et al., 2005).
Figure 2. Sortilin receptor blockade increases MN survival in response to muscle-derived factors in vitro.
MNs were cultured from plating with either ACM or MCM in the presence or absence of sortilin receptor neutralizing antibodies, neurotensin, or optimal concentrations of both, and viability determined after 3 days. Neutralization of the sortilin receptor with either function-blocking antibodies or with saturating concentrations of its endogenous ligand, neurotensin, resulted in a significant increase in MN survival in response to MCM (***p<0.001; ANOVA with Tukey-Kramer post hoc test; n=5 cultures of 3 wells / condition / culture).
MCM-induced caspase 3/7 activity is reduced in response to BDNF and sortilin receptor inhibition
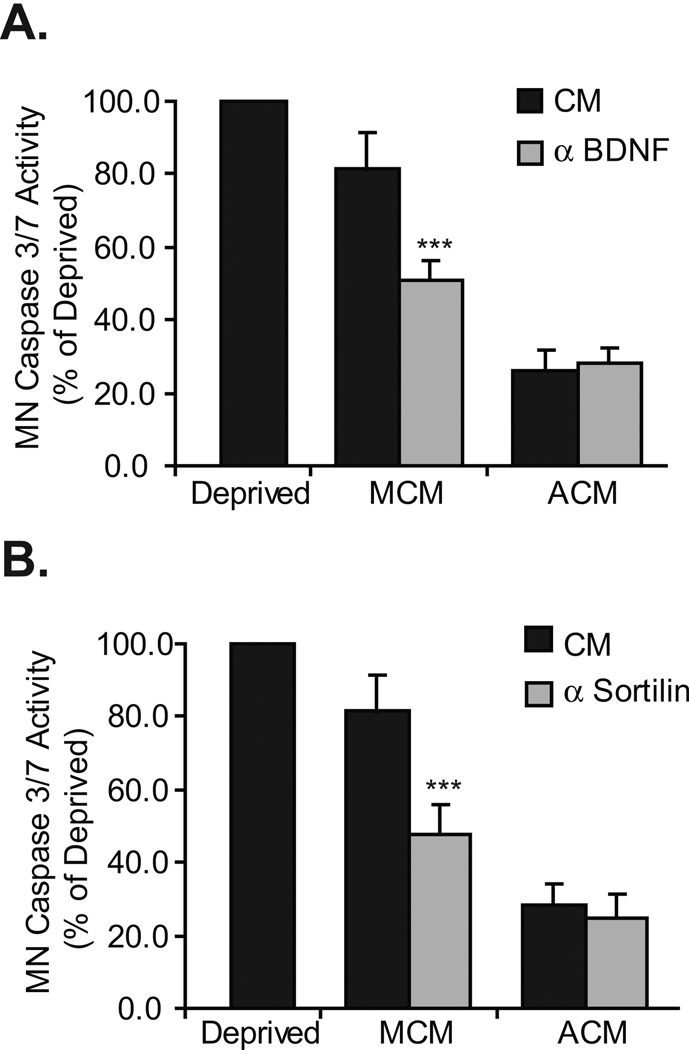
We have previously shown that within 24 hours in vitro, caspase 3/7 activity is increased in MCM-treated MNs, whereas no change in activity was seen in ACM-treated MNs. The MCM-induced caspase 3/7 activity was reduced by p75NTR blockade (Taylor et al., 2007a). Based on our findings that implicate p75NTR, the sortilin receptor and a BDNF-like neurotrophin in the death of a subpopulation of MCM-treated MNs, we analyzed caspase 3/7 activity in MN cultures that were treated with sortilin receptor- or BDNF-blocking antibodies. As expected, inhibition of BDNF or the sortilin receptor had no effect on the caspase 3/7 activity of ACM treated MNs; in MCM treated cells, caspase 3/7 was significantly reduced by blockade of these receptors (Figure 3).
Figure 3. Blockade of BDNF and the sortilin receptor reduces MCM-induced MN caspase 3/7 activation.
A and B. MNs were cultured with either ACM or MCM in the presence or absence of either BDNF or sortilin receptor neutralizing antibodies. After 24 hours in culture, protein extracts were collected, and caspase 3/7 activity measured. Although BDNF (A) and sortilin receptor (B) blockade did not affect ACM-induced MN caspase 3/7 activation, it did significantly reduce caspase 3/7 activation in response to treatment with MCM (***p<0.001; ANOVA with Tukey-Kramer post hoc test; n=5).
Target-derived proBDNF promotes MN apoptosis in vitro
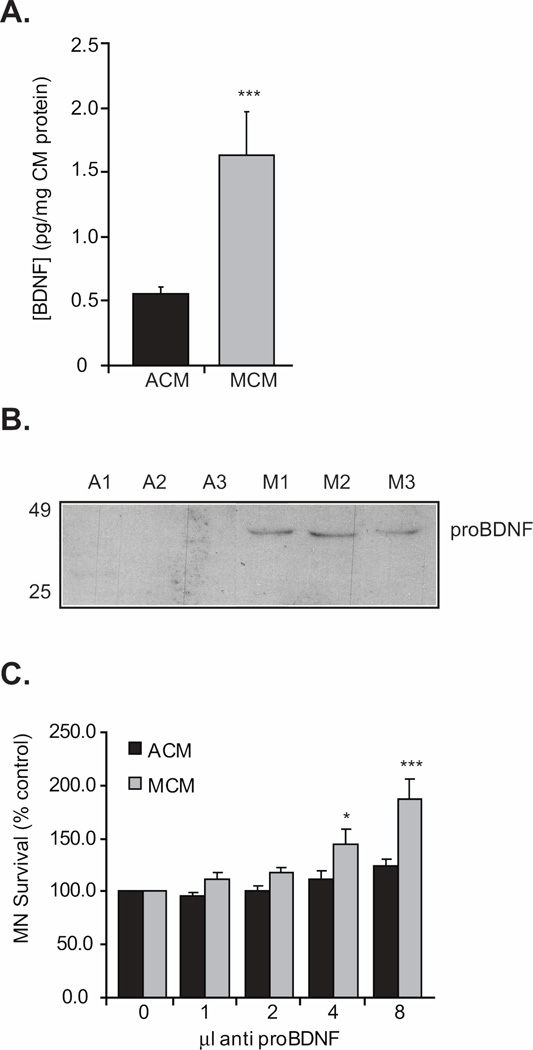
Collectively, our findings support the hypothesis that BDNF from the target signals through a complex of the p75NTR and sortilin receptors, and acts as a pro-apoptotic signal for a subpopulation of MNs. To address this, we measured the levels of BDNF in muscle cell and astrocyte-derived conditioned media. The BDNF detected in MCM was more than three times the amount detected in ACM (Figure 4A). Although neurotrophins are classically classified as pro-survival molecules, recent studies have implicated the unprocessed, pro form of these proteins in neuronal death (Teng et al., 2005; Volosin et al., 2006; Domeniconi et al., 2007; Koshimizu et al., 2009). While the assay utilized to measure BDNF expression is extremely sensitive (Ma et al., 1998; Pollock et al., 2001), it is not designed to distinguish between the pro form and the mature form of BDNF. Additionally, the epitope for the BDNF function-blocking antibody used in the culture experiments above is present in both pro- and mature BDNF. This was confirmed when we used the function-blocking BDNF antibody with MCM for immunoprecipitation and then the anti-proBDNF antibody for immunoblot. This experiment resulted in a single 32 kD band corresponding to the predicted size for proBDNF (not shown). To further test whether proBDNF was enriched in MCM, we performed western blots using conditioned media and an antibody that specifically recognizes proBDNF. Our data indicate that proBDNF is present in MCM, and not detected in ACM (Figure 4B). Furthermore, using the antibody to BDNF, we could not detect the mature form in MCM suggesting that if the mature form of BDNF is present, it is below the level of detection. We next tested whether proBDNF in MCM is responsible for the apoptosis a subset of MNs in vitro. In the presence of a proBDNF-blocking antibody, a significant number of MCM-treated MNs were rescued, further implicating this protein in the apoptosis of some MNs in response to target-derived factors (Figure 4C).
Figure 4. Target-derived proBDNF is present in MCM and promotes MN apoptosis in vitro.
A. BDNF from MCM was three times more abundant than that measured in ACM (***p<0.001; Student T-test; n=4). B. Western blot analysis revealed that MCM but not ACM contained proBDNF while ACM did not. Three different samples of MCM (M1, M2 or M3) or ACM (A1, A2 or A3) were used with consistent results in each. We never detected mature BDNF in MCM (not shown). C. MNs were cultured with either ACM or MCM in the presence or absence of a proBDNF neutralizing antibody and viability determined three days later. Increasing amounts of this antibody resulted in significantly increased survival in MCM-treated MNs (*p<0.05, ***p<0.001; ANOVA with Tukey-Kramer post hoc test; n=6 cultures of 3 wells / condition / culture).
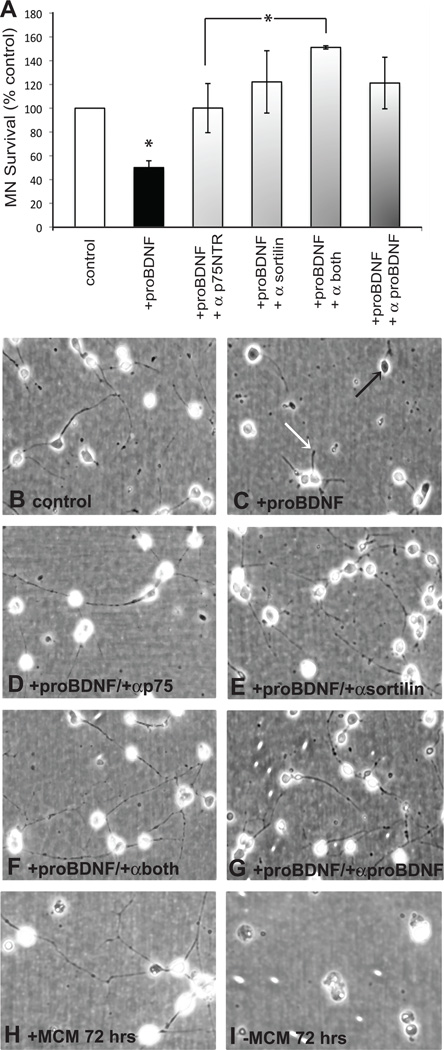
To directly determine if proBDNF induces the death of MNs, we cultured cells either with or without proBDNF and determined survival after 24 hours. We found a statistically significant reduction in the number of surviving cells in conditions with proBDNF, and this result was abolished when the antibody to proBDNF was included in the culture (Figure 5). To confirm that proBDNF exerted its anti-survival activity via p75NTR or sortilin, cultures supplied with proBDNF were also treated with function-blocking antibodies to p75NTR, sortilin or both. Treatment with the antibodies blocked proBDNF’s death promoting activity, and addition of both antibodies resulted in enhanced survival as compared to cultures treated with either antibody alone.
Figure 5. Blockade of both p75NTR and sortilin results in significantly enhanced MN survival in the presence of proBDNF.
A. MNs were plated with or without proBDNF (4 ng/ml). Cells treated with proBDNF were also cultured with function-blocking antibodies to p75NTR, sortilin, both p75NTR and sortilin, or proBDNF. MN survival was determined 24 hours after plating. There were significantly fewer surviving cells in cultures supplied with proBDNF as compared to cultures without proBDNF or with proBDNF plus inhibition of p75NTR, sortilin, or proBDNF (*p<0.05, ANOVA with Newman-Keuls Multiple Comparison post hoc test; n=3 cultures of 3 wells / condition / culture). Inhibition of both p75NTR and sortilin resulted in enhanced survival as compared to inhibition of either receptor alone, although significance was only reached against p75NTR. (*p<0.05). Cells cultured with the antibodies alone exhibited no change in survival as compared to control cultures (not shown). B–G. MNs in culture for 24 hours without proBDNF show phase bright cell bodies and neurite outgrowth (B), while cells cultured with proBDNF do not exhibit phase-bright cells bodies (C, black arrow) and have reduced neurite outgrowth (C, white arrow). Cells cultured with proBDNF and the function-blocking antibodies exhibit characteristics similar to cells cultured without proBDNF (D–G). Images are also shown of cells cultured with MCM for 72 hours indicating phase-bright cell bodies and extensive neurite outgrowth (H). At 24 hours in culture cells with or without MCM are not distinguishable (see Milligan et al., 1994); however after 72 hours in conditions without trophic support few cells remain in culture (I).
proBDNF/sortilin/p75NTR signaling complex in vivo
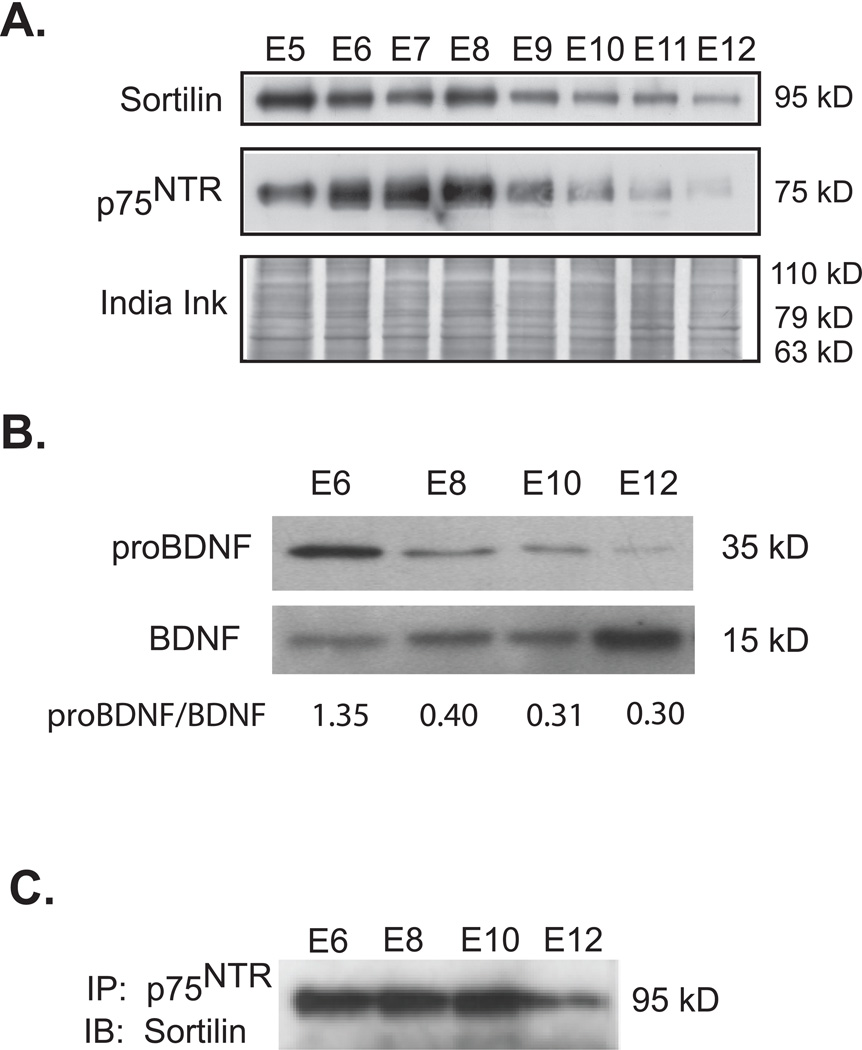
Our results thus far suggest that target-derived proBDNF acts through a p75NTR and sortilin receptor complex to result in the death of a subpopulation of MNs in vitro. We therefore analyzed the expression of these proteins during the major period of naturally occurring programmed cell death in the developing chick embryo that occurs between embryonic day (E) 5–12 with the peak at E7.5–8.0 (Hamburger 1958; 1975; Chu-Wang and Oppenheim 1978a,b; Oppenheim et al., 1978). In the lumbar spinal cord, the expression of p75NTR and sortilin was high between E5 and E8, and gradually declined between E9 and E12 (Figure 6A). The presence of proBDNF in MCM could be the result of culture conditions. We therefore also examined the expression of proBDNF in the chick hindlimb. ProBDNF levels were greatest at E6 and decreased after this time point, whereas the levels of mature BDNF are low at E6 and appear to increase until E12 (Figure 6B). As proBDNF is thought to signal through a receptor complex of p75NTR and sortilin, we immunoprecipitated spinal cord extracts with a p75NTR antibody and analyzed its association with sortilin (Teng et al., 2005). The complex of p75NTR and sortilin was greatest at E6–10, reminiscent of the expression of proBDNF (Figure 6C). We confirmed these results with three unique p75NTR antibodies, although the results from only one of these are presented (Figure 6C; data not shown).
Figure 6. p75NTR, Sortilin and proBDNF expression in developing spinal cord and muscle corresponds and contributes to the period of naturally occurring MN cell death.
A. Expression of the sortilin receptor and p75NTR in the spinal cord was analyzed throughout the period of naturally occurring PCD. Western blots indicate that both of these proteins were highly expressed early in the cell death period, but their expression was gradually reduced after reaching a peak at E8. B. Expression of proBDNF or BDNF in the hindlimb muscles was analyzed throughout PCD. Western blots indicate that the expression of proBDNF in hindlimb muscles is highest at E6 and decreases at later stages. BDNF’s expression shows an opposite pattern to proBDNF with the highest expression of BDNF occurring at E12. The ratio of proBDNF/BDNF densitometry also indicates this pattern. C. Spinal cord protein extracts were immunoprecipitated with a p75NTR antibody, and subsequently immunoblotted with a sortilin receptor antibody. p75NTR and sortilin complexes were greatest during PCD, similar to the proBDNF expression.
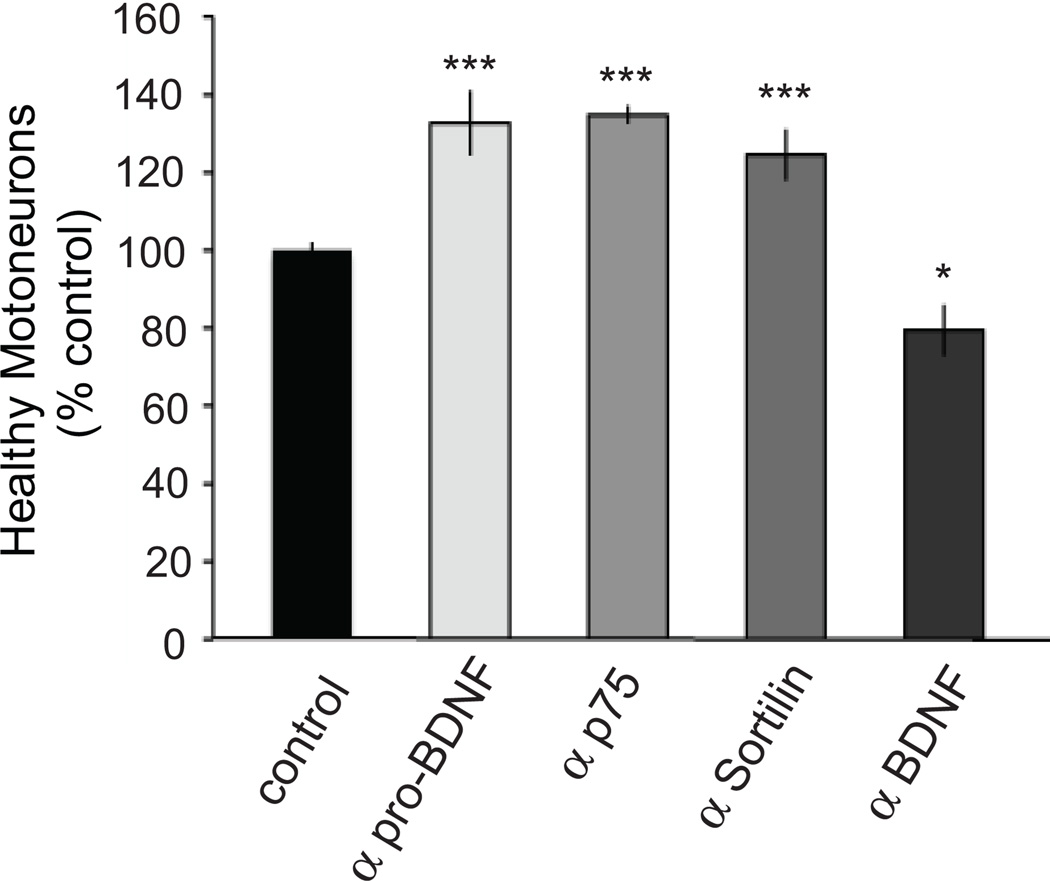
To determine if sortilin, p75NTR, BDNF or proBDNF blockade can rescue MNs from PCD in ovo, we treated embryos with receptor blocking antibodies applied to the highly-vascularized chorioallantoic membrane daily between E6 to E8. Healthy MNs were counted in the lumbar spinal cord of E9 embryos using well-established methods (Chu-Wang and Oppenheim, 1978a,b; Clarke and Oppenheim, 1995). Similar to our in vitro findings, daily treatment with the p75NTR or sortilin receptor blocking antibody, or the pro-BDNF neutralizing antibody rescued a significant number of MNs from naturally occurring PCD (Figure 7). In contrast, daily treatment with a BDNF neutralizing antibody resulted in fewer MNs at E9 than in controls, confirming the survival-promoting activity of BDNF previously reported (Oppenheim et al., 1992). We should note that there was a higher incidence of embryo death in the anti-proBDNF group compared to the other groups, presumably because the neutralizing antibody is produced in chick.
Figure 7. Functional blockade of sortilin, p75NTR or proBDNF results in increased MN survival during the period of naturally occurring cell death.
Treatment with the pro-BDNF function-blocking antibody (10 µl antibody in 100 µl saline from E6–8) resulted a significant increase in the number of healthy MNs on E9. Treatment with a sortilin receptor or p75NTR function-blocking antibody (20 µg/day from E6–8) also resulted in a significant increase in healthy MNs on E9. In ovo treatment with a BDNF function-blocking antibody resulted in a decrease in the number of surviving MNs. Results are expressed as % control, where control represents the number of healthy MNs on E9 in embryos that were treated with vehicle (PBS + 0.1% BSA; p<0.05, **p<0.01; ANOVA with Tukey-Kramer post hoc test; n=13 controls, 3 anti-proBDNF, 5 anti-p75NTR, 5 anti-sortilin, 4 anti-BDNF).
Discussion
Our results indicate that muscle cells produce factors that promote both cell survival and death, and that proBDNF appears to play a specific role in mediating MN programmed cell death. MNs are polytrophic, in that they require multiple factors to maximally sustain their survival (Nishi, 1994; Gould and Oppenheim, 2004); however, even with treatment of muscle extract (MEx), one of the most potent experimental sources of trophic support, not all MNs can be rescued from naturally occurring or induced PCD (Oppenheim et al., 1988). This result suggests two possible hypotheses that are not necessarily mutually exclusive. One such possibility is that the target is not the sole trophic source for MNs (Okado and Oppenheim, 1984; Eagleson et al., 1985; Furber et al., 1987; Yin et al., 1994; Riethmacher et al., 1997; Arce et al., 1998; Wong et al., 1999). The other is that the target provides MNs with a complex balance of survival and pro-apoptotic signals that in sum, determine the fate of any given cell (Oppenheim, 1991; Pettmann and Henderson, 1998; Raoul et al., 2000; Ricart et al., 2006). Therefore, provision of MNs with increasing amounts of MEx (or by augmentation of target size) may result in the increase of both trophic and toxic factors, thereby making the rescue of all MNs impossible by these measures. Our data suggest that both of these possibilities may be true. We have previously shown that astrocytes are a potent source of trophic support for MNs in vitro and following induced PCD in vivo (Taylor et al., 2007a). Here, we report that in addition to trophic factor production, muscle produces proBDNF that may promote MN death via interaction with the p75NTF receptor and sortilin. This interaction appears to be temporally regulated because expression of proBDNF in muscle and the receptor complex in lumbar spinal cord are highest during the period of programmed cell death.
The most common hypothesis underlying the survival of MNs during the PCD is that neurons participate in a competition for limited amounts of trophic factor from the target, and the cells that lose this competition ultimately die (Cunningham, 1982; Oppenheim, 1991; Sendtner et al., 2000; Yuan and Yankner, 2000). This implies that survival is an active process and that death occurs by default, although the presence of one or more pro-apoptotic signals from the target has been suggested (Oppenheim, 1991; Pettmann and Henderson, 1998; Raoul et al., 2000; Ricart et al., 2006). There are several lines of evidence that indirectly suggest the presence of a pro-apoptotic signal for MNs during development. First, doubling of the target size does not result in a doubling of the number of surviving MNs, though inadequate limb innervation remains an alternative explanation (Hollyday and Hamburger, 1976). Second, activity blockade, or genetic inhibition of NMJ function results in greatly increased, but not total MN survival following PCD (Pittman and Oppenheim, 1978; Pittman and Oppenheim, 1979; Houenou et al., 1991; Yaginuma et al., 1996; Caldero et al., 1998; Banks et al., 2001; Banks and Noakes, 2002). Finally, treatment with high concentrations of MEx results in MN death in vitro, perhaps due to saturation of Trk receptors and subsequent activation of p75NTR (Henderson et al., 1984).
p75NTR is highly expressed on MNs during PCD and following injury, and its activation can result in MN death in a context-dependent manner (Mckay et al., 1996; Ferri et al., 1998; Johnson et al., 1999; Wiese et al., 1999; Boyd and Gordon, 2001; Copray et al., 2003; Gschwendtner et al., 2003). Several reports suggest that MNs can die as a result of p75NTR activation; however, much of this death is in response to injury or the reactive state of astrocytes, which stimulate the production of NGF, proNGF and other deleterious factors (reviewed in Barbeito et al., 2004; Domeniconi et al., 2007). A critical role for p75NTR in MN development has been questioned based on work examining the effects of axotomy on MN survival in the p75NTR knock-out mouse, in which the baseline number of MNs before axotomy of both p75NTR KO and WT was the same (Ferri et al., 1998). While these results suggest that p75NTR has no effect on developmental PCD there is no published report of changes in the timing or extent of naturally occurring MN death in the p75NTR knock-out mouse. Additionally, while unlikely, it is possible that our results are specific to the chick embryo. Interestingly, when embryonic mouse MNs isolated from either the p75NTR KO or WT mice were treated with either NGF or BDNF, MN death was increased in WT motoneurons, while there was no effect on p75NTR KO MN death (Wiese et al., 1999). Although these results were interpreted as a role for p75NTR in inducing MN death, they also are in agreement with our results indicating the p75NTR is a critical mediator of proBDNF’s death promoting activity. In the same study, when MNs in culture were treated with GDNF or CNTF, the effects of NGF or BDNF on WT MNs were negated. The authors discuss the possibility that GDNF/CNTF signaling overrides potential p75NTR death-inducing signals. We believe that this latter possibility can explain why there is no overt change in MN cell death or survival during normal development in the p75NTR KO animal (Ferri et al., 1998; Wiese et al., 1999). Furthermore, treatment of trophic factor-deprived MNs with β-neuregulin in combination with NGF, BDNF, NT-3 or NT-4, results in the p75NTR-dependent death of MNs in vitro (Boyd and Gordon, 2001; Ricart et al., 2006). β-neuregulin’s trophic support was counterbalanced by BDNF or NGF activation of the p75NTR (Ricart et al., 2006), further suggesting a balance of trophic and toxic mechanisms that mediate final MN number. If MN death is actually the result of a balance between survival and pro-apoptotic factors, a balance that favors pro-apoptotic factors, it is clear that following the period of PCD, some alteration(s) of either the MN or the target occurs inhibiting the response from this presumably deleterious signal. p75NTR expression may be a critical mediator of a MN’s response to a potential pro-apoptotic signal, because it is transiently expressed in the developing chick embryo between E3 and E12 exclusively in MNs (Mckay et al., 1996). Finally, our results in the current study indicating that blockade of p75NTR results in increased MN survival during the period of PCD further suggests a critical role of this receptor during development.
In addition to p75NTR, several studies have demonstrated that interactions of proneurotrophins require the co-receptor sortilin. Indeed, the interaction of proNGF with the p75NTR/sortilin complex initiates apoptosis in sympathetic neurons in culture (Nykjaer et al., 2004). Cell death could be blocked in this situation when the sortilin antagonist, neurotensin was included in the cultures, suggesting that the sortilin receptor is critical in mediating proneurotrophin cell death in neurons (Nykjaer et al., 2004). Our data suggest that the inactivation of the p75NTR/sortilin complex represents a critical switch in MNs limiting the death-promoting activity of proBDNF and promoting subsequent survival and maturation, though the mechanisms mediating this are not clear. Interestingly, it was previously shown that in MNs, p75NTR expression decreased after the period of PCD, while at this same time expression of TrkB increased (McKay et al., 1996). These results further suggest a switch in the MNs ability to respond to survival- or death-promoting factors.
In addition to their well-characterized role in promoting neuronal survival, the neurotrophins have been shown to have a role in synaptic modulation, neurotransmitter phenotype, growth cone guidance and protection from injury (reviewed in Poo, 2001; Hempstead 2006; Lykissas et al., 2007; Luther and Birren, 2009). The neurotrophins are synthesized as pro-forms that were originally thought to be inactive; however, this notion was proven incorrect when it was demonstrated that pro-NGF could preferentially bind to p75NTR vs. TrkA, and later that proBDNF mediates superior cervical ganglion death via a p75NTR/Sortilin receptor complex (Lee et al., 2001; Teng et al., 2005). ProBDNF has also been shown to facilitate long-term depression in the hippocampus (Woo et al., 2005). ProBDNF can be cleaved by intra- or extra-cellular mechanisms to generate mature (m) BDNF (Seidah et al 1996; Mowla et al., 2001; Lee et al., 2001). The extent of secretion of proBDNF as compared to the mature form may vary dependent on cell type, neuronal activity and age. For example, mouse hippocampal cells appear to secrete little to no proBDNF because of rapid intracellular cleavage to the mature form (Matsumoto et al., 2008). On the other hand, neuronal activity has been shown to also regulate the extracellular cleavage of proBDNF to mature BDNF: low frequency stimulation results in more proBDNF secretion, whereas high frequency stimulation results in more BDNF secretion (Nagappan et al., 2009). As reviewed above, NMJ activity regulates MN survival. Together these results suggest that neuronal activity may regulate the diametrically opposed actions of proBDNF and mBDNF. Additionally, proBDNF may also directly regulate neuronal activity because it has been shown to induce synaptic depression and terminal retraction in Xenopus nerve-muscle co-culture (Yang et al., 2009).
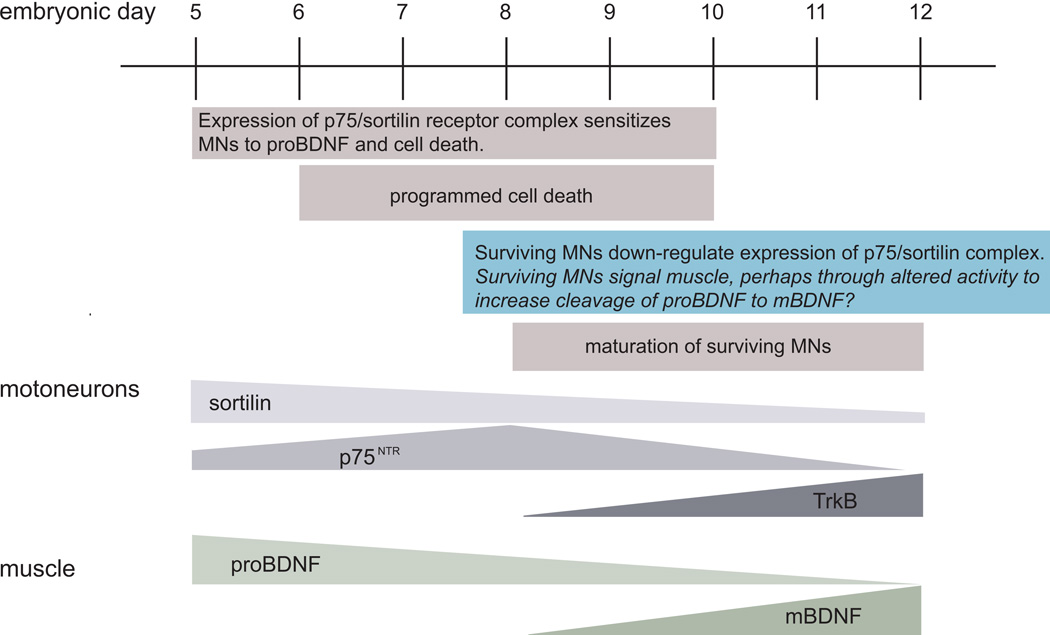
In sympathetic neurons, a three stage, target-initiated process is proposed to regulate cell survival. These cells appear to utilize two neurotrophin receptors and three neurotrophins to mediate neuronal sensitization, paracrine death signaling and finally protection from the two earlier events thereby promoting cell survival (Deppmann et al. 2008). Our results indicate that a similar competitive mechanism may regulate the survival of developing spinal MNs (Figure 8). While target-derived trophic factors may promote survival via their specific receptors, expression of p75NTR and sortilin may sensitize MNs for cell death mediated by proBDNF. During the period of PCD, MNs that respond more to proBDNF are “weakened” and die, while those that respond more to trophic factors are “strengthened” and survive. Surviving MNs contribute to maturation of NMJs that alters activity levels. The change in activity level may cause an increase in intra- or extracellular convertases that cleave pro-BDNF to mBDNF. The availability of mBDNF reinforces MN maturation, synapse development and survival. This model involves several hypotheses that serve as directions for further research that will contribute to our understanding of the role of survival and pro-apoptotic factors during MN development.
Figure 8. Summary diagram illustrating a model of MN development and programmed cell death that includes receptor expression on MNs and pro- and mature-BDNF expression in muscle.
The mechanisms that regulate muscle production and secretion of pro- vs. mature-BDNF are not known. We propose that alterations in surviving motoneuron/NMJ activity may play a role, although this remains a topic for further investigation (blue box).
Acknowledgements
This work was supported by grants NS036081 and NS53527 from NINDS, funds from the Brian White ALS Foundation at WFUSM and The Mr. & Mrs. A. Tab Williams, Jr. and Family Neuroscience Research and Program Development Endowment to CEM.
Literature Cited
- Arce V, Pollock RA, Philippe JM, Pennica D, Henderson CE, deLapeyriere O. Synergistic effects of Schwann- and muscle-derived factors on motoneuron survival involve GDNF and cardiotrophin-1 (CT-1) J Neurosci. 1998;18:1440–1448. doi: 10.1523/JNEUROSCI.18-04-01440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks GB, Noakes PG. Elucidating the molecular mechanisms that underlie the target control of motoneuron death. Int J Dev Biol. 2002;46:551–558. [PubMed] [Google Scholar]
- Banks GB, Chau TN, Bartlett SE, Noakes PG. Promotion of motoneuron survival and branching in rapsyn-deficient mice. J Comp Neurol. 2001;429:156–165. doi: 10.1002/1096-9861(20000101)429:1<156::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Barbeito LH, Pehar M, Cassina P, Vargas MR, Peluffo H, Viera L, Estevez AG, Beckman JS. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev. 2004;47:263–274. doi: 10.1016/j.brainresrev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Barnes NY, Li L, Yoshikawa K, Schwartz LM, Oppenheim RW, Milligan CE. Increased production of amyloid precursor protein provides a substrate for caspase-3 in dying motoneurons. J Neurosci. 1998;18(15):5869–5880. doi: 10.1523/JNEUROSCI.18-15-05869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch-Gallego E, Huchet M, el M'Hamdi H, Xie FK, Tanaka H, Henderson CE. Survival in vitro of motoneurons identified or purified by novel antibody-based methods is selectively enhanced by muscle-derived factors. Development. 1991;111(1):221–232. doi: 10.1242/dev.111.1.221. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. The neurotrophin receptors, trkB and p75, differentially regulate motor axonal regeneration. J Neurobiol. 2001;49:314–325. doi: 10.1002/neu.10013. [DOI] [PubMed] [Google Scholar]
- Caldero J, Prevette D, Mei X, Oakley RA, Li L, Milligan C, Houenou L, Burek M, Oppenheim RW. Peripheral target regulation of the development and survival of spinal sensory and motor neurons in the chick embryo. J Neurosci. 1998;18:356–370. doi: 10.1523/JNEUROSCI.18-01-00356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu-Wang IW, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. I. A light and electron microscopic study of naturally occurring and induced cell loss during development. J Comp Neurol. 1978a;177:33–57. doi: 10.1002/cne.901770105. [DOI] [PubMed] [Google Scholar]
- Chu-Wang I, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. II. A quantitative and qualitative analysis of degeneration in the ventral root, including evidence for axon outgrowth and limb innervation prior to cell death. J Comp Neurol. 1978b;177:59–86. doi: 10.1002/cne.901770106. [DOI] [PubMed] [Google Scholar]
- Clarke PG, Oppenheim RW. Neuron death in vertebrate development: in vitro methods. Methods Cell Biol. 1995;46:277–321. [PubMed] [Google Scholar]
- Copray JC, Jaarsma D, kust BM, Bruggeman RW, Mantingh I, Brouwer N, Boddeke HW. Expression of the low affinity neurotrophin receptor p75 in spinal motoneurons in a transgenic mouse model for amyotrophic lateral sclerosis. Neuroscience. 2003;116:685–694. doi: 10.1016/s0306-4522(02)00755-8. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ. Naturally occurring neuron death and its regulation by developing neural pathways. Int Rev Cytol. 1982;74:163–186. doi: 10.1016/s0074-7696(08)61172-9. [DOI] [PubMed] [Google Scholar]
- Deppmann CD, Mihalas S, Sharma N, Lonze BE, Niebur E, Ginty DD. A model for neuronal competition during development. Science. 2008;320(5874):369–373. doi: 10.1126/science.1152677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Hempstead BL, Chao MV. Pro-NGF secreted by astrocytes promotes motor neuron cell death. Mol Cell Neurosci. 2007;34:271–279. doi: 10.1016/j.mcn.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson KL, Raju TR, Bennett MR. Motoneurone survival is induced by immature astrocytes from developing avian spinal cord. Brain Res. 1985;349:95–104. doi: 10.1016/0165-3806(85)90135-x. [DOI] [PubMed] [Google Scholar]
- Easton RM, Deckwerth TL, Parsadanian AS, Johnson EM., Jr Analysis of the mechanism of loss of trophic factor dependence associated with neuronal maturation: a phenotype indistinguishable from Bax deletion. J Neurosci. 1997;17(24):9656–9666. doi: 10.1523/JNEUROSCI.17-24-09656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Henschen A, Olson L, Persson H. Expression of nerve growth factor receptor mRNA is developmentally regulated and increased after axotomy in rat spinal cord motoneurons. Neuron. 1989;2:1605–1613. doi: 10.1016/0896-6273(89)90049-4. [DOI] [PubMed] [Google Scholar]
- Escandon E, Soppet D, Rosenthal A, Mendoza-Ramirez JL, Szonyi E, Burton LE, Henderson CE, Parada LF, Nikolics K. Regulation of neurotrophin receptor expression during embryonic and postnatal development. J Neurosci. 1994;14:2054–2068. doi: 10.1523/JNEUROSCI.14-04-02054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri CC, Moore FA, Bisby MA. Effects of facial nerve injury on mouse motoneurons lacking the p75 low-affinity neurotrophin receptor. J Neurobiol. 1998;34:1–9. [PubMed] [Google Scholar]
- Fox MA, Sanes JR, Borza DB, Eswarakumar VP, Fassler R, Hudson BG, John SW, Ninomiya Y, Pedchenko V, Pfaff SL, Rheault MN, Sado Y, Segal Y, Werle MJ, Umemori H. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell. 2007;129:179–193. doi: 10.1016/j.cell.2007.02.035. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Furber S, Oppenheim RW, Prevette D. Naturally-occurring neuron death in the ciliary ganglion of the chick embryo following removal of preganglionic input: evidence for the role of afferents in ganglion cell survival. J Neurosci. 1987;7:1816–1832. doi: 10.1523/JNEUROSCI.07-06-01816.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29(41):12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbeck O, Parsadanian AS, Sendtner M, Thoenen H. Expression o neurotrophins in skeletal muscle: quantitative comparison and significance for motoneuron survival and maintenance of function. J Neurosci Res. 1995;42:21–33. doi: 10.1002/jnr.490420104. [DOI] [PubMed] [Google Scholar]
- Gould TW, Oppenheim RW. The function of neurotrophic factor receptors expressed by the developing adductor motor pool in vivo. J Neurosci. 2004;24(19):4668–4682. doi: 10.1523/JNEUROSCI.0580-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwendtner A, Liu Z, Hucho T, Bohatschek M, kalla R, Dechant G, Raivich G. Regulation, cellular localization, and function of the p75 neurotrophin receptor (p75NTR) during the regeneration of facial motoneurons. Mol Cell Neurosci. 2003;24:307–322. doi: 10.1016/s1044-7431(03)00167-2. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morphology. 1951;88:49–92. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hamburger V. Regression versus peripheral control of differentiation in motor hypoplasia. Am J Anat. 1958;102:365–410. doi: 10.1002/aja.1001020303. [DOI] [PubMed] [Google Scholar]
- Hamburger V. Cell death in the development of the lateral motor column of the chick embryo. J Comp Neurol. 1975;160:535–546. doi: 10.1002/cne.901600408. [DOI] [PubMed] [Google Scholar]
- Havercroft JC, Cleveland DW. Programmed expression of beta-tubulin genes during development and differentiation of the chicken. J Cell Biol. 1984;99:1927–1935. doi: 10.1083/jcb.99.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL. Dissecting the diverse actions of pro- and mature neurotrophins. Curr Alzheimer Res. 2006;3(1):19–24. doi: 10.2174/156720506775697061. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Huchet M, Changeux JP. Neurite-promoting activities for embryonic spinal neurons and their developmental changes in the chick. Dev Biol. 1984;104:336–347. doi: 10.1016/0012-1606(84)90089-7. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Yamamoto Y, Livet J, Arce V, Garces A, deLapeyrière O. Role of neurotrophic factors in motoneuron development. J Physiol Paris. 1998;92(3–4):279–281. doi: 10.1016/s0928-4257(98)80033-8. [DOI] [PubMed] [Google Scholar]
- Hollyday M, Hamburger V. Reduction of the naturally occurring motor neuron loss by enlargement of the periphery. J Comp Neurol. 1976;170:311–320. doi: 10.1002/cne.901700304. [DOI] [PubMed] [Google Scholar]
- Houenou LJ, McManaman JL, Prevette D, Oppenheim RW. Regulation of putative muscle-derived neurotrophic factors by muscle activity and innervation: in vivo and in vitro studies. J Neurosci. 1991;11:2829–2837. doi: 10.1523/JNEUROSCI.11-09-02829.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LJ, Chao MV. Mesenchymal and neuronal cell expression of the p75 neurotrophin receptor gene occur by different mechanisms. Dev Biol. 1995;167(1):227–238. doi: 10.1006/dbio.1995.1019. [DOI] [PubMed] [Google Scholar]
- Johnson H, Hokfelt T, Ulfhake B. Expression of p75(NTR), trkB and trkC in nonmanipulated and axotomized motoneurons of aged rats. Brain Res Mol Brain Res. 1999;69:21–34. doi: 10.1016/s0169-328x(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Hazama S, Hara T, Ogura A, Kojima M. Distinct signaling pathways of precursor BDNF and mature BDNF in cultured cerebellar granule neurons. Neurosci Lett. 2010;473(3):229–232. doi: 10.1016/j.neulet.2010.02.055. Epub 2010 Feb 26. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, Zaitsev E, Hirokawa T, Tatsu Y, Ogura A, Lu B, Kojima M. Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain. 2009;2(1):27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulwichit W, Edwards RH, Davenport EM, Baskar JF, Godfrey V, Raab-Traub N. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc Natl Acad Sci U S A. 1998;95:11963–11968. doi: 10.1073/pnas.95.20.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Levison S, McCarthy KD. Astroglia in culture. In: Banker GAGK, editor. Culturing Nerve Cells. Cambridge: MIT Press; 1991. pp. 309–336. [Google Scholar]
- Li L, Oppenheim RW, Milligan CE. Characterization of the execution pathway of developing motoneurons deprived of trophic support. J Neurobiol. 2001;46(4):249–264. doi: 10.1002/1097-4695(200103)46:4<249::aid-neu1006>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Li L, Prevette D, Oppenheim RW, Milligan CE. Involvement of specific caspases in motoneuron cell death in vivo and in vitro following trophic factor deprivation. Mol Cell Neurosci. 1998;12(3):157–167. doi: 10.1006/mcne.1998.0709. [DOI] [PubMed] [Google Scholar]
- Luther JA, Birren SJ. Neurotrophins and target interactions in the development and regulation of sympathetic neuron electrical and synaptic properties. Auton Neurosci. 2009;151(1):46–60. doi: 10.1016/j.autneu.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr Neurovasc Res. 2007;4(2):143–151. doi: 10.2174/156720207780637216. [DOI] [PubMed] [Google Scholar]
- Ma YT, Hsieh T, Forbes ME, Johnson JE, Frost DO. BDNF injected into the superior colliculus reduces developmental retinal ganglion cell death. J Neurosci. 1998;18(6):2097–2107. doi: 10.1523/JNEUROSCI.18-06-02097.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa SM, Xie Y, Yang T, Harrington AW, Kim ML, Yoon SO, Kraemer R, Moore LA, Hempstead BL, Longo FM. Small, nonpeptide p75NTR ligands induce survival signaling and inhibit proNGF-induced death. J Neurosci. 2006;26:5288–5300. doi: 10.1523/JNEUROSCI.3547-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckay SE, Garner A, Caldero J, Tucker RP, Large T, Oppenheim RW. The expression of trkB and p75 and the role of BDNF in the developing neuromuscular system of the chick embryo. Development. 1996;122:715–724. doi: 10.1242/dev.122.2.715. [DOI] [PubMed] [Google Scholar]
- Michler-Stuke A, Bottenstein JE. Proliferation of glial-derived cells in defined media. J Neurosci Res. 1982;7:215–228. doi: 10.1002/jnr.490070212. [DOI] [PubMed] [Google Scholar]
- Milligan CE, Oppenheim RW, Schwartz LM. Motoneurons deprived of trophic support in vitro require new gene expression to undergo programmed cell death. J Neurobiol. 1994;25:1005–1016. doi: 10.1002/neu.480250809. [DOI] [PubMed] [Google Scholar]
- Milligan CE, Prevette D, Yaginuma H, Homma S, Cardwell C, Fritz LC, Tomaselli KJ, Oppenheim RW, Schwartz LM. Peptide inhibitors of the ICE protease family arrest programmed cell death of motoneurons in vivo and in vitro. Neuron. 1995;15(2):385–393. doi: 10.1016/0896-6273(95)90042-x. [DOI] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276(16):12660–12666. doi: 10.1074/jbc.M008104200. 2001. [DOI] [PubMed] [Google Scholar]
- Nagappan G, Zaitsev E, Senatorov VV, Jr, Yang J, Hempstead BL, Lu B. Control of extracellular cleavage of ProBDNF by high frequency neuronal activity. Proc Natl Acad Sci U S A. 2009;106(4):1267–1272. doi: 10.1073/pnas.0807322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville C, Rosenthal N, McGrew M, Bogdanova N, Hauschka S. Methods in cell biology. Vol 52. San Diego: Academic; 1998. Skeletal muscle cultures; pp. 85–114. [PubMed] [Google Scholar]
- Newbern J, Taylor A, Robinson M, Lively MO, Milligan CE. c-Jun N-terminal kinase signaling regulates events associated with both health and degeneration in motoneurons. Neuroscience. 2007;147(3):680–692. doi: 10.1016/j.neuroscience.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Newbern J, Taylor A, Robinson M, Li L, Milligan CE. Decreases in phosphoinositide-3-kinase/Akt and extracellular signal-regulated kinase 1/2 signaling activate components of spinal motoneuron death. J Neurochem. 2005;94(6):1652–1665. doi: 10.1111/j.1471-4159.2005.03320.x. [DOI] [PubMed] [Google Scholar]
- Nishi R. Neurotrophic factors: two are better than one. Science. 1994;265:1052–1053. doi: 10.1126/science.8066443. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Okado N, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. IX. The loss of motoneurons following removal of afferent inputs. J Neurosci. 1984;4:1639–1652. doi: 10.1523/JNEUROSCI.04-06-01639.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW, Haverkamp LJ, Prevette D, McManaman JL, Appel SH. Reduction of naturally occurring motoneuron death in vivo by a target-derived neurotrophic factor. Science. 1988;240:919–922. doi: 10.1126/science.3363373. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Chu-Wang I, Maderdrot JL. Cell death of motoneurons in the chick embryo spinal cord. III. The differentiation of motoneurons prior to their induced degeneration following limb-bud removal. J Comp Neurol. 1978;177:87–112. doi: 10.1002/cne.901770107. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Yin QW, Prevette D, Yan Q. Brain-derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature. 1992;360:755–757. doi: 10.1038/360755a0. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Neurotrophic survival molecules for motoneurons: and embarrassment of riches. Neuron. 1996;17:195–197. doi: 10.1016/s0896-6273(00)80151-8. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306(5695):487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Pettmann B, Henderson CE. Neuronal cell death. Neuron. 1998;20:633–647. doi: 10.1016/s0896-6273(00)81004-1. [DOI] [PubMed] [Google Scholar]
- Pittman R, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. IV. Evidence that a functional neuromuscular interaction is involved in the regulation of naturally occurring cell death and the stabilization of synapses. J Comp Neurol. 1979;187:425–446. doi: 10.1002/cne.901870210. [DOI] [PubMed] [Google Scholar]
- Pittman RH, Oppenheim RW. Neuromuscular blockade increases motoneurone survival during normal cell death in the chick embryo. Nature. 1978;271:364–366. doi: 10.1038/271364a0. [DOI] [PubMed] [Google Scholar]
- Pollock GS, Vernon E, Forbes ME, Yan Q, Ma YT, Hsieh T, Robichon R, Frost DO, Johnson JE. Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levels in the visual system, hippocampus, and cerebellum. J Neurosci. 2001;21(11):3923–3931. doi: 10.1523/JNEUROSCI.21-11-03923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2(1):24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Harris CA, Moulder KL, Easton RM, Thompson CB, Johnson EM., Jr Intrinsic and extrinsic pathway signaling during neuronal apoptosis: lessons from the analysis of mutant mic. J Cell Biol. 2002;157(3):441–453e. doi: 10.1083/jcb.200110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC. Social controls on cell survival and cell death. Nature. 1992;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Raoul C, Pettmann B, Henderson CE. Active killing of neurons during development and following stress: a role for p75(NTR) and Fas? Curr Opin Neurobiol. 2000;10:111–117. doi: 10.1016/s0959-4388(99)00055-0. [DOI] [PubMed] [Google Scholar]
- Ricart KJ, Pearson RJ, Viera L, Cassina P, Kamaid A, Carroll SL, Estevez AG. Interactions between beta-neuregulin and neurotrophins in motor neuron apoptosis. J Neurochem. 2006;97:222–233. doi: 10.1111/j.1471-4159.2006.03739.x. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Tidwell JL, Gould T, Taylor AR, Newbern JM, Graves J, Tytell M, Milligan CE. Extracellular heat shock protein 70: a critical component for motoneuron survival. J Neurosci. 2005;25(42):9735–9745. doi: 10.1523/JNEUROSCI.1912-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Pareek S, Chrétien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379(3):247–250. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Pei G, Beck M, Schweizer U, Wiese S. Developmental motoneuron cell death and neurotrophic factors. Cell Tissue Res. 2000;301:71–84. doi: 10.1007/s004410000217. [DOI] [PubMed] [Google Scholar]
- Tauris J, Gustafsen C, Christensen EI, Jansen P, Nykjaer A, Nyengaard JR, Teng KK, Schwarz E, Ovesen T, Madsen P, Petersen CM. Proneurotrophin-3 may induce Sortilin-dependent death in inner ear neurons. Eur J Neurosci. 2011 Feb;33(4):622–631. doi: 10.1111/j.1460-9568.2010.07556.x. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AR, Gifondorwa DJ, Newbern JM, Robinson MB, Strupe JL, Prevette D, Oppenheim RW, Milligan CE. Astrocyte and muscle-derived secreted factors differentially regulate motoneuron survival. J Neurosci. 2007a;27:634–644. doi: 10.1523/JNEUROSCI.4947-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AR, Robinson MB, Milligan CE. In vitro methods to prepare astrocyte and motoneuron cultures for the investigation of potential in vivo interactions. Nat Protoc. 2007b;2:1499–1507. doi: 10.1038/nprot.2007.208. [DOI] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ. Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J Neurosci. 2006;26:7756–7766. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese S, Metzger F, Holtmann B, Sendtner M. The role of p75NTR in modulating neurotrophin survival effects in developing motoneurons. Eur J Neurosci. 1999;11:1668–1676. doi: 10.1046/j.1460-9568.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- Wong kC, Meyer T, Harding DI, Dick JR, Vrbova G, Greensmith L. Integrins at the neuromuscular junction are important for motoneuron survival. Eur J Neurosci. 1999;11:3287–3292. doi: 10.1046/j.1460-9568.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8(8):1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Yaginuma H, Tomita M, Takashita N, Mckay SE, Cardwell C, yin QW, Oppenheim RW. A novel type of programmed neuronal death in the cervical spinal cord of the chick embryo. J Neurosci. 1996;16:3685–3703. doi: 10.1523/JNEUROSCI.16-11-03685.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Je H-S, Ji Y, Nagappan G, Hempstead B, Lu B. Pro-BDNF-induced synaptic depression and retraction at developing neuromuscular synapses. J. Biol. Chem. 2009;185(4):727–741. doi: 10.1083/jcb.200811147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin QW, Johnson J, Prevette D, Oppenheim RW. Cell death of spinal motoneurons in the chick embryo following deafferentation: rescue effects of tissue extracts, soluble proteins, and neurotrophic agents. J Neurosci. 1994;14:7629–7640. doi: 10.1523/JNEUROSCI.14-12-07629.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]