Abstract
Background
Gastrointestinal bleeding (GIB) after percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS) and coronary artery disease (CAD) is associated with high morbidity and mortality.
Methods
The Nationwide Inpatient Sample (NIS) database from 1998 to 2006 was utilized to identify 1,216,759 PCIs performed for ACS and CAD. We sought to analyze temporal trends in the incidence and in-hospital outcomes of GIB associated with PCI along with its predictors.
Results
The overall incidence of GIB was 1.04% (95% confidence interval (CI), 1.02%–1.06%). The incidence of GIB decreased over the study period (p for trend <0.0001). The overall mortality in the GIB group was 6.0% (95% CI, 5.6%–6.4%). The adjusted odds ratio (OR) for in-hospital mortality and GIB was 4.70 (95% CI, 4.23–5.23; p<0.0001); this remained high and essentially unchanged over the study period. Independent predictors of GIB included rectum/anal cancer (OR, 4.64; 95% CI, 3.20–6.73; p<0.0001), stomach cancer (OR, 2.74; 95% CI, 1.62–4.66; p=0.0002), esophageal cancer (OR, 1.99; 95% CI, 1.08–3.69; p=0.0288), colon cancer (OR, 1.69; 95% CI, 1.43–2.02; p<0.0001), congestive heart failure (OR, 1.43; 95% CI, 1.35–1.52; p<0.0001), and acute myocardial infarction (OR, 1.23; 95% CI, 1.13–1.35; p<0.0001).
Conclusions
Although the incidence of GIB associated with PCI decreased from 1998–2006 in the face of aggressive therapies for ACS and CAD, the risk of GIB-associated death remained high. Underlying GI malignancy is a significant independent predictor of GIB associated with PCI; identifying these patients may reduce the rate of GIB.
INTRODUCTION
Advances in invasive interventional procedures, anti-platelet and anti-thrombotic therapies have led to significant reductions in cardiovascular morbidity and mortality in patients with symptomatic coronary artery disease (CAD) and acute coronary syndrome (ACS);1–4 however, the risk of overall bleeding, which includes access site and gastrointestinal bleeding (GIB), still remains a significant problem.5–7 The reported incidence of GIB in patients undergoing percutaneous coronary intervention (PCI) ranges between 1.1% and 3.0%8–12 and development of post-PCI GIB is associated with a 10% in-hospital mortality.6, 11, 12 We hypothesized that changes in the utilization of antiplatelet, anticoagulant agents, and coronary stents would impact the overall incidence of GIB and associated mortality. The Healthcare Cost and Utilization Project (HCUP) is a family of health care databases which encompasses the most extensive collection of longitudinal hospital care data in the United States enabling research on a broad range of health care policy issues including medical practice patterns and outcomes of treatments.13 Given the introduction of drug-eluting stents, new antiplatelet and anticoagulation strategies and wider implementation of guideline-recommended care in this period, we sought to examine the temporal trend of in-hospital GIB events among patients with ACS and CAD undergoing PCI in a broad range of patients representing real-world clinical practice in the US from 1998 to 2006.
METHODS
Data Source
The Nationwide Inpatient Sample (NIS) is the largest all-payer U.S. inpatient care database that contains over a hundred clinical and nonclinical data elements from approximately 8 million hospital stays each year.13 Included in these data elements are primary and secondary diagnoses, primary and secondary procedures, admission and discharge status, patient demographics, expected payment source, length of stay, hospital characteristics. All patients are considered for inclusion. The most recent NIS database contains data from about 1050 hospitals from 44 States in the U.S. sampled to approximate a 20% stratified sample of U.S. community hospitals as defined by the American Hospital Association. NIS was developed as part of HCUP, which is sponsored by the Agency for Healthcare Research and Quality. NIS data are available yearly, beginning with 1988, and allow for analyzing trends over time. It is the only national hospital database with charge information on all patients regardless of payer.
Study Patients and Definitions
From 1998 to 2006, a total of 1,216,759 PCI procedures performed in patients for symptomatic CAD and acute myocardial infarction (AMI) diagnoses, which encompass ST elevation myocardial infarction (STEMI) and non-ST elevation myocardial infarction (NSTEMI), were identified. The Clinical Classifications Software (CCS) developed by HCUP was used in analyzing our dependent and independent variables. CCS is a diagnosis and procedure categorization scheme that is based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM); a uniform and standardized coding system. The multitude of ICD-9-CM codes are collapsed into a manageable number of clinically meaningful categories. CCS consists of a single-level and multi-level classification systems. The single-level CCS system classifies all diagnoses and procedures into unique groups, and the multi-level CCS expands the single-level CCS into a hierarchical system and also splits single-level CCS categories to provide more detail. The specific single-level CCS diagnosis category used to define GIB in this study was “153 – Gastrointestinal Hemorrhage.” GIB was the dependent variable. Additional data on covariates were collected and include year, age, race, gender, in-hospital mortality, length of stay, cost of hospitalization, esophageal cancer, stomach cancer, colon cancer, rectum/anal cancer, gastroduodenal ulcer/gastritis/duodenitis, diabetes mellitus (DM), dyslipidemia, heart valve disorder, hypertension, AMI, CAD, congestive heart failure (CHF), atrial fibrillation/flutter, transient ischemic attack (TIA)/cerebrovascular accident (CVA), peripheral vascular disease (PVD), chronic obstructive pulmonary disease (COPD), diverticulosis/diverticulitis, chronic renal insufficiency (CRI), upper endoscopy, lower endoscopy, pulmonary artery catheter monitoring, blood transfusion, bare-metal stents (BMS), drug-eluting stents (DES), and percutaneous transluminal coronary angioplasty (PTCA).
Primary Analysis
Our primary analysis was to assess the incidence of GIB and its trend over the study period.
Secondary Analysis
Our secondary analysis looked at independent predictors of GIB, mortality rate in patients with GIB, and temporal trend in mortality rate in patients with GIB over the study years.
Statistical Analysis
The study population were separated into two groups – those with GIB and those without GIB. The summary statistics with baseline characteristics were generated for the entire population separated into the “GIB” and “No GIB” groups as well as for the subpopulations stratified by the year.
All tests were 2-tailed, and a P value of less than 0.05 was considered significant for all tests. Univariate analysis was initially conducted to summarize the data. The Pearson chi-square tests were used to test for categorical variables and are presented as percentages. The nonparametric Wilcoxon rank sum tests were employed to test for all continuous variables and are presented as mean ± standard deviation.
The logistic regressions were fit to the data to evaluate the trend for incidence of GIB over the years 1998 to 2006. Wald test with a 0.05 level of significance was used to test the null hypothesis of no trend. The logistic regression model was then used to assess independent predictors of GIB after adjusting for the observed baseline demographic and clinical characteristics. The logistic regression model was also used to investigate the trends for incidence of in-hospital mortality with and without GIB as well as to assess the trends for the adjusted and unadjusted odds ratio (OR) for the association between death and GIB over the study years.
Finally, we used propensity-score method to evaluate the effect of GIB on the mortality rate. Propensity scores were estimated using a logistic regression model with GIB as the outcome and all the observed baseline demographic and clinical characteristic variables. We then used the method of regression adjustment by the estimated propensity scores to estimate the effect of GIB on the mortality rate, taking into account all the other observed baseline demographic and clinical characteristic variables. Advantage of this two-step propensity score procedure is that this allows us to fit a complicated propensity score model with interactions and higher order terms for more accurate estimation of GIB probability.14
The missing data were omitted as follows: in the No GIB group (n=1204065) age (n=20, 0.002%), death (n=296, 0.02%), female gender (n=97, 0.008%), length of stay (n=20, 0.002%), mean financial cost (n=17295, 1.4%), race (n=337586, 28%). In the GIB group (n=12694) death (n=13, 0.1%), female gender (n=1, 0.008%), mean financial cost (n=211, 1.7%), race (n=3672, 29%).
All analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc., Cary, NC, USA).
All authors have read and agree to the manuscript as written. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. This study has been approved by the University of Illinois at Chicago Institutional Review Board. This study is supported in part by the Division of Cardiology, University of Illinois at Chicago (Chicago, Illinois) and the University of Illinois at Chicago Center for Clinical and Translational Science, which is funded in part by the National Center for Research Resources, National Institutes of Health (Bethesda, Maryland), grant number UL1RR029879.
RESULTS
GIB after PCI
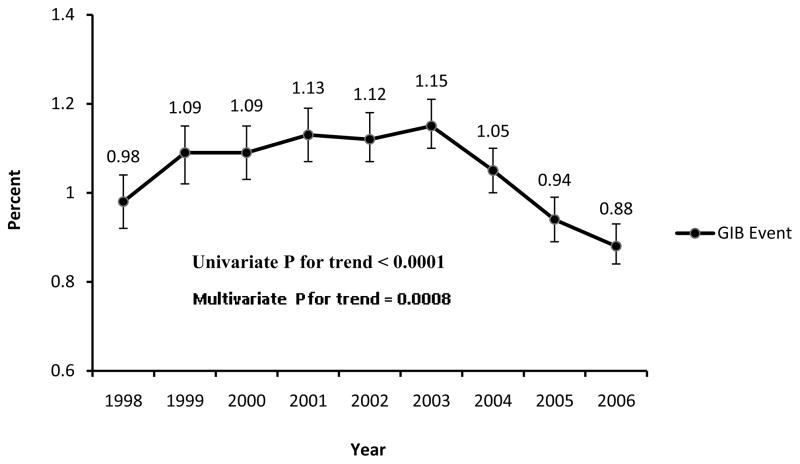
From 1998 to 2006, there were 1,216,759 PCI procedures performed for AMI and CAD diagnoses. Patients’ baseline characteristics and clinical presentation are shown in Table I. GIB during the PCI hospitalization occurred in 12,694 (1.04%, 95% confidence interval (CI), 1.02%–1.06%) patients. The overall incidence in GIB associated with PCI decreased during the study period from 0.98% in 1998 to 0.88% in 2006 (p for trend <0.0001 univariate analysis, p=0.0008 multivariate analysis, Figure 1).
Table I.
Baseline patient characteristics
| No Gastrointestinal Bleeding (n = 1,204,065) | Gastrointestinal Bleeding (n = 12694) | p-value | |
|---|---|---|---|
| Age, mean ± SD | 64.2 ± 12.2 | 70.5 ± 11.4 | < 0.001 |
| Women, % | 34.2 | 44.3 | < 0.001 |
| Race, % | <0.0001 | ||
| White | 82.9 | 81.1 | |
| Black | 6.3 | 8.1 | |
| Hispanic | 6.0 | 6.2 | |
| Asian | 1.6 | 1.9 | |
| Native American | 0.3 | 0.2 | |
| Other | 3.1 | 2.6 | |
| Died during hospitalization, total (%) | 9199 (0.8) | 763 (6.0) | < 0.001 |
| Length of stay, mean ± SD | 2.78 ± 3.1 | 7.61 ± 7.1 | < 0.001 |
| Financial cost, mean ($) | 36758 | 60094 | < 0.001 |
|
| |||
|
Medical History, total number (%)
| |||
| Diabetes mellitus | 334395 (27.8) | 3411 (26.9) | 0.024 |
| Hypertension | 736634 (61.2) | 6837 (53.9) | < 0.001 |
| CRI | 18803 (1.6) | 443 (3.5) | < 0.001 |
| Dyslipidemia | 620215 (51.5) | 3863 (30.4) | < 0.001 |
| CAD | 972236 (80.7) | 9797 (77.2) | < 0.001 |
| AMI | 44367 (3.7) | 944 (7.4) | < 0.001 |
| CHF | 116754 (9.7) | 3469 (27.3) | < 0.001 |
| Heart Valve Disorder | 82980 (6.9) | 1495 (11.8) | < 0.001 |
| Atrial fibrillation/Atrial flutter | 12490 (1.0) | 292 (2.3) | < 0.001 |
| TIA and CVA | 11115 (0.9) | 186 (1.5) | < 0.001 |
| PVD | 76305 (6.3) | 967 (7.6) | < 0.001 |
| COPD | 113365 (9.4) | 2437 (19.2) | < 0.001 |
| Diverticulosis or diverticulitis | 6777 (0.6) | 589 (4.6) | < 0.001 |
| GD ulcer/Gastritis/Duodenitis | 22223 (1.9) | 1706 (13.4) | < 0.001 |
| Esophageal cancer | 475 (0.0) | 24 (0.2) | < 0.001 |
| Stomach cancer | 378 (0.0) | 35 (0.3) | < 0.001 |
| Colon cancer | 8234 (0.7) | 266 (2.1) | < 0.001 |
| Rectum/Anal cancer | 714 (0.1) | 62 (0.5) | < 0.001 |
|
| |||
|
Procedures, total number (%)
| |||
| Upper endoscopy | 8143 (0.7) | 3382 (26.6) | < 0.001 |
| Lower endoscopy | 2192 (0.2) | 1000 (7.9) | < 0.001 |
| Pulmonary artery catheter monitoring | 3233 (0.3) | 226 (1.8) | < 0.001 |
| Blood transfusion | 17137(1.4) | 2123 (16.7) | < 0.001 |
| BMS | 664707 (55.2) | 7787 (61.3) | < 0.001 |
| DES | 419782 (34.9) | 3534 (27.8) | < 0.001 |
CRI indicates chronic renal insufficiency; CAD, coronary artery disease; AMI, acute myocardial infarct; CHF, congestive heart failure; TIA, transient ischemic attack; CVA, cerebrovascular accident; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; GD ulcer, gastroduodenal ulcer; BMS, bare-metal stent; DES, drug eluting stent.
Figure 1.
Incidence of gastrointestinal bleeding from 1998 to 2006.
The error bars indicate 95% confidence intervals.
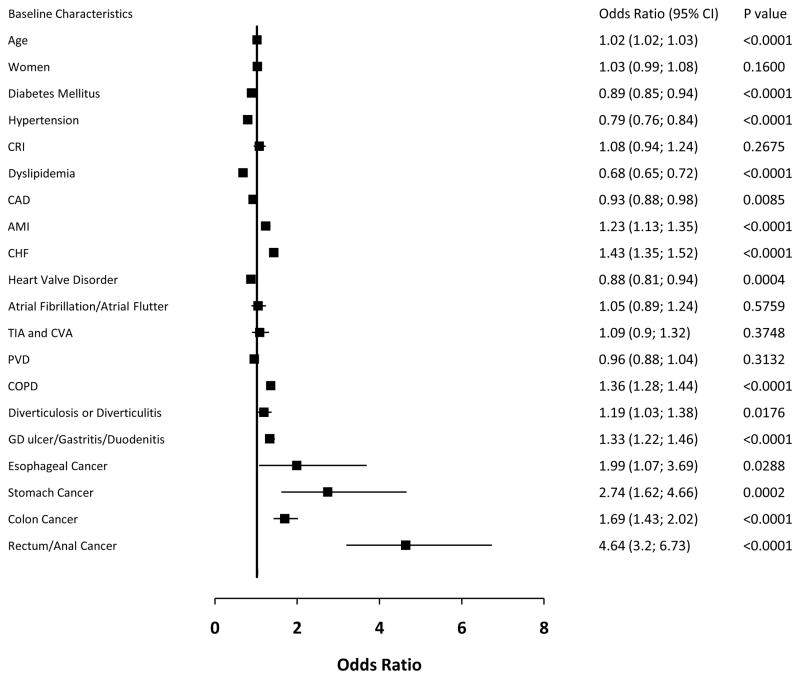
The independent predictors for GIB after adjusting for covariates were identified (Figure 2). Important independent predictors included rectum/anal cancer (adjusted OR, 4.64; 95% CI, 3.20–6.73; p<0.0001), stomach cancer (OR, 2.74; 95% CI, 1.62–4.66; p=0.0002), esophageal cancer (OR, 1.99; 95% CI, 1.07–3.69; p=0.0288), colon cancer (OR, 1.69; 95% CI, 1.43–2.02; p<0.0001), and CHF (OR, 1.43; 95% CI, 1.35–1.52; p<0.0001). Other predictors included COPD, gastroduodenal ulcer/gastritis/duodenitis and AMI. Female gender and atrial fibrillation/atrial flutter were not significant independent predictors for GIB.
Figure 2.
Independent predictors for GIB after adjusting for covariates.
The number of procedures performed (upper endoscopy, lower endoscopy, pulmonary artery catheter monitoring, and blood transfusion [p<0.0001 for all]), length of stay (p<0.0001), mean financial cost (p<0.0001), utilization of bare-metal stents (p<0.0001) and drug-eluting stents (p=0.0004) were significantly associated with GIB after adjusting for covariates.
Death and GIB Associated with PCI
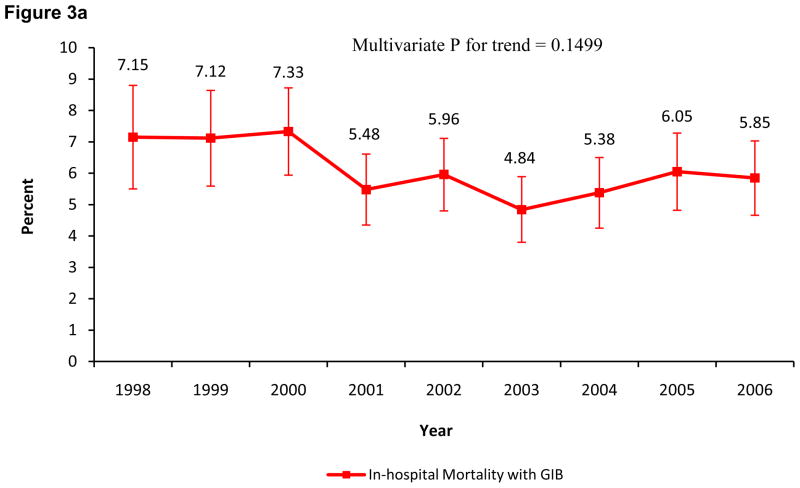
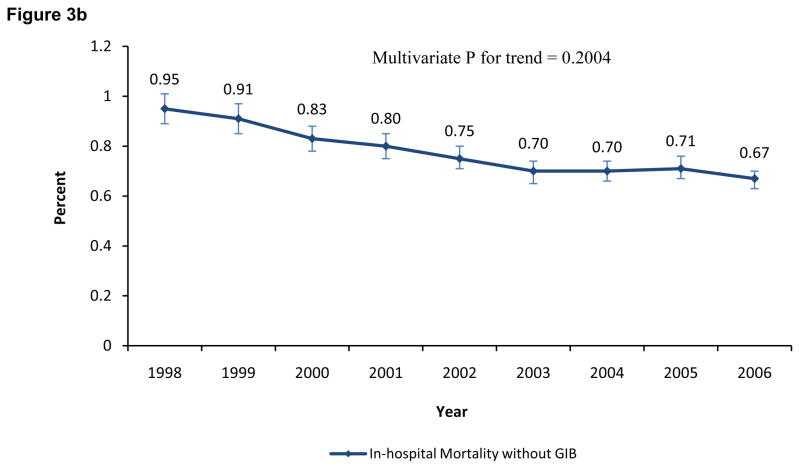
The overall mortality in the GIB group was 6.0% (95% CI, 5.6%–6.4%). Patients who died were 8.31 (95% CI, 7.71–8.97; p<0.0001) times more likely to have GIB than the patients who were alive; this OR decreased to 4.70 (95% CI, 4.23–5.23; p<0.0001) after adjusting for covariates. The in-hospital mortality in patients with GIB (p=0.1499 multivariate analysis) and without GIB (p=0.2004 multivariate analysis) was unchanged from 1998 to 2006 (Figures 3A and 3B respectively). Both the adjusted and unadjusted OR for in-hospital mortality associated with GIB were high for each individual year when compared to in-hospital mortality without GIB (p<0.0001 for all years). The temporal trend of the unadjusted and adjusted OR for in-hospital mortality associated with GIB (p for trend = 0.64 and 0.72, respectively) remained unchanged over the study period (Table II).
Figure 3.
Figure 3A. Incidence of in-hospital mortality with gastrointestinal bleeding from 1998 to 2006.
The error bars indicate 95% confidence intervals.
Figure 3B. Incidence of in-hospital mortality without gastrointestinal bleeding from 1998 to 2006.
The error bars indicate 95% confidence intervals.
Table II.
Association between death and gastrointestinal bleed by year with and without adjusting for covariates.
| Without adjusting for covariates | With adjusting for covariates* | |||
|---|---|---|---|---|
| Variable | Odds Ratio (95% Confidence Interval) | P-value | Odds Ratio (95% Confidence Interval) | P-value |
| Overall | ||||
| Died | 8.31 (7.71, 8.97) | < 0.0001 | 4.70 (4.23, 5.23) | < 0.0001 |
| Individual Year | ||||
| 1998 | 8.04 (6.22, 10.40) | < 0.0001 | 5.43 (3.92, 7.52) | < 0.0001 |
| 1999 | 8.35 (6.57, 10.61) | < 0.0001 | 4.58 (3.26, 6.42) | < 0.0001 |
| 2000 | 9.48 (7.66, 11.74) | < 0.0001 | 5.11 (3.76, 6.94) | < 0.0001 |
| 2001 | 7.19 (5.74, 9.01) | < 0.0001 | 4.22 (3.13, 5.67) | < 0.0001 |
| 2002 | 8.38 (6.76, 10.38) | < 0.0001 | 5.37 (3.99, 7.21) | < 0.0001 |
| 2003 | 7.24 (5.73, 9.15) | < 0.0001 | 3.84 (2.71, 5.43) | < 0.0001 |
| 2004 | 8.07 (6.42, 10.16) | < 0.0001 | 4.14 (2.95, 5.79) | < 0.0001 |
| 2005 | 8.95 (7.14, 11.2) | < 0.0001 | 5.30 (3.93, 7.14) | < 0.0001 |
| 2006 | 9.27 (7.42, 11.59) | < 0.0001 | 4.41 (3.21, 6.07) | < 0.0001 |
The adjusting covariates are year, age, gender, race, length of stay, financial cost, diabetes mellitus, hypertension, chronic renal insufficiency, dyslipidemia, coronary artery disease, acute myocardial infarction, congestive heart failure, heart valve disorder, atrial fibrillation/flutter, transient ischemic attack/cerebrovascular accident, peripheral vascular disease, chronic obstructive pulmonary disease, diverticulosis/diverticulitis, gastroduodenal ulcer/gastritis/duodenitis, esophageal cancer, stomach cancer, colon cancer, rectum/anal cancer, upper endoscopy, lower endoscopy, pulmonary artery catheter monitoring, blood transfusion, bare-metal stents, drug-eluting stents, and percutaneous transluminal coronary angioplasty.
Propensity matching analysis was also performed to reduce the confounding effects of baseline demographics and clinical characteristic variables. After propensity matching the association of GIB with in-hospital mortality remained statistically significant (p<0.001).
DISCUSSION
GI bleeding is a serious and often fatal complication after PCI.5, 15, 16 Although post-PCI bleeding most frequently occurs at the access site, the GI tract is the second most common site of hemorrhage.10, 12, 17, 18 The overall observed incidence of GIB associated with PCI from 1998 to 2006 in this large, contemporary, national registry was 1.04%. This is lower compared with the reported incidence of GIB associated with PCI in previous studies which ranged from 1.1% to 3.0%.8–12 This finding is important since more intensive anti-platelet and anti-thrombotic therapies associated with increased GIB rates were introduced during this period.1, 12, 19, 20
During the observation period, bivalirudin, a direct thrombin inhibitor was approved for use in 2000. In the National Cardiovascular Data Registry (NCDR) Catheterization PCI (CathPCI) registry, the use of UFH, LMWH and glycoprotein IIb/IIIa inhibitors (GPI) significantly decreased and the use of bivalirudin increased among patients with ACS and non-ACS from 2005 to 2009.3 GIB occurred significantly more frequently in patients randomized to heparin + GPI when compared with bivalirudin monotherapy (0.6% vs. 0.1%).6, 12, 21, 22 A sharp decline in the incidence of GIB was noted from 2003 to 2006 in this study, which coincides with the publication of the REPLACE-2 results that brought the reduction in bleeding complications with the use of bivalirudin to the forefront. Similarly, the use of thienopyridines underwent dramatic changes in this period.
Among the NSTE-ACS patients enrolled in 4 large prospective, multicenter US registries from 1999 to 2008, the use of thienopyridines increased from 8.9% to 76.2% with a significant increase in dual anti-platelet therapy use.23 Dual antiplatelet therapy with clopidogrel and aspirin increases GI bleeding complications – GI is the site of major bleeding in 1.3% of patients treated with clopidogrel and aspirin versus 0.7% in those treated with aspirin alone.24 Prasugrel, a more potent thienopyridine, further increases the risk of major, life-threatening and fatal bleeding; and the GI site was one of the most frequent site for life-threatening bleeding.25 It is very encouraging that the wide adoption of intensive dual antiplatelet regimens did not result in an increase in GIB in the NIS patient population.
With an increase in PCI with stents, utilization of anticoagulants, dual oral anti-platelet medications, and GPI in patients with AMI and NSTE-ACS as noted from 1990 to 2008,23, 26 a parallel increase in the incidence of GIB would have been expected. The observation of risk factors for GIB associated with PCI, including age, AMI, and CRI (Table I) are consistent with other trials.10, 12 Focused efforts in identifying these high bleeding risk patients 5, 27 and tailoring therapies 23 to reduce GIB in these patients may have contributed partially to the lower observed GIB rates. Implementation of societal guidelines 26 and quality-improvement techniques targeting bleeding complications, such as renal function and weight-adjusted dosing of anti-platelet/anti-thrombotic therapies, preferential use of medications such as bivalirudin that cause less bleeding,4, 22 prophylactic treatment of high bleeding risk patients with proton pump inhibitors (PPIs),11, 28, 29 may have also contributed to the observed decrease in the rates of GIB in this period.
Previous studies have reported a 10% in-hospital mortality with development of GIB associated with PCI.8 The overall mortality in this NIS GIB group was 6.0%. The high mortality noted in patients developing GIB associated with PCI is likely multifactorial in etiology. The practice of discontinuing effective anti-platelet/anti-thrombotic agents in patients who develop GIB, has been associated with the increased risk of further ischemia, infarction, stent thrombosis and need for repeat PCI and death.5, 10, 30 Aspirin and/or thienopyridines were noted to be frequently discontinued in patients with GIB.12 Among patients triaged to PCI, 5.8% of patients with GIB developed stent thrombosis compared with 2.4% of patients without GIB.12 Other proposed mechanisms for the association between GIB and mortality are anemia and hypotension-related reduction in myocardial oxygen delivery, hemodynamic instability, blood transfusions and thrombocytopenia.8, 9, 12 The risk-adjusted in-hospital mortality did not change significantly among the ACS and non-ACS patients in the NCDR CathPCI registry from 2005 to 2009.3 A very concerning finding in this NIS PCI registry is that the adjusted risk of death after developing GIB associated with PCI remained high and largely unchanged from 1998 to 2006 (Table II); this finding is consistent with the findings of the NCDR CathPCI registry and was noted over a longer study period.
The significant association between GI malignancies and GIB associated with PCI has not been previously observed. Patients with malignancies are generally excluded from clinical trials, and other large nationwide registries have not reported this particular information. Our findings indicate that the presence of an underlying GI malignancy is an additional significant independent predictor of GIB associated with PCI. Identifying patients with GI malignancies may reduce the rate of GIB associated with PCI. Currently, there are no systematic strategies to screen for GI malignancy in the elective PCI population. Such measures, however, may not be effective in the emergent STEMI or NSTEMI setting. While the rate of GIB in the elective PCI population was lower than that in the AMI population, elective PCI may allow substantial improvement in the processes of care and procedural outcomes. It is important to emphasize that the findings of this study does not imply causality but only an association of GI malignancy with GIB.
Study Limitations
Data about management patterns, utilization rates or dosing of the various anti-platelet/anti-thrombotic medications and utilization rates of PPIs that have been associated with decreased GIB were not collected. The database does not allow for evaluation of practice patterns that account for the noted reduction in GIB. This precludes us from confirming an association between the trends in the utilization of specific anti-platelet/anti-thrombotic medications and PPI with the incidence of GIB. Data on other bleeding complications such as access site and retroperitoneal bleeds were not available in our dataset. The details on the type and temporal relationship of GIB to PCI were not available. The identification of GIB was determined by the local sites and not centrally adjudicated. The underreporting of smaller GIB cannot be excluded, and could have biased the estimation of the true incidence of GIB, in addition to the mortality rate associated with GIB. Importantly, the cause-effect relationship between PCI, anti-platelet/anti-thrombotic therapy, GIB and the observed outcome of mortality cannot be determined. Additional unmeasured confounders may have accounted for the observed differences. The race data was missing in about 29% of all cases. NIS does not collect long-term outcomes of GIB associated with PCI. Finally, participation in the NIS registry is voluntary and only selected centers may have participated in this registry, the results may not be generalized to all U.S. hospitals/population at large.
Summary
A temporal trend in GIB associated with PCI has not been studied, and as such this study addresses an important gap in the literature. This very large sample study represents the contemporary, real-world practice in the US and encompasses a broad range of patients who would have otherwise been excluded from randomized clinical trials. This study describes a period during which important changes in PCI management have occurred. From 1998 to 2006 the incidence of GIB associated with PCI decreased in the face of more aggressive therapies for ACS and CAD. The risk of GIB-associated death has remained high and unchanged. Underlying GI malignancy is a significant independent predictor of GIB associated with PCI. The findings of this study may help develop a basis for future investigation to better define causes of GIB associated with PCI and provide pertinent information regarding preventive management strategies to reduce morbidity and mortality.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aronow HD, Steinhubl SR, Brennan DM, Berger PB, Topol EJ. Bleeding risk associated with 1 year of dual antiplatelet therapy after percutaneous coronary intervention: Insights from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J. 2009;157(2):369–74. doi: 10.1016/j.ahj.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Mehta SR, Yusuf S. The Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial programme; rationale, design and baseline characteristics including a meta-analysis of the effects of thienopyridines in vascular disease. Eur Heart J. 2000;21(24):2033–41. doi: 10.1053/euhj.2000.2474. [DOI] [PubMed] [Google Scholar]
- 3.Roe MT, Messenger JC, Weintraub WS, Cannon CP, Fonarow GC, Dai D, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. 56(4):254–63. doi: 10.1016/j.jacc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358(21):2218–30. doi: 10.1056/NEJMoa0708191. [DOI] [PubMed] [Google Scholar]
- 5.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114(8):774–82. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 6.Nikolsky E, Mehran R, Stone GW. Gastrointestinal bleeding in percutaneous coronary intervention and acute coronary syndromes. Am J Cardiol. 2009;104(5 Suppl):22C–9C. doi: 10.1016/j.amjcard.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Rao SV, O’Grady K, Pieper KS, Granger CB, Newby LK, Van de Werf F, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96(9):1200–6. doi: 10.1016/j.amjcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 8.Abbas AE, Brodie B, Dixon S, Marsalese D, Brewington S, O’Neill WW, et al. Incidence and prognostic impact of gastrointestinal bleeding after percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. 2005;96(2):173–6. doi: 10.1016/j.amjcard.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mallah M, Bazari RN, Jankowski M, Hudson MP. Predictors and outcomes associated with gastrointestinal bleeding in patients with acute coronary syndromes. J Thromb Thrombolysis. 2007;23(1):51–5. doi: 10.1007/s11239-006-9005-8. [DOI] [PubMed] [Google Scholar]
- 10.Foley P, Foley S, Kinnaird T, Anderson RA. Clinical review: gastrointestinal bleeding after percutaneous coronary intervention: a deadly combination. Qjm. 2008;101(6):425–33. doi: 10.1093/qjmed/hcm112. [DOI] [PubMed] [Google Scholar]
- 11.Ng FH, Wong SY, Lam KF, Chang CM, Lau YK, Chu WM, et al. Gastrointestinal bleeding in patients receiving a combination of aspirin, clopidogrel, and enoxaparin in acute coronary syndrome. Am J Gastroenterol. 2008;103(4):865–71. doi: 10.1111/j.1572-0241.2007.01715.x. [DOI] [PubMed] [Google Scholar]
- 12.Nikolsky E, Stone GW, Kirtane AJ, Dangas GD, Lansky AJ, McLaurin B, et al. Gastrointestinal bleeding in patients with acute coronary syndromes: incidence, predictors, and clinical implications: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol. 2009;54(14):1293–302. doi: 10.1016/j.jacc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Introduction to the Nationwide Inpatient Sample (NIS) Rockville, MD: Healthcare Cost and Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality; 2008. [Google Scholar]
- 14.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Willis P, Voeltz MD. Anemia, hemorrhage, and transfusion in percutaneous coronary intervention, acute coronary syndromes, and ST-segment elevation myocardial infarction. Am J Cardiol. 2009;104(5 Suppl):34C–8C. doi: 10.1016/j.amjcard.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Rao SV, O’Grady K, Pieper KS, Granger CB, Newby LK, Mahaffey KW, et al. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol. 2006;47(4):809–16. doi: 10.1016/j.jacc.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 17.Mehta SK, Frutkin AD, Lindsey JB, House JA, Spertus JA, Rao SV, et al. Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv. 2009;2(3):222–9. doi: 10.1161/CIRCINTERVENTIONS.108.846741. [DOI] [PubMed] [Google Scholar]
- 18.Moscucci M, Fox KA, Cannon CP, Klein W, Lopez-Sendon J, Montalescot G, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE) Eur Heart J. 2003;24(20):1815–23. doi: 10.1016/s0195-668x(03)00485-8. [DOI] [PubMed] [Google Scholar]
- 19.Feit F, Voeltz MD, Attubato MJ, Lincoff AM, Chew DP, Bittl JA, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 Trial. Am J Cardiol. 2007;100(9):1364–9. doi: 10.1016/j.amjcard.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Stone GW, Bertrand M, Colombo A, Dangas G, Farkouh ME, Feit F, et al. Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial: study design and rationale. Am Heart J. 2004;148(5):764–75. doi: 10.1016/j.ahj.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Lincoff AM, Bittl JA, Harrington RA, Feit F, Kleiman NS, Jackman JD, et al. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. Jama. 2003;289(7):853–63. doi: 10.1001/jama.289.7.853. [DOI] [PubMed] [Google Scholar]
- 22.Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, et al. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355(21):2203–16. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 23.Elbarouni B, Elmanfud O, Yan RT, Fox KA, Kornder JM, Rose B, et al. Temporal trend of in-hospital major bleeding among patients with non ST-elevation acute coronary syndromes. Am Heart J. 160(3):420–7. doi: 10.1016/j.ahj.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 24.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 25.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 26.Peterson ED, Shah BR, Parsons L, Pollack CV, Jr, French WJ, Canto JG, et al. Trends in quality of care for patients with acute myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156(6):1045–55. doi: 10.1016/j.ahj.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 27.Segev A, Strauss BH, Tan M, Constance C, Langer A, Goodman SG. Predictors and 1-year outcome of major bleeding in patients with non-ST-elevation acute coronary syndromes: insights from the Canadian Acute Coronary Syndrome Registries. Am Heart J. 2005;150(4):690–4. doi: 10.1016/j.ahj.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Chan FK, Ching JY, Hung LC, Wong VW, Leung VK, Kung NN, et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med. 2005;352(3):238–44. doi: 10.1056/NEJMoa042087. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda H, Yamada M, Sawada S, Endo Y, Inoue K, Asano F, et al. Upper gastrointestinal bleeding in patients receiving dual antiplatelet therapy after coronary stenting. Intern Med. 2009;48(19):1725–30. doi: 10.2169/internalmedicine.48.2031. [DOI] [PubMed] [Google Scholar]
- 30.Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49(12):1362–8. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]