Abstract
BACKGROUND
Although whole lung irradiation (WLI) is used to treat pulmonary metastases of pediatric solid malignancies, few studies have addressed its long-term pulmonary consequences.
METHODS
We conducted a retrospective study of longitudinal changes in 171 pulmonary function tests (PFTs) and their relationship with clinical features in 48 survivors of pediatric malignant solid tumors treated with WLI.
RESULTS
Although active respiratory symptoms were seen in only 9 (18.8%) patients, abnormalities in forced vital capacity (FVC; 58.3%), forced expiratory volume in 1 second (FEV1; 64.6%), total lung capacity (TLC; 72.9%), and diffusion capacity of carbon monoxide corrected for hemoglobin (DLCOcorr; 70.8%) were common. At a median follow-up of 9.7 years after WLI, FVC, FEV1, and TLC significantly declined longitudinally (P = .04, .03, and .02, respectively). Focal pulmonary boost irradiation was significantly associated with abnormal FEV1/FVC (P = .03), forced expiratory flow between 25% and 75% forced vital capacity (FEF25%–75%; P = .005), residual volume (RV; P = .005), and RV/TLC (P = .002). Ten patients had baseline PFTs, and FVC, FEV1, TLC and DLCOcorr worsened immediately after radiation, followed by transient improvement but subsequent decline. Thirteen of 32 (40.6%) patients over the age of 18 were smokers.
CONCLUSIONS
Pulmonary dysfunction, especially in lung volumes, was prevalent after WLI and worsened over time although most patients were asymptomatic. Boost irradiation impaired pulmonary function, and a significant proportion of patients were smokers. Further studies are planned to assess the predictors and clinical consequences of progressive PFT abnormalities and to evaluate educational interventions.
Keywords: pulmonary function tests, solid tumor, whole lung irradiation, pediatric cancer, follow-up
INTRODUCTION
Because of the increased survival rates of pediatric patients with cancer, more attention is being paid to the long-term effects of their treatment.1,2 Whole lung irradiation (WLI) has been used to treat children with pulmonary metastasis of Wilms tumor, Ewing sarcoma, and rhabdomyosarcoma. Evaluating pulmonary function during and after WLI is important because growing lung tissue is sensitive to radiation. The Childhood Cancer Survivor Study showed that 5-year survivors who received chest or total body irradiation had a significantly increased relative risk of reporting long-term pulmonary complications, such as lung fibrosis, a need for supplemental oxygen, recurrent pneumonia, chronic cough or shortness of breath, and abnormal chest wall development.3
The purpose of this study was to determine the long-term changes in pulmonary function after WLI and to determine the relationship between abnormal pulmonary function values and clinical parameters in a cohort of pediatric patients with malignant solid tumors.
PATIENTS AND METHODS
Patients
This retrospective medical record review was approved by the St. Jude Children’s Research Hospital Institutional Review Board. We reviewed clinic notes and pulmonary function tests (PFTs) of patients who survived for more than 1 year after WLI that was administered between 1971 and 2003 to treat pulmonary metastasis of a solid malignancy. Each patient’s smoking history and second-hand smoke exposure were addressed during follow-up visits.
Pulmonary Function Testing
PFTs were performed only in children 6 years of age and older. All tests were performed in the same laboratory, by experienced pediatric pulmonary function technicians, and according to the guidelines of the American Thoracic Society.4,5 PFTs included spirometry, lung volume measurements, and single-breath carbon monoxide–diffusion capacity. Spirometry was performed by using a pneumotachograph, and lung volumes were determined by using the open-circuit nitrogen washout method. The diffusion capacity of the lung for carbon monoxide (DLCO) was measured by using the single-breath technique and corrected for hemoglobin concentration to yield the DLCOcorr. The observed values of each patient were compared with those predicted for the patient’s age, race, gender, and height on the basis of reference equations.5–8
The forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), total lung capacity (TLC), and DLCOcorr measurements were considered abnormal if they were less than 80% of the predicted normal values. The measurement of forced expiratory flow between 25% and 75% forced vital capacity (FEF25%–75%) was considered abnormal if it was less than 67% of the predicted value. The value of DLCOcorr for alveolar volume (DLCO/VA) was considered abnormal if it was below the 95th percentile value of normal population. The functional residual capacity (FRC) and residual volume (RV) measurements were classified as abnormal if they were more than 120% of the predicted normal values. FEV1/FVC was considered abnormal if the ratio was less than 80%. RV/TLC was considered abnormal if the ratio was greater than 30%.
Statistical Analyses
In this study, 10 PFT outcome measures were of interest: FVC, FEV1, FEV1/FVC, FEF25%–75%, TLC, FRC, RV, RV/TLC, DLCOcorr, and DLCO/VA. The predicted values of these PFT parameters were plotted against time to assess their longitudinal changes and a smooth spline fit to the data was obtained by using a spline routine. We further classified each PFT measurement as normal or abnormal according to the predicted values.5–7 Then, for each PFT outcome measure, logistic regression analysis was applied to model the probability of having an abnormal measurement on 3 independent variables: years since WLI, focal pulmonary boost radiation, and pulmonary wedge resection. The analysis was conducted by using a generalized linear mixed model with the logit link function and the power covariance structure.9 An odds ratio significantly greater than 1 indicates an increased chance of having abnormal PFT results. All the analyses were performed by SAS 9.2 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Patient Characteristics
Forty-eight patients who were treated with WLI and had PFTs after completion of WLI were identified. This comprises 45% of all 107 patients who survived one or more years after WLI in our institution. Thirty-nine (81%) were alive in the last follow-up. On the other hand, among 59 patients who did not have PFTs, only 15 (25%) were alive. A total of 171 evaluable PFTs was analyzed; each patient performed a median of 3 PFTs (range, 1 to 10 PFTs). The median follow-up time was 9.7 years (range, 0.7 to 21 years), and the solid tumor diagnoses represented were Wilms tumor, rhabdomyosarcoma, Ewing sarcoma, synovial sarcoma, and thymoma (Table 1). The median age at diagnosis was 5.8 years (range, 0 to 17.4 years), and the median age at lung irradiation was 6.2 years (range, 0.5 to 20.2 years); a median WLI dose of 12 Gy was given (range, 10.5 to 18 Gy). Of the 48 patients, 17 (35.4%) received focal pulmonary boost radiation treatment (median dose, 18 Gy and range, 8.5 to 36 Gy), and 20 (41.7%) underwent partial pulmonary resections (Table 1).
Table 1.
Characteristics of Study Patients
| Characteristic | No. of patients (%) N = 48 |
|---|---|
| Sex | |
| Female | 23 (48) |
| Male | 25 (52) |
| Race | |
| White | 32 (67) |
| Black | 13 (27) |
| Other | 3 (6) |
| Primary diagnosis | |
| Wilms tumor | 38 (79) |
| Rhabdomyosarcoma | 5 (10) |
| Ewing sarcoma | 3 (6) |
| Synovial sarcoma | 1 (2) |
| Thymoma | 1 (2) |
| Chemotherapeutic agents | |
| Vincristine | 47 (98) |
| Doxorubicin | 45 (94) |
| Actinomycin D | 44 (92) |
| Etoposide | 28 (58) |
| Ifosfamide | 24 (50) |
| Carboplatin | 19 (40) |
| Cyclophosphamide | 14 (29) |
| Topotecan | 9 (19) |
| Bleomycin | 0 (0) |
| Focal pulmonary boost | |
| Yes | 17 (35) |
| No | 31 (65) |
| Pulmonary wedge resection | |
| Yes | 20 (42) |
| No | 28 (58) |
Respiratory Symptoms
At the last clinic visit, most patients did not have active pulmonary symptoms. Of the 48 patients, only 9 (18.9%) reported symptoms; 4 had shortness of breath with exertion, 3 had coughs, and 2 reported a history of asthma.
Pulmonary Function Testing
The prevalence and patterns of patients with abnormal PFT values are shown in Table 2. A high percentage (> 50%) of patients had at least one abnormal value, particularly of FVC, FEV1, TLC, and DLCOcorr. Abnormal values of FEV1/FVC, FEV25%–75%, FRC, RV, and DLCO/VA were less frequent.
Table 2.
Frequency of Lung Function Abnormality After Whole Lung Irradiation
| Pulmonary defect | No. patients with abnormal values at any time point (%) N = 48 |
|---|---|
| FVC | 28 (58) |
| FEV1 | 31 (65) |
| FEV1/FVC | 11 (23) |
| FEF25%–75% | 12 (25) |
| TLC | 35 (73) |
| FRC | 3 (6) |
| RV | 11 (23) |
| RV/TLC | 19 (40) |
| DLCOcorr* | 34 (74) |
| DLCO/VA* | 6 (13) |
DLCOcorr, diffusion capacity of the lung for carbon monoxide corrected for hemoglobin; FEF25%–75%, forced expiratory flow between 25% and 75% forced vital capacity; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FRC, functional residual capacity; RV, residual volume; TLC, total lung capacity
Forty-six patients
Longitudinal Changes in Pulmonary Function Tests
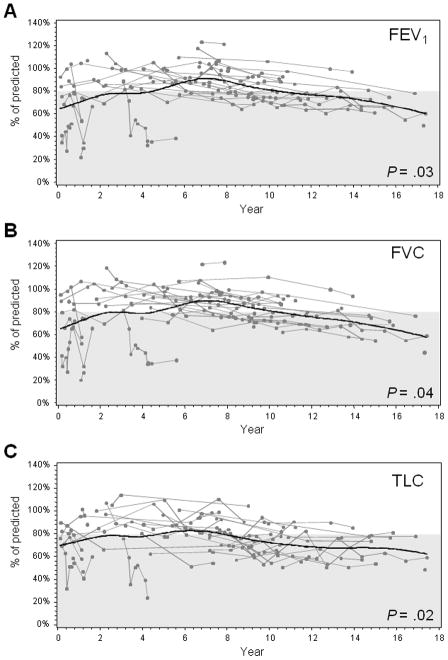
At a median follow-up of 9.7 years from WLI therapy, FVC, FEV1, and TLC values had decreased (Figure 1, A–C). Every year after WLI, the odds of having abnormal values of FVC, FEV1, and TLC increased by a factor of 1.11 (P = .04), 1.12 (P = .03), and 1.12 (P = .02), respectively, indicating that the proportion of patients with abnormal values of FVC, FEV1, and TLC increased over time (Table 3). Among the clinical parameters examined, focal boost lung irradiation was associated with significant declines in pulmonary function values. Compared to patients who did not receive boost irradiation, patients who received boost irradiation had a significantly higher chance to have abnormal FEV1/FVC (P = .03), FEF25%–75% (P = .005), RV (P = .005), and RV/TLC(P = .002) (Table 3). Compared to patients who did not receive pulmonary wedge resection, patients who received pulmonary wedge resection had a significantly lower risk of having an abnormal RV/TLC (P=.002) (Table 3).
Figure 1.
Longitudinal changes in pulmonary function tests after whole lung irradiation. During follow-up, the patients’ FEV1 (A), FVC (B), and TLC (C) values were measured and compared to values predicted by measurements in the control population. Each line plotted represents the percent of the predicted test value that was measured from an individual patient at the time of each PFT. A smooth line was fitted to the data by using a spline-interpolation method for each response variable along the year after whole lung irradiation. The gray shaded area indicates the range of abnormal test values.
Table 3.
Clinical Factors and Abnormal Values in Pulmonary Function Tests
| Outcome (Y) | Years after WLI (X1) | Pulmonary boost radiation (X2) | Pulmonary wedge resection (X3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PFT parameter | OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value |
| FVC | 1.11 | 1.01–1.23 | 0.04 | 1.57 | 0.55–4.51 | 0.41 | 0.47 | 0.17–1.28 | 0.15 |
| FEV1 | 1.12 | 1.01–1.24 | 0.03 | 1.8 | 0.61–5.34 | 0.29 | 0.59 | 0.21–1.64 | 0.31 |
| FEV1/FVC | 0.95 | 0.82–1.1 | 0.47 | 4.43 | 1.19–16.53 | 0.03 | 3.32 | 0.84–13.05 | 0.09 |
| FEF25%–75% | 1.06 | 0.97–1.17 | 0.22 | 3.83 | 1.56–9.39 | 0.005 | 1.85 | 0.75–4.55 | 0.19 |
| TLC | 1.13 | 1.02–1.24 | 0.02 | 1.31 | 0.5–3.48 | 0.59 | 1.21 | 0.47–3.09 | 0.7 |
| FRC | 0.88 | 0.68–1.14 | 0.33 | 8.43 | 0.49–145.14 | 0.15 | 0.95 | 0.11–8.11 | 0.96 |
| RV | 0.93 | 0.83–1.05 | 0.24 | 6.01 | 1.85–19.54 | 0.005 | 0.73 | 0.26–2.04 | 0.55 |
| RV/TLC | 0.96 | 0.88–1.05 | 0.41 | 4.17 | 1.76–9.88 | 0.002 | 0.31 | 0.13–0.77 | 0.002 |
| DLCOcorr | 1.02 | 0.93–1.12 | 0.71 | 0.63 | 0.25–1.58 | 0.33 | 0.94 | 0.38–2.32 | 0.89 |
| DLCO/VA | 0.97 | 0.81–1.17 | 0.77 | 3.08 | 0.51–18.51 | 0.23 | 0.78 | 0.13–4.78 | 0.79 |
Y, the response variable in the model; X1, X2,, and X3, the independent variables in the model; CI, confidence interval; DLCOcorr, diffusion capacity of the lung for carbon monoxide corrected for hemoglobin; FEF25%–75%, forced expiratory flow between 25% and 75% forced vital capacity; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FRC, functional residual capacity; OR, odds ratio; PFT, pulmonary function test: RV, residual volume; TLC, total lung capacity; VA, alveolar volume; WLI, whole lung irradiation
Changes from Baseline Pulmonary Function Tests
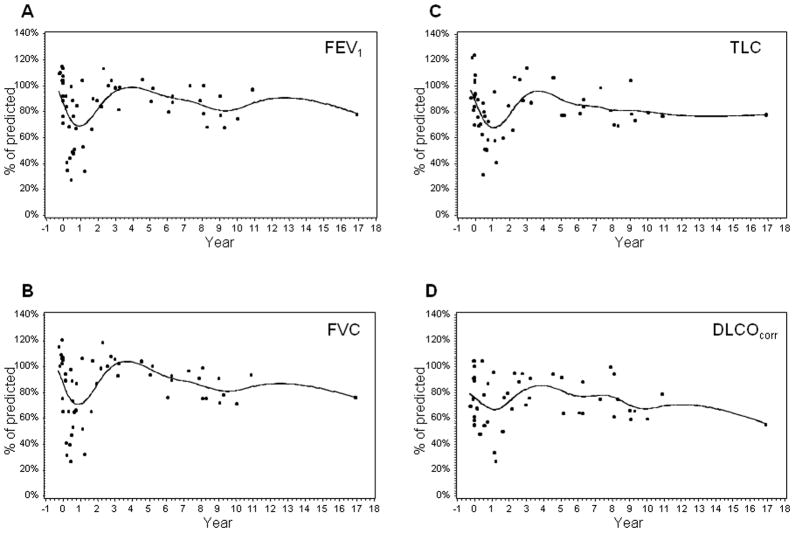
To evaluate the immediate change in pulmonary function after WLI, we assessed PFTs in a subgroup of patients who underwent PFTs both before and after WLI. Because of the young age of our patient population at diagnosis, only 10 patients were old enough to have baseline PFTs prior to WLI. Figures 2A–D show time plots to compare their PFT parameters before and after WLI. Profile plots of FVC, FEV1, TLC, and DLCOcorr show an immediate reduction from baseline values after radiation, then an increase over the next 3 years, and a subsequent gradual decline.
Figure 2.
Longitudinal changes in pulmonary function, including FEV1 (A), FVC (B), TLC (C), and DLCOcorr(D), before and after whole lung irradiation in survivors having baseline pulmonary function tests. A smooth line was fitted to the data by using a spline-interpolation method for each response variable along the year after whole lung irradiation.
Smoking Incidence
At the last visit, 13 of the 32 patients (40.6%) older than 18 years and 1 of the 16 patients (6.2%) younger than 18 years reported any history of smoking. Information on secondhand smoke exposure was obtained from 17 of the 34 non-smokers, and 7 had family members in their residence who smoked. The patients’ smoking histories were not associated with worse pulmonary function (data not shown).
DISCUSSION
Upon review of 171 PFTs in 48 pediatric patients who received WLI for solid malignancies, we found that the prevalence of abnormal PFT parameters was high and increased over time. Boost radiation also contributed to the decline of pulmonary function. Despite the known adverse consequences of smoking on pulmonary function, 40% of at-risk adult survivors were smokers.
Among the PFT parameters, FVC, FEV1, TLC, and DLCOcorr were frequently abnormal in our study. Littman et al. found that patients treated with WLI had lower vital capacity, FEV1, and TLC values than did non-irradiated patients.10 A recent retrospective study of children who received WLI to treat metastatic disease also showed reduced FVC (53% of patients), FEV1 (50%), TLC (60%), and DLCO (81%) values.11 These findings suggest that WLI leads to restrictive pulmonary defects as well as impairments in diffusion capacity. As there was a high incidence of abnormalities in TLC and DLCOcorr, we evaluated the DLCO/VA to determine if the decrease in DLCOcorr was solely due to decreased TLC or, in addition, to the presence of parenchymal disease. Among the 46 patients evaluated, only 6 (13%) had abnormal DLCO/VA, suggesting that the majority of patients have a decrease in lung volume and diffusion area. In our study, only 48 of the 107 patients who survived more than 1 year after WLI had PFTs. Although it is possible that they are a selected subgroup of patients who were in their routine follow-up, few complained of respiratory problems. Eighty-one percent of the studied population were long-term survivors whereas the majority of patients who did not have PFT had expired due to progressive disease. We believe that our study represents the natural history of pulmonary function of long-term survivors. However, this study was based on retrospective review of PFTs and clinical notes, and prospective studies of routinely performed PFTs and evaluation of clinical symptoms are necessary.
In our study, FVC, FEV1, and TLC values deteriorated significantly over time. When the longitudinal effects of WLI were evaluated in children with Wilms tumor, declines in VC, TLC, FRC, and dynamic compliance were observed during follow-up.12 This radiation-induced pulmonary impairment occurred in 3 chronological phases. The acute phase occurred within the first few months after radiation therapy when initial worsening of pulmonary function was seen. The second phase, during which no further deterioration occurred, covered the next 2 years. The third phase occurred when pulmonary function progressively deteriorated again. The first-phase changes are thought to be due to acute lung injury from radiation, and the later effects are attributed to parenchymal sequelae of acute lung injury (i.e., destruction of or failure to develop alveoli and peripheral airways) and impaired chest wall growth.12 The PFT changes in our small subset of patients who had PFTs performed prior to WLI support these observations. We also observed a similar longitudinal pattern of pulmonary function decline in a separate study of survivors of hematopoietic stem cell transplantation, most of whom received total body irradiation.13
The type of chemotherapy, its timing in relation to the WLI, and the boost dose may all influence the pulmonary outcome. Most of the patients received vincristine, doxorubicin and actinomycin D, which can affect long-term pulmonary function.14 Vincristine can cause dysplasia of alveolar lining cells and inflammation of interstitial and alveolar cells. High cumulative dose of doxorubicin is associated with cardiopulmonary sequelae. Furthermore, both doxorubicin and actinomycin D are known for a radiation sensitizing effect. In addition to chemotherapy, PFT changes may be caused by less physical activity, poor neurocognitive status preventing from good performance of PFT, and pulmonary opportunistic infections. Thus, it would be important to compare these results between those treated with and without WLI in a larger number of survivors. In addition, the thoracic wall deformations following irradiation can affect PFT results, especially lung volume.12 We evaluated the height of our patient population and mean z-score was −0.79 compared with sex, race and age matched control (p< 0.001, data not shown), suggesting that patients in our study are significantly shorter. For this purpose, we evaluate lung parenchymal volumes with CT scan as well as sitting height in a prospective study.
We examined risk factors associated with pulmonary dysfunction after WLI. Patients who received focal pulmonary boost radiation had worse FEV1/FVC and FEF25%–75% as well as RV and RV/TLC. The boost radiation more than doubled the radiation exposure in most of the cases and this outcome is consistent with current knowledge that larger radiation doses are associated with increased pulmonary morbidity, and careful monitoring is necessary in this population.10,15–17 In addition, those who receive boost radiation have advanced metastatic disease which typically requires additional chemotherapy. They can have further inflammatory reactions from disease itself, chemotherapy effect, and opportunistic infections, which can lead to additional pulmonary abnormalities such as obstructive defects and air trapping.
In our study population, there was a significant incidence of smoking, especially among those older than 18 years. Smoking is a major risk factor in the pathogenesis of chronic obstructive pulmonary disorder (COPD) and early exposure to cigarette smoke is linked to higher rates of childhood asthma.18,19 Smoking cessation improves respiratory symptoms and prevents accelerated pulmonary impairments in patients with either COPD or asthma.20,21 In patients previously treated with WLI, smoking may further compromise already impaired pulmonary function and cessation efforts should be particularly vigorous. Smoking was not a predictor of poor pulmonary function in this cohort of patients. This might be due to our small sample size and/or because most of the PFTs were performed before they started smoking. It is also possible that those young people and teenagers are only experimenting with cigarette use and they may not be really addicted as the percentage of smokers seems higher than that in the whole American society.22 Nevertheless, patient counseling should include information about smoking cessation, the benefits of living in an area with minimal air pollution, and vaccinations to avoid influenza and pneumococcal infections.23–25 Prospective longitudinal studies of lung function after WLI may better define the risks of smoking, family history, and co-morbidities such as asthma and COPD on the decline of lung function.
In conclusion, our study emphasizes the importance of continuous long-term follow-up of pulmonary function as well as the development of measures to minimize pulmonary morbidity and mortality in pediatric patients who receive WLI. Although most patients in the current study did not have respiratory symptoms at the last clinic visit, the follow-up was relatively short and the cohort was quite young. Because deterioration of pulmonary function can occur longitudinally, we suggest long-term follow-up of these patients even when they are asymptomatic and have normal PFTs. We recommend that PFT monitoring be continued beyond the third year after radiation and that patients who received boost radiation be carefully observed. We have initiated prospective studies to better define the clinical consequences of progressive PFT abnormalities as well as to identify risk factors associated with significant pulmonary dysfunction after WLI and to promote counseling and education of long-term survivors.
Acknowledgments
Supported in part by Cancer Center Support Grant CA 21765 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC).
We would like to acknowledge the expertise of Cherise Guess, PhD, who edited the manuscript and Paul W. Mackert and Doug Harper who performed all pulmonary function testing.
Footnotes
The authors have no conflicts of interest, including specific financial interests, relationships, or affiliations, relevant to the subject of this manuscript.
References
- 1.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–18. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudson MM, Mulrooney DA, Bowers DC, et al. High-risk populations identified in Childhood Cancer Survivor Study investigations: implications for risk-based surveillance. J Clin Oncol. 2009;27:2405–14. doi: 10.1200/JCO.2008.21.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer. 2002;95:2431–41. doi: 10.1002/cncr.10978. [DOI] [PubMed] [Google Scholar]
- 4.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 5.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J. 1995;8:492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Dockery DW, Wypij D, et al. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 7.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 8.Paoletti P, Viegi G, Pistelli G, et al. Reference equations for the single-breath diffusing capacity. A cross-sectional analysis and effect of body size and age. Am Rev Respir Dis. 1985;132:806–13. doi: 10.1164/arrd.1985.132.4.806. [DOI] [PubMed] [Google Scholar]
- 9.Inc SI: SAS/STAT® 9.2 User’s Guide. 2008 Available from URL: http://support.sas.com/documentation/cdl/en/statugglm/61789/PDF/default/statugglm.pdf.
- 10.Littman P, Meadows AT, Polgar G, et al. Pulmonary function in survivors of Wilm’s tumor. Patterns of impairment. Cancer. 1976;37:2773–6. doi: 10.1002/1097-0142(197606)37:6<2773::aid-cncr2820370631>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Weiner DJ, Maity A, Carlson CA, et al. Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatr Blood Cancer. 2006;46:222–7. doi: 10.1002/pbc.20457. [DOI] [PubMed] [Google Scholar]
- 12.Benoist MR, Lemerle J, Jean R, et al. Effects of pulmonary function of whole lung irradiation for Wilm’s tumour in children. Thorax. 1982;37:175–80. doi: 10.1136/thx.37.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inaba H, Yang J, Pan J, et al. Pulmonary dysfunction in survivors of childhood hematologic malignancies after allogeneic hematopoietic stem cell transplantation. Cancer. 2010;116:2020–30. doi: 10.1002/cncr.24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol. 2001;13:242–8. doi: 10.1097/00001622-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Marks LB, Munley MT, Bentel GC, et al. Physical and biological predictors of changes in whole-lung function following thoracic irradiation. Int J Radiat Oncol Biol Phys. 1997;39:563–70. doi: 10.1016/s0360-3016(97)00343-x. [DOI] [PubMed] [Google Scholar]
- 16.Bolling T, Schuck A, Paulussen M, et al. Whole lung irradiation in patients with exclusively pulmonary metastases of Ewing tumors. Toxicity analysis and treatment results of the EICESS-92 trial. Strahlenther Onkol. 2008;184:193–7. doi: 10.1007/s00066-008-1810-x. [DOI] [PubMed] [Google Scholar]
- 17.Wohl ME, Griscom NT, Traggis DG, et al. Effects of therapeutic irradiation delivered in early childhood upon subsequent lung function. Pediatrics. 1975;55:507–16. [PubMed] [Google Scholar]
- 18.Soltani A, Reid DW, Sohal SS, et al. Basement membrane and vascular remodelling in smokers and chronic obstructive pulmonary disease: a cross-sectional study. Respir Res. 2010;11:105. doi: 10.1186/1465-9921-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodwin RD, Cowles RA. Household smoking and childhood asthma in the United States: a state-level analysis. J Asthma. 2008;45:607–10. doi: 10.1080/02770900802126982. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhuri R, Livingston E, McMahon AD, et al. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am J Respir Crit Care Med. 2006;174:127–33. doi: 10.1164/rccm.200510-1589OC. [DOI] [PubMed] [Google Scholar]
- 21.Willemse BW, Postma DS, Timens W, et al. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J. 2004;23:464–76. doi: 10.1183/09031936.04.00012704. [DOI] [PubMed] [Google Scholar]
- 22.Klosky JL, Tyc VL, Hum A, et al. Establishing the predictive validity of intentions to smoke among preadolescents and adolescents surviving cancer. J Clin Oncol. 2010;28:431–6. doi: 10.1200/JCO.2008.21.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avol EL, Gauderman WJ, Tan SM, et al. Respiratory effects of relocating to areas of differing air pollution levels. Am J Respir Crit Care Med. 2001;164:2067–72. doi: 10.1164/ajrccm.164.11.2102005. [DOI] [PubMed] [Google Scholar]
- 24.Glezen WP. Asthma, influenza, and vaccination. J Allergy Clin Immunol. 2006;118:1199–206. doi: 10.1016/j.jaci.2006.08.032. quiz 1207–8. [DOI] [PubMed] [Google Scholar]
- 25.Thorburn AN, Hansbro PM, Gibson PG. Pneumococcal vaccines for allergic airways diseases. Expert Opin Biol Ther. 2009;9:621–9. doi: 10.1517/14712590902916999. [DOI] [PubMed] [Google Scholar]