Abstract
AIM: To investigate the effectiveness of low-volume plus ascorbic acid [polyethylene glycol plus ascorbic acid (PEG + Asc)] and high-volume plus simethicone [polyethylene glycol plus simethicone (PEG + Sim)] bowel preparations.
METHODS: A total of one hundred and forty-four outpatients (76 males), aged from 20 to 84 years (median age 59.5 years), who attended our Department, were divided into two groups, age and sex matched, and underwent colonoscopy. Two questionnaires, one for patients reporting acceptability and the other for endoscopists evaluating bowel cleansing effectiveness according to validated scales, were completed. Indications, timing of examination and endoscopical findings were recorded. Biopsy forceps were used as a measuring tool in order to determine polyp endoscopic size estimation. Difficulty in completing the preparation was rated in a 5-point Likert scale (1 = easy to 5 = unable). Adverse experiences (fullness, cramps, nausea, vomiting, abdominal pain, headache and insomnia), number of evacuations and types of activities performed during preparation (walking or resting in bed) were also investigated.
RESULTS: Seventy-two patients were selected for each group. The two groups were age and sex matched as well as being comparable in terms of medical history and drug therapies taken. Fourteen patients dropped out from the trial because they did not complete the preparation procedure. Ratings of global bowel cleansing examinations were considered to be adequate in 91% of PEG + Asc and 88% of PEG + Sim patients. Residual Stool Score indicated similar levels of amount and consistency of residual stool; there was a significant difference in the percentage of bowel wall visualization in favour of PEG + Sim patients. In the PEG + Sim group, 12 adenomas ≤ 10 mm diameter (5/left colon + 7/right colon) vs 9 (8/left colon + 1/right colon) in the PEG + Asc group were diagnosed. Visualization of small lesions seems to be one of the primary advantages of the PEG + Sim preparation.
CONCLUSION: PEG + Asc is a good alternative solution as a bowel preparation but more improvements are necessary in order to achieve the target of a perfect preparation.
Keywords: Bowel preparation, Polyethylene glycol, Ascorbic acid, Colonoscopy, Simethicone
INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancers diagnosed in Western countries and it is the major cause of cancer-associated morbidity and mortality[1]. The increased demand for colonoscopy can be attributed to widespread CRC screening and surveillance[2,3]. A screening procedure, to be effective, must ensure high sensitivity and it must be both safe and tolerable in order to warrant adequate compliance in asymptomatic individuals[4].
Colonoscopy has been accepted as the gold standard for colon exploration and is considered the most effective method for assessing colonic lesions. In fact, this procedure performed in asymptomatic individuals ≥ 50 years old with no history of CRC or adenomas, and in younger high-risk patients[1,5], permits an early detection of CRC.
Several specific pre-procedure quality indicators were selected in 2006 by the American Society for Gastrointestinal Endoscopy (ASGE)/American College of Gastroenterology (ACG) taskforce on quality in endoscopy with the aim of establishing competence in colonoscopy performance. They are: (1) appropriate indication; (2) informed consent obtained; (3) use of recommended surveillance intervals; (4) use of recommended ulcerative colitis and Crohn’s colitis surveillance; and (5) patient preparation[5].
An inadequate preparation can be costly in terms of missed lesions, increased risk of complications, time required for procedure, and need for repeated colonoscopies[5,6]. Moreover, patient compliance to the preparation process is often poor[7] and it remains a deterrent for patients in whom colonoscopy is required[8]. Independent predictors of an inadequate colon preparation include a later colonoscopy starting time, failure to follow preparation instructions, inpatient status, indication of constipation, use of tricyclic antidepressants, male gender, and a history of cirrhosis, stroke, or dementia[9].
The ideal colon cleansing for diagnostic and surgical procedures would reliably empty the colon of fecal material, have no effect on the gross or microscopic appearance of the colon, require a short period for ingestion and evacuation, cause no discomfort and produce no significant shifts of fluids or electrolytes[10-13]. Moreover, the cleansing regimen should be simple and suitable for inpatients and outpatients. Nowadays, available methods do not completely meet these criteria, and problems with patient compliance, safety, and adequacy of cleansing prompt continued investigation for alternative forms of cleansing.
Polyethylene glycol (PEG)-based gut lavage is an isosmotic solution that passes through the bowel without absorption or secretion. PEG has been safely used in patients with serum electrolyte imbalances, advanced hepatic dysfunction, acute and chronic renal failure and congestive heart failure[14,15]. PEG does not alter the histological features of colonic mucosa and may be used in patients suspected of having inflammatory bowel disease without obscuring the diagnostic capabilities of colonoscopy or biopsy analysis. Several PEG lavage solutions have added simethicone which is an oral antifoaming agent that decreases bloating, abdominal discomfort and abdominal pain by promoting the clearance of excessive gas along the gastrointestinal tract by reducing the surface tension of air bubbles. This combination is safe and effective (significant fluid and electrolyte shifts are avoided) but requires the consumption of large volumes of fluid in order to achieve a cathartic effect[16]. Nowadays, a low-volume PEG oral solution for colon cleansing that combines PEG with electrolytes plus ascorbic acid and sodium sulphate is gaining popularity over large volume oral lavage solutions[17]. The megadose of ascorbic acid that is not completely absorbed remains in the colonic lumen where it exerts an osmotic effect so a smaller quantity of PEG is required.
The aim of our randomized trial was to compare the PEG + ascorbic acid and sodium sulphate preparation (MoviPrep®; Norgine BV; PEG + Asc) with a PEG + simethicone preparation (Selg®-Esse 1000, Promefarm Srl, IT; PEG + Sim) in terms of cleansing effectiveness, patient compliance, physical tolerability, endoscopic findings and costs.
MATERIALS AND METHODS
A total of one hundred and forty-four outpatients (76 males), aged from 20 to 84 years (median age 59.5 years), who attended our Department of Surgical Sciences of “Sapienza” University of Rome over the period May 2009 to October 2010 and who underwent elective colonoscopy for routine clinical indications were randomized. Patients were 1:1 randomized to receive the commercially available bowel cleansing regimens: (1) 2 L of PEG + ASC (MoviPrep®; Norgine BV); and (2) 4 L of PEG + Sim (Selg®-Esse 1000, Promefarm Srl, IT). A computer-generated randomization chart was used to determine allocation. Allocation was concealed with an opaque envelope. The envelope was opened when the patient met the inclusion criteria and provided informed consent. Exclusion criteria were as follows: hospitalized patients, allergy or hypersensitivity to any constituent of both lavage solutions, and inability to fill in a questionnaire. Patient demographics, mean time of examination, indications and colonoscopy findings are shown in Table 1.
Table 1.
Patient demographics, indications and colonoscopy findings
| PEG + Asc | PEG + Sim | |
| ITT patients | 72 | 72 |
| Compliant patients (%) | 69 (96) | 61 (85) |
| Cecal intubation (%) | 62 (86) | 68 (94) |
| Median age (range) | 60.1 (20-84) | 57.6 (33-81) |
| Male (%) | 40 (55) | 36 (50) |
| Median timing of colonoscopy (min) | 22 | 21 |
| Indications (%) | ||
| Follow-up | 27 (37) | 18 (25) |
| Surveillance | 8 (11) | 11 (15) |
| CRC sreening | 15 (21) | 8 (11) |
| Hematochezia | 13 (18) | 16 (22) |
| Change in bowel habits | 3 (4) | 7 (10) |
| Anemia | 2 (3) | 1 (2) |
| Abdominal pain | 4 (6) | 11 (15) |
| Findings (%) | ||
| No abnormalities | 40 (55) | 24 (33) |
| Diverticular disease | 14 (19) | 14 (19) |
| Polyps/Malignancy | 13/2 (18/3) | 22/0 (31/0) |
| IBD | 1 (2) | 3 (4) |
| Other | 2 (3) | 9 (13) |
ITT: Intention to treat; CRC: Colorectal cancer; IBD: Inflammatory bowel disease; PEG + Asc: Polyethylene glycol plus ascorbic acid; PEG + Sim: Polyethylene glycol plus simethicone.
Written instructions on how to prepare and ingest the bowel preparation solution (Table 2) and dietary advice, randomly alternating between PEG + Asc and PEG + Sim, were given and explained by the endoscopists or paramedical staff at the time of exam scheduling. The coordinator told all patients not to reveal to the physicians performing the colonoscopy which preparation they had taken. An informed consent form was obtained from each study subject.
Table 2.
Colonoscopy preparation schedules
| PEG + Asc | 2 L from 6:00 to 8:00 PM (250 mL every 15 min) plus 500 mL of clear fluid for every L of solution, evening before colonoscopy |
| Each liter of PEG + Asc (MoviPrep®) contains 100 g macrogol 3350, 7.5 g sodium sulfate, 2.7 g sodium chloride, 1 g potassium chloride, 4.7 g ascorbic acid, 5.9 g sodium ascorbate, and lemon flavoring | |
| PEG + Sim | 2 L from 3:00 to 5:00 PM and 2 L from 6:00 to 8:00 PM (250 mL every 15 min), evening before colonoscopy |
| Each liter of PEG + Sim (Selg®-Esse 1000) contains 58.3 g macrogol 4000, 0.08 g simethicone, 5.68 g sodium sulfate, 1.68 g sodium bicarbonate, 1.46 g sodium chloride and 0.74 g potassium chloride and mandarin aroma |
A low-fiber diet (mainly the avoidance of fruits and vegetables) was recommended for three days before the endoscopy to all subjects and, the day before, they were advised to have regular breakfast, a light lunch and only clear liquids for dinner. PEG + Asc: Polyethylene glycol plus ascorbic acid; PEG + Sim: Polyethylene glycol plus simethicone.
Upon arrival at the endoscopy suite, patients filled in a questionnaire and were interviewed about their compliance to the assigned bowel preparation method (Appendix A). Feasibility of instructions and willingness to retake the exam in the future if needed was recorded. Difficulty in completing the preparation was rated in a 5-point Likert scale (1 = easy to 5 = unable). Adverse experiences (fullness, cramps, nausea, vomiting, abdominal pain, headache and insomnia), number of evacuations and types of activities performed during preparation (walking or resting in bed) were also investigated. The exams, performed by experienced endoscopists, were scheduled between 8:30 AM and 2:00 PM. Standard colonoscopies (EVIS EXERA II video colonoscope CF-Q165I®, Olympus Europa Holding GmbH) were used for colonoscopic examinations. A minimum 6-min withdrawal time was spent. After the procedure, endoscopists filled in a questionnaire in order to evaluate the global bowel cleansing score with an Aronchick scale, as indicated in Table 3[18].
Table 3.
Colonoscopy preparation assessments
| Aronchick scale | |
| 1 Excellent | Small volume of clear liquid or greater than 95% of surface seen |
| 2 Good | Large volume of clear liquid covering 5% to 25% of surface but greater than 90% of surface seen |
| 3 Fair | Some semi-solid stool that could be suctioned or washed away but greater than 90% of surface seen |
| 4 Poor | Semi-solid stool that could not be suctioned or washed away and less than 90% of surface seen |
| 5 Inadequate | Repreparation needed |
| Residual stool score (total in sum of three score) | |
| Amount of residual stool | 0 = none |
| 1 = small | |
| 2 = moderate | |
| 3 = large | |
| Consistency of residual stool | 0 = none |
| 1 = clear liquid | |
| 2 = colored liquid | |
| 3 = stool particles | |
| 4 = semi-solid stool | |
| 5 = solid stool | |
| Percent bowel wall visualized | 0 ≥ 75% |
| 1 = 50%-75% | |
| 2 = 25%-49% | |
| 3 ≤ 25% |
A Residual Stool Score (Table 3), based on the amount and consistency of residual stool and on the percent of bowel wall visualization[19,20], was recorded for each of five colon segments: cecum, right colon, transverse colon, left colon/sigmoid, and rectum. The three component scores from each colon segment were averaged and then summed to calculate a total residual stool score for each subject (range 0-11 for total score, 0 = best). Overall, colon cleansing efficacy was considered adequate if the ranking was 1-3 Aronchick scale score. Indications, timing of examination and endoscopical findings were recorded. Biopsy forceps were used as a measuring tool in order to determine polyp endoscopic size estimation.
χ2 test including Yates’ continuity correction was used as appropriate. A significant difference was considered when the P value was ≤ 0.05. All analyses were performed using GraphPad InStat version 2.04a.
RESULTS
Seventy-two patients were selected for the PEG + Asc group and seventy-two for the PEG + Sim group. The two groups were age and sex matched as well as comparable in terms of medical history and drug therapies taken. Fourteen patients dropped out from the trial because they did not complete the preparation procedure (Table 4). Among these patients, some were unable to complete their preparations because of nausea (13 patients) and others because of vomiting. There were no significant differences in reported side effects between the PEG + Asc and the PEG + Sim groups. The most common reported side effects were nausea and vomiting (Tables 4 and 5). In 14 cases, endoscopists were unable to achieve cecal intubation: 6 patients due to lack of bowel cleansing, 6 due to a poor tolerance and 2 patients because of the presence of a malignant stricture (both in the PEG + Asc group). Rating global bowel cleansing using the Aronchick scale (Table 6): examinations were considered to be adequate in 91% of PEG + Asc and 88% of PEG + Sim patients. Residual Stool Score indicated similar levels of amount and consistency of residual stool; there was a significant difference in the percentage of bowel wall visualization in favour of PEG + Sim patients (Figure 1).
Table 4.
Patient drop-out: global bowel cleansing, side effects and findings
| Drop out gut cleansing | PEG + Asc | PEG + Sim |
| No. of patients | 3 | 11 |
| Global bowel cleansing (%) | ||
| Aronchick 1 | (1) | (2) |
| Aronchick 2 | (1) | (5) |
| Aronchick 3 | - | (2) |
| Aronchick 4 | (1) | (2) |
| Aronchick 5 | - | - |
| Cecal Intubation (patients) | 2 | 11 |
| Findings | ||
| No abnormalities | 1 | 9 |
| Polyps/malignancy | 0/1 | 2/0 |
| Diverticular disease | 1 | - |
| Other | - |
PEG + Asc: Polyethylene glycol plus ascorbic acid; PEG + Sim: Polyethylene glycol plus simethicone.
Table 5.
Side effects in compliant patients (1multiple side effects possible)
| PEG + Asc | PEG + Sim | |
| No. of patients | 72 | 72 |
| Side effects patients1 | 14 | 21 |
| Nausea | 7 | 16 |
| Vomiting | 4 | 5 |
| Headache | 3 | 1 |
| Insomnia | 1 | 1 |
| Abdominal pain | 2 | 1 |
PEG + Asc: Polyethylene glycol plus ascorbic acid; PEG + Sim: Polyethylene glycol plus simethicone.
Table 6.
Overall gut cleansing and cecal intubation performed
| Overall gut cleansing | PEG + Asc | PEG + Sim |
| No. of patients | 69 | 61 |
| Aronchick 1 | 8/5 | 17/16 |
| Aronchick 2 | 29/27 | 13/12 |
| Aronchick 3 | 26/26 | 24/24 |
| Aronchick 4 | 5/2 | 5/5 |
| Aronchick 5 | 1/0 | 2/0 |
PEG + Asc: Polyethylene glycol plus ascorbic acid; PEG + Sim: Polyethylene glycol plus simethicone.
Figure 1.
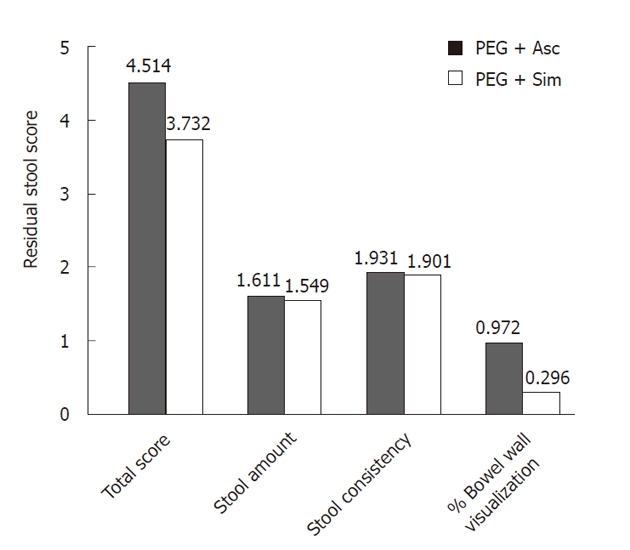
Residual stool score. A lower score indicates better bowel cleansing. Subjects in the PEG + Sim group demonstrated significantly lower scores for percentage of colon visualization. PEG + Asc: Polyethylene glycol plus ascorbic acid; PEG + Sim: Polyethylene glycol plus simethicone.
In the PEG + Sim group, 12 adenomas ≤ 10 mm in diameter (5/left colon + 7/right colon) vs 9 (8/left colon + 1/right colon) in the PEG + Asc group were diagnosed (Figure 2). Furthermore, in the PEG + Sim group, 12 adenomas ≤ 5 mm in diameter vs 5 (left colon only) in the PEG + Asc group were diagnosed.
Figure 2.
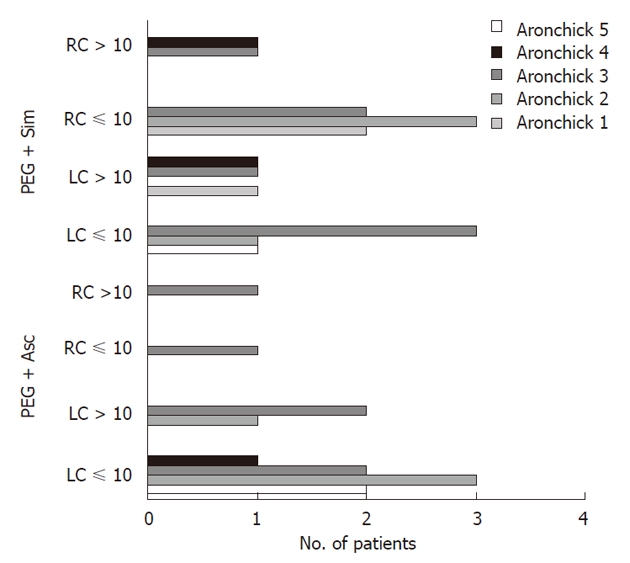
Patients with at least one newly diagnosed polyp in relation to the level of cleanliness achieved (LC = Left colon; RC = Right colon). PEG + Asc: Polyethylene glycol plus ascorbic acid; PEG + Sim: Polyethylene glycol plus simethicone.
The average time of examination was about 22 min. Moreover, the median timing of colonoscopy was longer in negative tests (24 min, range 20-40) than in colonoscopies with polyp diagnosis (19 min, range 18-25). In patients with a score of 4 or 5 on the Aronchick scale of bowel preparation, the average time for colonoscopy completion was approximately 27 min. The average number of bowel movements obtained during the preparation did not appear to be related to the degree of cleanliness achieved, with 13 movements for 1 or 2 Aronchick scale, 11 movements for 3 Aronchick scale and 11 movements for 4 or 5 Aronchick scale score. Conversely, the presence of clear liquid at the time of the last evacuation is a reliable parameter of effective colonic cleansing. In fact, 93% of patients who achieved a 1, 2 or 3 Aronchick scale score reported the presence of clear liquids during the last evacuation, while patients with 4 or 5 Aronchick scale score reported this in only 40% of cases. Sixty-three percent of the subjects taking PEG + Sim and 39% of the subjects taking PEG + Asc (P = 0.005) reported that they would rather try another preparation for a future colonoscopy. Other patient questionnaire findings rated by preparation group tolerability are reported in Table 7.
Table 7.
Patient questionnaire findings by preparation group tolerability
| Question | PEG + Asc | PEG + Sim |
| Clear liquid at the time of the last evacuation | ||
| Yes | 51 | 62 |
| No | 21 | 10 |
| Is this the first time you took a preparation for colonoscopy? | ||
| Yes | 33 | 43 |
| No | 39 | 29 |
| Discomfort: | ||
| None | 18 | 28 |
| Slightest | 32 | 36 |
| Moderate | 17 | 6 |
| Severe | 5 | 2 |
| How much would you be prepared to repeat this preparation for colonoscopy? | ||
| A little | 18 | 15 |
| Fairly | 32 | 42 |
| Much | 15 | 11 |
| I would never repeat | 7 | 4 |
PEG + Asc: Polyethylene glycol plus ascorbic acid; PEG + Sim: Polyethylene glycol plus simethicone.
DISCUSSION
Currently, the most significant disadvantage of performing colonoscopies is the need for adequate bowel preparation and poor bowel preparation impacts on the efficiency of colonoscopy[21]. Moreover, the major obstacle preventing a large scale implementation of CRC screening is the low level of patient compliance[21], and patient compliance is limited because of embarrassment[20,21], the bowel preparation procedure[8] and the fear of pain and discomfort associated with the examination[22-24].
Cleansing methods for colonoscopy have evolved by attempting to achieve a high efficiency together with a high patient compliance. A consensus of the American Society for Gastrointestinal Endoscopy, the American Society of Colon and Rectal Surgeons and the Society of American Gastrointestinal and Endoscopic Surgeons, indicated that PEG is the gold standard for colonoscopic bowel preparation (Grade IA), and aqueous sodium phosphate (NaP) is an alternative regimen to PEG solutions (Grade IA)[24].
Several meta-analyses on the available bowel preparations have favored NaP, concluding that it was effective and better tolerated by patients than PEG solutions[25-28]. However, the disadvantages of NaP are the associated side effects. Significant changes in serum electrolyte levels[29], even in patients without renal failure, have prompted recommendations for serum electrolyte evaluation prior to the administration of sodium phosphate[30,31]. On the other hand, osmotically balanced electrolyte lavage solutions (PEG-ELS, SF-ELS) offer safe and effective cleansing[22-24,32-34] but volume related discomfort and adverse experiences have decreased the percentage of patients completing the pre-examination preparation. This is mainly due to the large volumes of fluid required for bowel preparation, the unpleasant taste and an increase in the incidence of side effects[15]. In order to bypass volume and taste problems, a PEG electrolyte lavage solution containing ascorbic acid was developed. This low-volume formulation has satisfied many of our requirements. In fact, in our study the subjects enrolled were outpatients, and it was not possible to carry out a complete clinical history and serum electrolyte evaluation. Thus, one of our major considerations was patient safety in colonic preparation, which is well documented for PEG solutions.
To help ensure compliance, the paramedical staff explained to the patient in detail the instructions containing the correct procedures to follow, with special attention paid to explaining the importance of additional fluid consumption with this procedure. Patients were then asked if they completely understood the procedure they had to follow.
Our study has limitations, such as number of patients, single center, lack of a practice calibration on the bowel preparation scoring system for all physicians involved before the study, and full-dose vs split-dose regimen comparisons. However, some conclusions on efficacy of bowel wall cleansing, adenoma detection rate and patient compliance can be made.
Bowel cleansing can be evaluated using different scoring systems such as the Aronchick[18], the Ottawa[35] and the Boston scale[36]. In our study, we decided to use the Aronchick scale assisted by a residual stool score to evaluate effectiveness, as previously adopted by Balaban et al[19] and Harenwood et al[20]. Our data on the bowel cleansing evaluation showed similar levels in both groups, that in most cases was found to be “excellent-fair” (Aronchick 1-3) allowing cecal intubation in 90% of cases. However, we noted better colonic wall visualization in the PEG + Sim group, probably due to the effect of the simethicone.
Only a small number of studies have compared the adenoma detection rate to the quality of bowel preparation[6,37,38]. Froehlich et al[6] and Harewood et al[37], however, demonstrated that a better bowel preparation led to a higher rate of colon lesion detection, enhancing the ability to discern smaller lesions and thus improved the thoroughness of colonoscopy. In our trial, only 3 out of 30 polyps (Aronchick 4 only) were diagnosed in the presence of inadequate bowel preparation and two of them were > 10 mm in diameter. Thus, we focused on the diagnosis of adenoma in relation to the degree of colonic preparation, paying attention to adenomas ≤ 10 mm or 5 mm and their distribution. Indeed, while there is no significant difference in total adenoma detection rates between groups, looking at the number of adenomas ≤ 10 mm and ≤ 5 mm in diameter and their distribution, there was significant evidence of a greater number of microadenomas diagnosed in favor of the PEG + SIM group. This result reinforces our observations that PEG + Sim has a better ability to clean the colon wall as represented by the residual stool score. Although the impact of detection and removal of micro-adenomas on CRC incidence or mortality is debated, this parameter, which is strongly influenced by the quality of bowel preparation, could be objectively representative of the view of the intestinal wall.
However, since colonoscopy is the best screening test for CRC, we cannot underestimate the importance of patient compliance which directly affects its acceptance and distribution. We must therefore consider whether it is more important to have a highly effective or highly popular test and search for a compromise. So, from the aspect of patient compliance, the majority of patients in both groups completed the bowel preparation in the specified schedule (96% for PEG + Asc and 85% for PEG + Sim). Both groups contained patients who reported side effects and did not finish the pre-procedure preparation. However, this occurred predominantly in the PEG + Sim group, although colonoscopy was still able to be performed and it did not affect the results of the bowel cleansing score. Thus, in this study, the inability to completely drink the PEG + Sim solution (75% of the total was always drunk) did not significantly reduce the effectiveness of the pre-procedure preparation. It is difficult to say the same for the PEG + Asc group considering the small number of patients with adverse events (n = 3). However, our data have shown, in agreement with Ell et al[16], that the PEG + Asc formulation was more acceptable to patients and a greater number of them finished the recommended dose.
In conclusion, we agree that PEG + Asc is a good alternative solution, in particular addressing patient compliance, but some improvements seem to be necessary in order to achieve the target of a perfect preparation. One area could be the visualization of small lesions. This seems to be one of the primary advantages of the PEG + Sim solution. Based on the data, the low-volume preparation represents a valid alternative to high-volume preparations, especially with regard to patient compliance. However, improvements are needed to reduce the side effects in both types of preparation and further studies should be carried out, giving the patient the choice of preparation to be taken.
ACKNOWLEDGMENTS
The authors thank Dr. Andrea Stoler for having edited the English version.
COMMENTS
Background
Colonoscopy has been accepted as the gold standard for colon exploration and is considered the most effective method for assessing colonic lesions. An inadequate preparation can be costly in terms of missed lesions, increased risk of complications, time required for procedure and need for repeated colonoscopies.
Research frontiers
A bowel cleansing regimen should be simple and suitable for inpatients and outpatients. Nowadays, available methods do not completely meet these criteria, and problems with patient compliance, safety, and adequacy of cleansing prompt continued investigation for alternative forms of cleansing.
Innovations and breakthroughs
Our randomized trial compared the polyethylene glycol plus ascorbic acid (PEG + Asc) and sodium sulphate preparation with a polyethylene glycol plus simethicone (PEG + Sim) preparation in terms of cleansing effectiveness, patient compliance, physical tolerability, endoscopic findings and adenoma detection rate.
Applications
The low-volume preparation represents a valid alternative to high-volume preparations, especially with regard to patient compliance. On the other hand, the optimal visualization of colonic wall seems to be one of the primary advantages of the PEG + Sim solution. Through this study, the authors suggest different preparation regimens for different indications.
Peer review
This randomized trial compared the polyethylene glycol plus ascorbic acid and sodium sulphate preparation (MoviPrep®; Norgine BV, PEG + Asc) with a polyethylene glycol plus simethicone preparation (Selg®-Esse 1000, Promefarm Srl, IT, PEG + Sim) in terms of cleansing effectiveness, patient compliance, physical tolerability, endoscopical finding. Bowel preparation is a specific quality indicator and it is a critical point in clinical practice.
Footnotes
Peer reviewer: Haruhiko Sugimura, MD, PhD, Professor, Department of Pathology, Hamamatsu University School of Medicine, 1-20-1 Handayama, Higashi-ku, Hamamatsu 431-3192, Japan
S- Editor Sun H L- Editor Logan S E- Editor Zhang DN
References
- 1.Arditi C, Peytremann-Bridevaux I, Burnand B, Eckardt VF, Bytzer P, Agréus L, Dubois RW, Vader JP, Froehlich F, Pittet V, Schusselé Filliettaz S, Juillerat P, Gonvers JJ. Appropriateness of colonoscopy in Europe (EPAGE II). Screening for colorectal cancer. Endoscopy. 2009;41:200–208. doi: 10.1055/s-0028-1119626. [DOI] [PubMed] [Google Scholar]
- 2.Nelson RS, Thorson AG. Colorectal cancer screening. Curr Oncol Rep. 2009;11:482–489. doi: 10.1007/s11912-009-0065-8. [DOI] [PubMed] [Google Scholar]
- 3.Tsikitis VL, Malireddy K, Green EA, Christensen B, Whelan R, Hyder J, Marcello P, Larach S, Lauter D, Sargent DJ, et al. Postoperative surveillance recommendations for early stage colon cancer based on results from the clinical outcomes of surgical therapy trial. J Clin Oncol. 2009;27:3671–3676. doi: 10.1200/JCO.2008.20.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitoun A, Ponchon T, Barthet M, Coffin B, Dugué C, Halphen M. Results of a prospective randomised multicentre controlled trial comparing a new 2-L ascorbic acid plus polyethylene glycol and electrolyte solution vs. sodium phosphate solution in patients undergoing elective colonoscopy. Aliment Pharmacol Ther. 2006;24:1631–1642. doi: 10.1111/j.1365-2036.2006.03167.x. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2006;63:S16–S28. doi: 10.1016/j.gie.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 7.Radaelli F, Meucci G, Sgroi G, Minoli G. Technical performance of colonoscopy: the key role of sedation/analgesia and other quality indicators. Am J Gastroenterol. 2008;103:1122–1130. doi: 10.1111/j.1572-0241.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 8.Parente F, Marino B, Crosta C. Bowel preparation before colonoscopy in the era of mass screening for colo-rectal cancer: a practical approach. Dig Liver Dis. 2009;41:87–95. doi: 10.1016/j.dld.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Ness RM, Manam R, Hoen H, Chalasani N. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96:1797–1802. doi: 10.1111/j.1572-0241.2001.03874.x. [DOI] [PubMed] [Google Scholar]
- 10.Berry MA, DiPalma JA. Review article: orthograde gut lavage for colonoscopy. Aliment Pharmacol Ther. 1994;8:391–395. doi: 10.1111/j.1365-2036.1994.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 11.DiPalma JA, Brady CE. Colon cleansing for diagnostic and surgical procedures: polyethylene glycol-electrolyte lavage solution. Am J Gastroenterol. 1989;84:1008–1016. [PubMed] [Google Scholar]
- 12.Toledo TK, DiPalma JA. Review article: colon cleansing preparation for gastrointestinal procedures. Aliment Pharmacol Ther. 2001;15:605–611. doi: 10.1046/j.1365-2036.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- 13.Nelson DB, Barkun AN, Block KP, Burdick JS, Ginsberg GG, Greenwald DA, Kelsey PB, Nakao NL, Slivka A, Smith P, et al. Technology Status Evaluation report. Colonoscopy preparations. May 2001. Gastrointest Endosc. 2001;54:829–832. [PubMed] [Google Scholar]
- 14.Lichtenstein G. Bowel preparations for colonoscopy: a review. Am J Health Syst Pharm. 2009;66:27–37. doi: 10.2146/ajhp080084. [DOI] [PubMed] [Google Scholar]
- 15.Belsey J, Epstein O, Heresbach D. Systematic review: adverse event reports for oral sodium phosphate and polyethylene glycol. Aliment Pharmacol Ther. 2009;29:15–28. doi: 10.1111/j.1365-2036.2008.03837.x. [DOI] [PubMed] [Google Scholar]
- 16.Ell C, Fischbach W, Bronisch HJ, Dertinger S, Layer P, Rünzi M, Schneider T, Kachel G, Grüger J, Köllinger M, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol. 2008;103:883–893. doi: 10.1111/j.1572-0241.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- 17.Shawki S, Wexner SD. Oral colorectal cleansing preparations in adults. Drugs. 2008;68:417–437. doi: 10.2165/00003495-200868040-00003. [DOI] [PubMed] [Google Scholar]
- 18.Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52:346–352. doi: 10.1067/mge.2000.108480. [DOI] [PubMed] [Google Scholar]
- 19.Balaban DH, Leavell BS, Oblinger MJ, Thompson WO, Bolton ND, Pambianco DJ. Low volume bowel preparation for colonoscopy: randomized, endoscopist-blinded trial of liquid sodium phosphate versus tablet sodium phosphate. Am J Gastroenterol. 2003;98:827–832. doi: 10.1111/j.1572-0241.2003.07380.x. [DOI] [PubMed] [Google Scholar]
- 20.Harewood GC, Wright CA, Baron TH. Assessment of patients’ perceptions of bowel preparation quality at colonoscopy. Am J Gastroenterol. 2004;99:839–843. doi: 10.1111/j.1572-0241.2004.04176.x. [DOI] [PubMed] [Google Scholar]
- 21.Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. 2002;97:1696–1700. doi: 10.1111/j.1572-0241.2002.05827.x. [DOI] [PubMed] [Google Scholar]
- 22.Pox C, Schmiegel W. Colorectal screening in Germany. Z Gastroenterol. 2008;46 Suppl 1:S31–S32. doi: 10.1055/s-2007-963563. [DOI] [PubMed] [Google Scholar]
- 23.Bleiker EM, Menko FH, Taal BG, Kluijt I, Wever LD, Gerritsma MA, Vasen HF, Aaronson NK. Screening behavior of individuals at high risk for colorectal cancer. Gastroenterology. 2005;128:280–287. doi: 10.1053/j.gastro.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, Coates RJ. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100:2093–2103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 25.Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Gastrointest Endosc. 2006;63:894–909. doi: 10.1016/j.gie.2006.03.918. [DOI] [PubMed] [Google Scholar]
- 26.Young CJ, Simpson RR, King DW, Lubowski DZ. Oral sodium phosphate solution is a superior colonoscopy preparation to polyethylene glycol with bisacodyl. Dis Colon Rectum. 2000;43:1568–1571. doi: 10.1007/BF02236740. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, McCallion K, Acheson AG, Irwin ST. A prospective randomised study comparing polyethylene glycol and sodium phosphate bowel cleansing solutions for colonoscopy. Ulster Med J. 1999;68:68–72. [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu CW, Imperiale TF. Meta-analysis and cost comparison of polyethylene glycol lavage versus sodium phosphate for colonoscopy preparation. Gastrointest Endosc. 1998;48:276–282. doi: 10.1016/s0016-5107(98)70191-9. [DOI] [PubMed] [Google Scholar]
- 29.Verghese VJ, Ayub K, Qureshi W, Taupo T, Graham DY. Low-salt bowel cleansing preparation (LoSo Prep) as preparation for colonoscopy: a pilot study. Aliment Pharmacol Ther. 2002;16:1327–1331. doi: 10.1046/j.1365-2036.2002.01295.x. [DOI] [PubMed] [Google Scholar]
- 30.DiPalma JA, Buckley SE, Warner BA, Culpepper RM. Biochemical effects of oral sodium phosphate. Dig Dis Sci. 1996;41:749–753. doi: 10.1007/BF02213131. [DOI] [PubMed] [Google Scholar]
- 31.Sharma VK, Schaberg JW, Chockalingam SK, Vasudeva R, Howden CW. The effect of stimulant laxatives and polyethylene glycol-electrolyte lavage solution for colonoscopy preparation on serum electrolytes and hemodynamics. J Clin Gastroenterol. 2001;32:238–239. doi: 10.1097/00004836-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Pox C, Schmiegel W, Classen M. Current status of screening colonoscopy in Europe and in the United States. Endoscopy. 2007;39:168–173. doi: 10.1055/s-2007-966182. [DOI] [PubMed] [Google Scholar]
- 33.Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 34.Gili M, Roca M, Ferrer V, Obrador A, Cabeza E. Psychosocial factors associated with the adherence to a colorectal cancer screening program. Cancer Detect Prev. 2006;30:354–360. doi: 10.1016/j.cdp.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482–486. doi: 10.1016/s0016-5107(03)02875-x. [DOI] [PubMed] [Google Scholar]
- 36.Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620–625. doi: 10.1016/j.gie.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76–79. doi: 10.1067/mge.2003.294. [DOI] [PubMed] [Google Scholar]
- 38.Chiu HM, Lin JT, Wang HP, Lee YC, Wu MS. The impact of colon preparation timing on colonoscopic detection of colorectal neoplasms--a prospective endoscopist-blinded randomized trial. Am J Gastroenterol. 2006;101:2719–2725. doi: 10.1111/j.1572-0241.2006.00868.x. [DOI] [PubMed] [Google Scholar]


