Abstract
Activated protein C (APC) plays active roles in preventing progression of a number of disease processes. These include thrombosis due to its direct anticoagulant activity which is likely augmented by its cytoprotective activity, thereby limiting exposure of procoagulant cellular membrane surfaces on cells. Beyond that, the pathway signals the cells to prevent apoptosis, to dampen inflammation, to increase endothelial barrier function, and to selectively downregulate some genes implicated in disease progression. Most of these functions are manifested to APC binding to endothelial protein C receptor (EPCR) allowing PAR1 activation, but activation of other PARS is also implicated in some cases. In addition to EPCR orchestrating these changes, CD11b is also capable of supporting APC signaling. Selective control of these pathways offers potential in new therapeutic approaches to disease.
Keywords: Thrombin, Histones, Thrombomodulin, Inflammation, Sepsis, Reperfusion injury
History
The protein C system is best known for its anticoagulant activity seen most clearly in the clinical observation that patients born with a total protein C deficiency exhibit massive neonatal thrombosis that is usually lethal unless treated, reviewed in [1]. Indeed this is also one aspect of the anti-inflammatory functions of the pathway since coagulation, particularly thrombin generation, can trigger a wide variety of pro-inflammatory events including expression of adhesion molecules like P-selectin and activating the Nf-κB pathway [2]. While this is an important aspect of the anti-inflammatory function of the pathway, it does not distinguish the pathway from other anticoagulants. Indeed, heparin has long been noted to have apparent anti-inflammatory functions, in part likely due to its anticoagulant activity.
Some of the first suggestions that this pathway might have additional anti-inflammatory activity came from the treatment of newborns with protein C deficiency. The thrombotic lesions that developed in the newborns were surrounded by an intense red area that retracted rapidly following the administration of protein C, suggesting that protein C was preventing the inflammation in addition to decreasing the thrombosis, reviewed in [1].
These studies were followed by examination of the roles of thrombosis in sepsis. In an early study, Hinshaw and colleagues [3] observed that heparin could prevent the consumptive coagulopathy associated with Escherichia coli-induced sepsis in baboons but did not rescue the animals. Later we demonstrated that an active site blocked form of factor Xa could prevent the disseminated intravascular coagulation (DIC) but again failed to protect against sepsis [4]. Subsequently, Hinshaw and colleagues showed that extracorporeal perfusion without exogenous anticoagulation was protective against endotoxin-induced sepsis [5]. They also observed that an associated anticoagulant was being generated during these studies. Interestingly, the pump could be removed subsequently and the animals were still protected from subsequent bacterial challenge. With the identification of thrombomodulin [6] and demonstration of thrombin-dependent protein C activation in vivo [7], it was possible to test whether the anticoagulant might be activated protein C (APC) generated by thrombin formed by the pump. Indeed, thrombin infusion into dogs challenged with endotoxin was protective [8] despite the fact that the animals would develop DIC without the thrombin infusion. Thrombin infusion decreased both the DIC and inflammation. With the advent of a rapid means for purification of protein C from human plasma [9], it was possible to test the ability of APC to protect baboons from E. coli-induced sepsis. When APC was administered with the E. coli, the animals survived a normally lethal dose and exhibited reduced coagulation, protection from shock, and decreased inflammation [10]. These older studies highlight that APC could protect against an inflammation-induced disease like sepsis when other comparable anticoagulants could not. In contrast, inhibition of the pathway in the E. coli sepsis model, in this case with C4 binding protein, elevated cytokine production in response to E. coli challenge [11].
Either reducing protein C levels [12, 13] in mice or blocking protein C activation [10] in baboons increased a sublethal to a lethal challenge with bacteria or endotoxin. In order to perform its full anti-inflammatory functions, the APC must bind to the endothelial protein C receptor (EPCR) [14]. Mice overexpressing EPCR are resistant to endotoxemia [15], whereas those with low-level expression are sensitized [16, 17]. Furthermore, mice with low levels of EPCR have cardiac dysfunction from the challenge [16]. These studies illustrate the important role of the pathway in regulating the host response to acute inflammatory challenges.
How does activated protein C influence inflammation directly?
One of the major mechanisms that augment inflammation is mediated through Nf-κB activation and nuclear translocation from the cytosol [18, 19]. This turns on synthesis of a variety of inflammatory mediators including cytokine production. APC can decrease the synthesis of Nf-κB components [19, 20] and decrease Nf-κB nuclear translocation [18]. Together these activities probably constitute the major mechanisms by which APC downregulates inflammatory cytokine production in inflamed endothelium in culture [21] and in animal models of sepsis [22, 23].
APC signaling
These effects are dependent on APC, EPCR, and protease-activated receptor 1 (PAR-1) [14, 24]. Activation of PAR-1 by the APC–PAR-1 complex leads to different cellular signaling than when thrombin activates PAR-1 despite cleaving the same site on the receptor [25] (Fig. 1). The mechanisms for this change in signaling are currently being elucidated. In one model, protein C binding to EPCR leads to migration of EPCR out of the lipid rafts at which time it interacts with PAR-1 coupled to a different G protein than when it was in the lipid rafts, thus resulting in the altered signaling profile [26–28]. In support of this model, EPCR did appear to migrate from rafts in the presence of protein C [26] and recombinant mutant molecules containing the protein C Gla domain that could elicit signaling similar to that of APC [26].
Fig. 1.
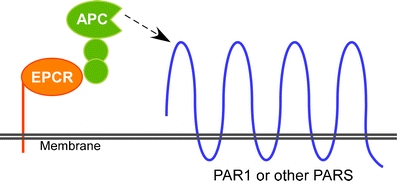
Cytoprotective signaling by APC. APC binds to EPCR at which time it cleaves PAR1 to generate the active signaling molecule. The APC cleaved PAR1 appears to be linked to a G protein that generates cytoprotective functions—see text for discussion. APC activated protein C, EPCR endothelial cell protein C receptor, PAR1 protease-activated receptor-1
Inhibition of leukocyte adhesion
Leukocyte adhesion and trafficking
APC reduces leukocyte adhesion and activation and protects capillary function in endotoxemia [22, 29–31] in part by reducing chemotaxis [32] and cytokine production [23]. This inhibition of leukocyte attachment could be mediated by decreases in thrombin-dependent mobilization of selectins from Weible Paladi bodies in the endothelium, suppression of ICAM, synthesis, and decreased synthesis of monocyte chemotactic protein-1 [21]. In central venous sinus thrombosis, APC decreases inflammatory cell recruitment and protects the microvasculature in this manner [33].
Endothelial barrier function
Endothelial barrier function is compromised in a number of diseases resulting in edema. Thrombin is known to decrease endothelial barrier function, a process that is reversed by APC [26, 34, 35]. APC accomplishes this, at least in part, through the generation of shingosine 1-phosphate receptor transactivation [34, 35]. Improving endothelial barrier function is likely to provide anti-inflammatory effects since it should reduce leukocyte trafficking into the extravascular space. While not directly related to inflammation, one of the features of APC is that it diminishes both endothelial cell and neuronal apoptosis [36–38]. Excessive apoptosis or cellular necrosis leads to release of relatively large amounts of nuclear material in the form of nucleosomes and also the release of mitochondrial contents. Both of these events will trigger inflammation. The histones on nucleosomes induce leukocyte migration into the tissue, platelet activation, and thrombosis [39] and induce cytokine formation, and the mitochondria induce leukocyte activation [40].
Histone neutralization
Extracellular histones are cytotoxic [39], and APC can cleave and neutralize this activity of histones. The importance of the latter observation was apparent in studies that demonstrated that histones were much more toxic in mice where the protein C pathway was blocked and that blocking histone function was protective in endotoxemia [39].
Signaling is required for APC protection in sepsis
Mutants of APC have been developed that retain signaling activity but have very low anticoagulant activity [41]. These mutant forms of APC (5A- aPC and other similar mutants) were effective in preventing mortality in mouse models of sepsis [24, 42].
The original signaling studies were done in endothelium [20]. More recent studies have detected EPCR on leukocytes, particularly CD8+ dendritic cells [43]. Mice with low levels of EPCR (EPCR low) were studied and were less effectively protected from endotoxin-induced sepsis toxicity by 5A aPC than wild-type mice [43]. When bone splenic CD11chi dendritic cells from wild-type mice were transplanted into EPCR-low mice, they supported protection from endotoxin by 5A- aPC whereas similar cells from EPCR-low mice did not. In vitro, 5A-aPC inhibited the inflammatory response of dendritic cells which appeared to be independent of a requirement for normal levels of EPCR [43]. Thus, protective function seems EPCR dependent but there are cell populations that are responsive to APC in suppressing inflammation that do not appear to require EPCR. A likely receptor for APC on macrophages is CDllb/CD18, also known as Mac-1. APC administration in wild type, but not CD11b null mice, reduced mortality. CD11b was also required for suppression of the endotoxin-induced macrophage inflammatory response [44]. These results indicate that the cellular signaling mechanisms play a dominant role in protection from endotoxemia [45].
Role of APC in specific disease states
Coronary reperfusion injury
One of the events that occur in reperfusion injury is apoptosis. Blocking protein C activation exacerbates reperfusion injury in pig hearts [46]. The ischemia reperfusion also leads to rapid protein C activation in this model [46]. In mouse models of coronary reperfusion, APC reduced coronary apoptosis and decreased inflammation and leukocyte adhesion resulting in improved heart function [47, 48].
Stroke protection
APC treatment of ischemic brain endothelium prevents apoptosis in part by blocking P53 function [49]. APC also prevents tissue plasminogen activation-induced Nf-κB-dependent upregulation of matrix metaloproteinase-9 and can prevent neuronal apoptosis by PARs 1 and 3 [37].
Diabetes
Diabetes also results in apoptosis of kidney cells. Increasing endogenous APC production decreased apoptosis and improved kidney function in mouse models of type 1 diabetes [50]. As mentioned above, the large degree of apoptosis and necrosis would be anticipated to increase inflammation which is thus indirectly prevented by APC.
Inflammatory bowel disease
In inflammatory bowel disease, EPCR and thrombomodulin are downregulated. APC treatment reduced cytokine production, inhibited leukocyte adhesion, diminished weight loss, and reduced the magnitude of the pathological lesions [51].
Tumor adhesion and propagation
Endogenous APC decreases the adhesion of tumor cells to the lung and reduces the number of metastatic sites [52, 53]. It does so in part by activating the sphengosine-phosphate-1 system [52] in a PAR-1 dependent reaction.
Trauma
Trauma, even sterile trauma, is associated with an increase in inflammation. This may be due to the release of intracellular components that activate the innate immune system, see [54] for a brief review. In mouse models of trauma, APC appears to contribute to the coagulopathy associated with trauma [55]. By use of a selective antibody to murine APC [45], the coagulopathy can be largely prevented by the selective inhibition of endogenous APC’s anticoagulant activity with preservation of its cytoprotective functions [55]. APC’s cytoprotective/anti-inflammatory functions play a key role in preventing death in this model, in part apparently by preventing excessive thrombosis that might result from tissue necrosis or apoptosis.
Amyotrophic lateral sclerosis
A mutant superoxide dismutase gene has been found in amyotrophic lateral sclerosis (ALS) patients, and this gene insertion will elicit ALS-like symptoms in mice [56]. APC mutants with cytoprotective activity can suppress the mutant gene expression and slow the progression of ALS symptoms apparently by crossing the blood–brain barrier and signaling through a PAR1- and PAR3-dependent pathway [56].
Conclusions
APC is a potent modulator of disease processes. Both direct anticoagulant activity and cell signaling are involved. The anti-histone activity of APC has also been implicated. Either the native protein or genetically modified versions of the molecule have potential therapeutic utility. In some clinical conditions like trauma, excess activation of the endogenous protein C seems to contribute to morbidity and mortality. With exogenous APC, mutations of the molecule can selectively alter its function, whereas antibodies can be utilized to modulate the functions of the endogenous APC. As we gain better understanding of the details of the interplay of APC with the complex regulatory systems in vivo, the potential is high to be able to exploit this system for even greater selectivity and clinical benefit. In particular, APC may be able to prevent the autoamplification of inflammation and coagulation depicted in Fig. 2.
Fig. 2.
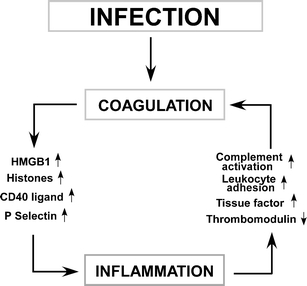
The links between infection, coagulation, and inflammation. Infection either directly triggers the activation of the intrinsic pathway through activation of factor XII or activates a series of toll-like receptors that can generate cytokines that initiate tissue factor expression. Coagulation leads to platelet activation, releasing CD40 ligand that amplifies inflammation, expression of P-selectin on cell surfaces which aids in leukocyte trafficking, and with ischemia reperfusion injury which leads to the release of HMGB 1 or histones that further trigger inflammation and tissue damage. The resultant amplified inflammatory response leads to additional tissue factor formation, thrombomodulin downregulation, complement activation, and leukocyte activation, further stimulating coagulation. Unchecked, this has the potential for devastating inflammatory and coagulation-mediated injury
Acknowledgments
CTE is an investigator of the Howard Hughes Medical Institute.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
This article is published as part of the Special Issue on Coagulation & Inflammation [34:1].
References
- 1.Esmon CT, Schwarz HP. An update on clinical and basic aspects of the protein C anticoagulant pathway. Trends Cardiovasc Med. 1995;5:141–148. doi: 10.1016/1050-1738(95)00054-D. [DOI] [PubMed] [Google Scholar]
- 2.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 3.Coalson JJ, Benjamin B, Archer LT, Beller B, Gilliam CL, Taylor FB, Hinshaw LB. Prolonged shock in the baboon subjected to infusion of E. coli endotoxin. Circ Shock. 1978;5:423–437. [PubMed] [Google Scholar]
- 4.Taylor FB, Jr, Chang ACK, Peer GT, Mather T, Blick K, Catlett R, Lockhart MS, Esmon CT. DEGR-factor Xa blocks disseminated intravascular coagulation initiated by Escherichia coli without preventing shock or organ damage. Blood. 1991;78:364–368. [PubMed] [Google Scholar]
- 5.Hinshaw LB, Chang ACK, Beller-Todd BK, Archer LT, Taylor FB., Jr Extracorporeal perfusion without exogenous anticoagulation: its protective role in endotoxin shock. Circ Shock. 1982;9:281–295. [PubMed] [Google Scholar]
- 6.Esmon CT, Owen WG. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981;78:2249–2252. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comp PC, Jacocks RM, Ferrell GL, Esmon CT. Activation of protein C in vivo. J Clin Invest. 1982;70:127–134. doi: 10.1172/JCI110584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor FB, Jr, Chang A, Hinshaw LB, Esmon CT, Archer LT, Beller BK. A model for thrombin protection against endotoxin. Thromb Res. 1984;36:177–185. doi: 10.1016/0049-3848(84)90339-6. [DOI] [PubMed] [Google Scholar]
- 9.Stearns DJ, Kurosawa S, Sims PJ, Esmon NL, Esmon CT. The interaction of a Ca2+ dependent monoclonal antibody with the protein C activation peptide region: evidence for obligatory Ca2+ binding to both antigen and antibody. J Biol Chem. 1988;263:826–832. [PubMed] [Google Scholar]
- 10.Taylor FB, Jr, Chang A, Esmon CT, D’Angelo A, Vigano-D’Angelo S, Blick KE. Protein C prevents the coagulopathic and lethal effects of E. coli infusion in the baboon. J Clin Invest. 1987;79:918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor F, Chang A, Ferrell G, Mather T, Catlett R, Blick K, Esmon CT. C4b-binding protein exacerbates the host response to Escherichia coli. Blood. 1991;78:357–363. [PubMed] [Google Scholar]
- 12.Ganopolsky JG, Castellino FJ. A protein C deficiency exacerbates inflammatory and hypotensive responses in mice during polymicrobial sepsis in a cecal ligation and puncture model. Am J Pathol. 2004;165:1433–1446. doi: 10.1016/S0002-9440(10)63401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levi M, Dörffler-Melly J, Reitsma P, Büller H, Florquin S, van der Poll T, Carmeliet P. Aggravation of endotoxin-induced disseminated intravascular coagulation and cytokine activation in heterozygous protein-C-deficient mice. Blood. 2003;101:4823–4827. doi: 10.1182/blood-2002-10-3254. [DOI] [PubMed] [Google Scholar]
- 14.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Zheng X, Gu J, Hunter J, Ferrell GL, Lupu F, Esmon NL, Esmon CT. Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin. J Thromb Haemost. 2005;3:1351–1359. doi: 10.1111/j.1538-7836.2005.01385.x. [DOI] [PubMed] [Google Scholar]
- 16.Iwaki T, Cruz DT, Martin JA, Castellino FJ. A cardioprotective role for the endothelial protein C receptor in lipopolysacchride-induced endotoxemia in the mouse. Blood. 2005;105:2364–2371. doi: 10.1182/blood-2004-06-2456. [DOI] [PubMed] [Google Scholar]
- 17.Zheng X, Li W, Song Y, Ferrell GL, Esmon NL, Esmon CT. Nonhematopoietic EPCR regulates the coagulation and inflammatory responses during endotoxemia. J Thromb Haemost. 2007;5:1394–1400. doi: 10.1111/j.1538-7836.2007.02592.x. [DOI] [PubMed] [Google Scholar]
- 18.White B, Schmidt M, Murphy C, Livingstone W, O’Toole D, Lawler M, O’Neill L, Kelleher D, Schwarz HP, Smith OP. Activated protein C inhibits lipopolysaccharide-induced nuclear translocation of nuclear factor kappaB (NF-kappaB) and tumour necrosis factor alpha (TNF-alpha) production in the THP-1 monocytic cell line. Br J Haematol. 2000;110:130–134. doi: 10.1046/j.1365-2141.2000.02128.x. [DOI] [PubMed] [Google Scholar]
- 19.Joyce DE, Grinnell BW. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit Care Med. 2002;30:S288–S293. doi: 10.1097/00003246-200205001-00019. [DOI] [PubMed] [Google Scholar]
- 20.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 21.Franscini N, Bachli EB, Blau N, Leikauf MS, Schaffner A, Schoedon G. Gene expression profiling of inflamed human endothelial cells and influence of activated protein C. Circulation. 2004;110:2903–2909. doi: 10.1161/01.CIR.0000146344.49689.BB. [DOI] [PubMed] [Google Scholar]
- 22.Hirose K, Okajima K, Taoka Y, Uchiba M, Tagami H, Nakano K-Y, Utoh J, Okabe H, Kitamura N. Activated protein C reduces the ischemia/reperfusion-induced spinal cord injury in rats by inhibiting neutrophil activation. Ann Surg. 2000;232:272–280. doi: 10.1097/00000658-200008000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, Takatsuki K. Activated protein C prevents LPS-induced pulmonary vascular injury by inhibiting cytokine production. Am J Physiol. 1997;272:L197–L202. doi: 10.1152/ajplung.1997.272.2.L197. [DOI] [PubMed] [Google Scholar]
- 24.Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, Castellino FJ, Mackman N, Griffin JH, Weiler H. Endotoxemia and sepsis mortality reduction by non-anticoagulant-activated protein C. J Exp Med. 2007;204:2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riewald M, Ruf W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J Biol Chem. 2005;280:19808–19814. doi: 10.1074/jbc.M500747200. [DOI] [PubMed] [Google Scholar]
- 26.Bae J-S, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bae J-S, Yang L, Rezaie AR. Lipid raft localization regulates the cleavage specificity of protease activated receptor 1 in endothelial cells. J Thromb Haemost. 2008;6:954–961. doi: 10.1111/j.1538-7836.2008.02924.x. [DOI] [PubMed] [Google Scholar]
- 28.Bae JS, Yang L, Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc Natl Acad Sci U S A. 2007;104:2867–2872. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann JN, Vollmar B, Laschke MW, Inthorn D, Fertmann J, Schildberg FW, Menger MD. Microhemodynamic and cellular mechanisms of activated protein C action during endotoxemia. Crit Care Med. 2004;32:1011–1017. doi: 10.1097/01.CCM.0000120058.88975.42. [DOI] [PubMed] [Google Scholar]
- 30.Mizutani A, Okajima K, Uchiba M, Noguchi T. Activated protein C reduces ischemia/reperfusion-induced renal injury in rats by inhibiting leukocyte activation. Blood. 2000;95:3781–3787. [PubMed] [Google Scholar]
- 31.Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, Takatsuki K. Activated protein C attenuates endotoxin-induced pulmonary vascular injury by inhibiting activated leukocytes in rats. Blood. 1996;87:642–647. [PubMed] [Google Scholar]
- 32.Nick JA, Coldren CD, Geraci MW, Poch KR, Fouty BW, O’Brien J, Gruber M, Zarini S, Murphy RC, Kuhn K, Richter D, Kast KR, Abraham E. Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood. 2004;104:3878–3885. doi: 10.1182/blood-2004-06-2140. [DOI] [PubMed] [Google Scholar]
- 33.Nagai M, Yilmaz CE, Kirchhofer D, Esmon CT, Mackman N, Granger DN. Role of coagulation factors in cerebral venous sinus and cerebral microvascular thrombosis. Neurosurgery. 2010;66:560–566. doi: 10.1227/01.NEU.0000365745.49583.FD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Acivated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 35.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphinosine 1-phospate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 36.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Dean R, Fernández JA, LaRue B, Griffin JH, Chopp M, Zlokovic BV. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1284. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 37.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/S0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 38.Mosnier LO, Griffin JH. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease-activated receptor-1 and endothelial cell protein C receptor. Biochem J. 2003;373:65–70. doi: 10.1042/BJ20030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosnier LO, Gale AJ, Yegneswaran S, Griffin JH. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood. 2004;104:1740–1744. doi: 10.1182/blood-2004-01-0110. [DOI] [PubMed] [Google Scholar]
- 42.Niessen F, Furlan-Freguia C, Fernandez JA, Mosnier LO, Castellino FJ, Weiler H, Rosen H, Griffin JH, Ruf W. Endogenous EPCR/aPC-PAR1 signaling prevents inflammation-induced vascular leakage and lethality. Blood. 2009;113:2859–2866. doi: 10.1182/blood-2008-12-192385. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Kerschen E, Hernandez I, Zogg M, Jia S, Hessner MJ, Fernandez JA, Griffin JH, Huettner CS, Castellino FJ, Weiler H. Activated protein C targets CD8+ dendritic cells to reduce the mortality of endotoxemia in mice. J Clin Invest. 2010;120:3167–3178. doi: 10.1172/JCI42629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao C, Gao Y, Li Y, Antalis TM, Castellino FJ, Zhang L. The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b. J Clin Invest. 2010;120:1971–1980. doi: 10.1172/JCI40380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J, Zhang X, Drake M, Esmon CT. Endogenous activated protein C signaling is critical to protection of mice from lipopolysaccaride-induced septic shock. J Thromb Haemost. 2009;7:851–856. doi: 10.1111/j.1538-7836.2009.03333.x. [DOI] [PubMed] [Google Scholar]
- 46.Snow TR, Deal MT, Dickey DT, Esmon CT. Protein C activation following coronary artery occlusion in the in situ porcine heart. Circulation. 1991;84:293–299. doi: 10.1161/01.cir.84.1.293. [DOI] [PubMed] [Google Scholar]
- 47.Loubele STBG, Spek CA, Leenders P, van Oerle R, Aberson HL, Hamulyák K, Ferrell G, Esmon CT, Sprongk HMH, ten Cate H. Activated protein C protects against myocardial ischemia/reperfusion injury via inhibition of apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2009;29:1087–1092. doi: 10.1161/ATVBAHA.109.188656. [DOI] [PubMed] [Google Scholar]
- 48.Pirat B, Muderrisoglu H, Unal MT, Ozdemir H, Yildirir A, Yucel M, Turkoglu S. Recombinant human-activated protein C inhibits cardiomyocyte apoptosis in a rat model of myocardial ischemia-reperfusion. Coron Artery Dis. 2007;18:61–66. doi: 10.1097/MCA.0b013e328010a44a. [DOI] [PubMed] [Google Scholar]
- 49.Cheng T, Liu D, Griffin JH, Fernández JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 50.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13:1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 51.Scaldaferri F, Sans M, Vetrano S, Graziani C, de Cristofaro R, Gerlitz B, Repici A, Malesci A, Panes J, Grinnell BW, Danese S. Crucial role of the protein C pathway in governing microvascular inflammation in inflammatory bowel disease. J Clin Invest. 2007;117:1951–1960. doi: 10.1172/JCI31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Sluis GL, Niers TMH, Esmon CT, Tigchelaar W, Richel DJ, Buller HR, Van Noorden CJF, Spek CA. Endogenous activated protein C limits cancer cell extravasation through sphingosine-1-phosphate receptor 1 mediated vascular endothelial barrier enhancement. Blood. 2009;114:1968–1973. doi: 10.1182/blood-2009-04-217679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bezuhly M, Cullen R, Esmon CT, Morris SF, West KA, Johnston B, Liwski RS. Role of activated protein C and its receptor in inhibition of tumor metastasis. Blood. 2009;113:3371–3374. doi: 10.1182/blood-2008-05-159434. [DOI] [PubMed] [Google Scholar]
- 54.Esmon CT. Far from the heart: counteracting coagulation. Nat Med. 2010;16:759–760. doi: 10.1038/nm0710-759. [DOI] [PubMed] [Google Scholar]
- 55.Chesebro BB, Rahn P, Carles M, Esmon CT, Xu J, Brohi K, Pittet J-F, Cohen MJ. Activated protein C mediates acute traumative coagulopathy in mice. Shock. 2009;32:659–665. doi: 10.1097/SHK.0b013e3181a5a632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong Z, Ilieva H, Hallagan L, Bell R, Singh I, Paquette N, Thiyagarajan M, Deane R, Fernandez JA, Lane S, Ziokovic AB, Liu T, Griffin JH, Chow N, Castellino FJ, Stojanovic K, Cleveland DW, Zlokovic BV. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119:3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]


