Abstract
Cichlid fishes are remarkably phenotypically diverse and species-rich. Therefore, they provide an exciting opportunity for the study of the genetics of adaptation and speciation by natural and sexual selection. Here, we review advances in the genomics and transcriptomics of cichlids, particularly regarding ecologically relevant differences in body shape, trophic apparatus, coloration and patterning, and sex determination. Research conducted so far has focused almost exclusively on African cichlids. To analyse genomic diversity and selection in a Neotropical radiation, we conducted a comparative transcriptomic analysis between sympatric, ecologically divergent crater-lake Midas cichlids (Lake Xiloá Amphilophus amarillo and Amphilophus sagittae). We pyrosequenced (Roche 454) expressed sequence tag (EST) libraries and generated more than 178 000 000 ESTs and identified nine ESTs under positive selection between these sister species (Ka/Ks > 1). None of these ESTs were found to be under selection in African cichlids. Of 11 candidate genes for ecomorphological differentiation in African cichlids, none showed signs of selection between A. amarillo and A. sagittae. Although more population-level studies are now needed to thoroughly document patterns of divergence during speciation of cichlids, available information so far suggests that adaptive phenotypic diversification in Neotropical and African cichlids may be evolving through non-parallel genetic bases.
Keywords: next-generation sequencing, cichlid fish, ecological speciation, coloration, body shape, expressed sequence tags
1. The genomics of adaptation and speciation in cichlids
Cichlid fishes are spectacularly species-rich and phenotypically diverse in morphology, behaviour and coloration, and therefore have become a ‘non-model’ model system for studying genomic diversification by natural and sexual selection [1–3]. Many of the more than 2000 species have diversified based on ecological niche within lakes and often in parallel within and across radiating lineages [2]. Frequently, many closely related species coexist that have diverged without geographical isolation and therefore presumably, or demonstrably, under divergent selection and through ecological speciation [4,5].
In part because of their relevance for basic evolutionary biology and speciation research, as well as the economic importance of tilapia, cichlid fishes are the focus of a multi-species genome sequencing effort [6]. As of early 2011, the tilapia genome (Oreochromis niloticus) was completed and assembled, and whole genome sequencing of four other African Rift Lake species is near completion (F. Di Palma 2011, personal communication). These species will be great models for studying genomic divergence during adaptive radiation [7]; some genomic, linkage mapping or transcriptomic resources already exist for many of them (e.g. for the basal haplochromine Astatotilapia burtoni [8–11]). Cichlids have a moderately sized genome (approx. 1.1 Gb), can be bred and crossed in the laboratory for mapping and encompass an amazing morphological and behavioural phenotypic variability. Further, being approximately 113 ± 11 Myr diverged from medaka (Oryzias latipes) [12]—a model fish species with a fully sequenced and well-annotated genome—means that even in advance of whole genome sequencing for a cichlid species, there has been a flurry of genomic and transcriptomic research.
The aims of our present paper are twofold. First, we review recent advances in understanding adaptive radiation in cichlid fishes using genetic, genomic and transcriptomic approaches. To date, this research has primarily been focused on African cichlids. Second, to address the dearth of information on Neotropical cichlid adaptive radiations, we present a new study on the transcriptome divergence of two young, sympatric Nicaraguan crater-lake cichlid species. Further, we address whether there are parallel genetic signals of selection across these African and Neotropical adaptive radiations and found surprisingly little congruence.
(a). Niche, body shape and trophic apparatus
Speciation in cichlid fishes involved extremely high levels of morphological divergence, primarily related to ecologically relevant variation in body shape and size, and the trophic apparatus [13]. Moulded by selective pressures of living in similar habitats or employing similar foraging preferences [14,15], African and Neotropical cichlid fishes have independently evolved parallel ecomorphs within and across their respective radiations [15,16], including elongate limnetic or predatory types, large jawed snail-crushing morphs, small-mouthed algae scrapers and thin-headed, thick-lipped crustacean suckers. Genomic differentiation associated with these rapidly evolving body, mouth and jaw shapes is an exciting—though so far little investigated—aspect of the genetics of speciation in cichlids.
Body shape varies greatly within cichlid adaptive radiations but may be relatively invariant in other, less species-rich lineages. Analysing transcriptome sequence data for African and Neotropical cichlid fishes, we recently identified accelerated evolution and signals of positive selection in the epithelial cell adhesion molecule (EPCAM) gene in the haplochromine ‘superflock’ cichlids in Lake Victoria relative to the species-poor basal tilapia (O. niloticus) lineage [17]. Functional analyses of EPCAM in zebrafish (Danio rerio) have shown its indispensable role in epithelial morphogenesis and skin development [18], making this a candidate gene for future analyses of body shape and trophic differentiation associated with adaptation in haplochromine cichlids.
Ecologically divergent cichlid species can differ dramatically in their jaw and dentition structures; there is a tight correlation between pharyngeal jaw morphology, dentition and foraging preference [4,19–21]. Thus, the pharyngeal jaw may be an evolutionary key innovation intrinsic to the rapid speciation of cichlid fishes [21,22] and the genetic basis of adaptive variation in jaws and dentition has been the focus of several studies. Albertson et al. [23,24] found that few loci were involved in the radiation of cichlid jaw apparatus but that these were under strong directional selection. In particular, bone morphogenetic protein 4 (Bmp4) is a critical locus responsible for mandibular morphological variation (reviewed in Albertson & Kocher [25]). Moreover, some genomic regions contain multiple quantitative trait loci (QTLs). For example, the QTLs for tooth, jaw and skull shape all mapped to the same interval in one linkage group [26]. Divergent selection on this genomic region may then affect multiple traits simultaneously and explain the covariation and parallel/convergent evolution often observed in cichlids [26]. Besides sequence variation in Bmp4 [26,27], craniofacial differences may result from variation in gene expression. Through microarray experiments, significant expression differences were observed in the genes cimp1 and magp4 during head and jaw development in closely related Lake Victoria cichlids [28,29], suggesting the importance of gene expression to phenotypic diversification within the species flock. However, the contributions of these differently expressed genes to morphological differences between species remain to be validated [30].
Dentition is also an excellent niche indicator for cichlid fishes: for example, the outer row of teeth of biting species is normally small but closely spaced and multi-cusped, in contrast to suction feeders' large and loosely spaced teeth [25]. Tooth shape and cusp number are positively correlated to the number of teeth in Malawi cichlids [31] and this trait appears to be mainly controlled by a single gene [32]. Transcriptomic experiments have shown that Malawi cichlids with different dentition have variable spatio-temporal gene expression [33] of conserved, ancient dental gene networks [34]. Knowledge of the genetic basis of the trophic apparatus in cichlids may therefore illuminate the genetics of their rapid adaptation and speciation.
(b). Coloration and patterning
Unlike complex traits such as body shape, across many vertebrate taxa coloration tends to be of a simple genetic basis and therefore a more tractable target for comparative genomics [35,36]. Cichlids show an amazing breadth of coloration and patterning, and this has recently been a fruitful topic of genomic investigation for Neotropical and African species. For example, various species of the Neotropical Midas cichlid complex (Amphilophus citrinellus complex) have a melanic (‘dark’) and amelanic (‘gold’) phenotype (figure 1a), with gold determined by the dominant allele of a single locus [37]. This colour polymorphism is not sex-linked (in contrast to the common genetic pattern for gold African Rift Lake cichlids) and is the basis of assortative mating resulting in intraspecific genetic divergence in sympatry in at least one Nicaraguan crater lake; therefore, it may be a trait that is involved in incipient sympatric speciation [38]. Henning et al. [37] found that, although the expression of the gene Mc1r (a common candidate gene for coloration [35]) was upregulated in the skin of gold fishes, comparative genomic analyses identified no sequence polymorphism in Mc1r between gold and dark Midas cichlids. Further, none of the nearby single nucleotide polymorphisms assorted with colour in the mapping crosses nor colour polymorphic populations from the wild. An analysis of conserved non-coding elements surrounding the Mc1r locus, compared with the genomes of five model fish species, failed to identify relevant polymorphisms. Combined, this suggests that mutations in Mc1r or surrounding regions have no effect on the gold Midas phenotype and the causal genetic locus remains to be found.
Figure 1.
Cichlid fishes show a rich array of coloration and behavioural phenotypes that have recently been investigated using genomic and transcriptomic approaches. (a) A breeding pair of Midas cichlids (Amphilophus xiloaensis) from crater Lake Xiloá, Nicaragua. The female has the ‘gold’ (amelanic) coloration and the male shows the typical ‘dark’ coloration of white and black bars. See §1b for more details. Photo credit: Ad Konings. (b) The haplochromine cichlid Paralabidochromis ‘red fin piebald’ from Lake Victoria, Africa, showing (i) a typical orange blotch (OB) phenotype and (ii) a typical white blotch (WB) phenotype. See §1b,c for more details. Photo credit: P. Eriksson. (c) Neolamprologus pulcher is a cooperatively breeding cichlid from Lake Tanganyika. This image shows two helpers-at-the-nest (left) and a breeder female (right). See §1d for more details. Photo credit: J. Desjardins.
In contrast, coloration is sex-linked in many African cichlids and may be associated with multiple loci [39]. Males of the Lake Malawi cichlid Pseudotropheus saulosi are blue and females are yellow. Gunter et al. [40] recently compared gene expression in the skin of both sexes/colours by cDNA microarray. Forty-five unique genes were differentially expressed in pooled tests and quantitative real-time PCR subsequently confirmed five at the individual level. The strongest candidate gene was Copz-1, which is known to have a conserved role in pigmentation [40] and is an interesting focus of future investigation of the genetic basis of colour polymorphisms.
Although no difference was found in expression levels of the xanthophore-related candidate gene colony-stimulating factor 1 receptor a (csf1ra) between yellow and blue skin of P. saulosi [40], csf1ra is involved in the yellow pigmentation of the egg dummy colour patterning in other African cichlids [41]. Salzburger et al. [41] found that csf1ra is expressed in the egg spots of the haplochromine and Ectodini lineages. The molecular basis of egg dummies in haplochromine cichlids is possibly derived from a de novo substitution in the ligand-binding portion of csf1ra; analyses indicated adaptive sequence evolution in the ancestral lineage, which coincided phylogenetically with the emergence of egg dummies [41].
Across African cichlid radiations, sexual selection on colour pattern is one of the most important forces for speciation [42], suggesting that positive selection may be acting on the gene(s) responsible for coloration. Comparing a zebrafish colour pattern gene (hagoromo) between riverine and lacustrine cichlids, Terai et al. [43] identified signals of positive selection in the lacustrine species famous for their splendid body colours (table 1) and also found increased species-level variation in hagoromo alternative splicing [49]. Accelerated evolution and a cichlid-specific isoform of the pigmentation candidate gene mitf were also suggested as relevant for the rapid evolution of different colorations [50].
Table 1.
Candidate genes of African cichlid species related to vision and light perception, bone and tooth development, and morphology. Gene functions, major findings and samples used are drawn from the cited literature. These candidate genes were analysed for signals of selection between Neotropical species Amphilophus amarillo and Amphilophus sagittae. ‘Shared coding region’ indicates the per cent of EST coding region overlapping between A. amarillo and A. sagittae compared with the relevant available African cichlid dataset.
| gene name and function | studied cichlid species and major findings | shared coding region (%) | selection signal in A. amarillo and A. sagittae |
|---|---|---|---|
| EPCAM morphology | two Midas cichlids, Oreochromis niloticus and three Lake Victoria cichlids. Positive selection on EPCAM was identified in the Lake Victoria lineage [17]. | 100 | none, sequences identical |
| MHC class II immune and olfactory | two Malawi species, Pseudotropheus fainzilberi and P. emmiltos. Higher genetic divergence at putative antigen binding sites (ABS) in exon 2 of MHC class II gene [44]. | 100 | Ka/Ks = 0.3249 |
| BMP4 bone development | sixteen lacustrine and riverine habitat cichlids. Accelerated rate of evolution of BMP4 in lacustrine habitat cichlids [27]. | 56 | Ka = 0, Ks = 0.0107 |
| SPP120 fertilization | Orechromis niloticus and six haplochromines. A subregion is under positive selection in African cichlids [45]. | 55 | none, sequences identical |
| CSF1Ra coloration and egg spot | nineteen cichlid species from Eastern Africa. Extracellular ligand-binding and receptor-interacting domain were under positive selection in the ancestral haplochromine lineage, which coincided with the egg-dummies [41]. | 38 | none, sequences identical |
| RH1 light perception | Oreochromis niloticus and 16 species from Lakes Tanganyika and Malawi. Different selection pressures were detected between species in the clear and turbid environments [46]. | 100 | none, sequences identical |
| RH2 light perception | Oreochromis niloticus and 16 species from Lakes Tanganyika and Malawi. Different selection pressures were detected between species in the clear and turbid environments [46]. | 100 | none, sequences identical |
| LWS light perception | twenty-two cichlid species from Lakes Malawi, Nabugabo and Victoria. Adaptive and functional genetic variations on this gene were identified in the Lake Victoria cichlids [47]. | 97 | none, sequences identical |
| SWS1 light perception | sixty-three cichlid species from Lakes Malawi and Victoria. SWS1 harbours the greatest diversity of functionally critical sites compared to other opsin genes in Malawi cichlids [48]. | 94 | none, sequences identical |
| SWS2a light perception | Oreochromis niloticus and 16 species from Lakes Tanganyika and Malawi. Different selection pressures were detected between species in the clear and turbid environments [46]. | 48 | Ka/Ks = 0.2740 |
Orange-blotched (OB) and white-blotched (WB) are incompletely sex-linked colour pattern phenotypes found in cichlid radiations in different African lakes and their basins. OB and WB patterning (figure 1b), while melanin-disrupting and female-linked in all species tested for the radiations in lakes Malawi and Victoria, are determined by different genes [51]. Blotched phenotype in general is correlated with increased aggressive behaviour [52] and associated with sexual selection by male preference in Lake Victoria OB and WB species [43] and Lake Malawi OB species [49], such that only in polymorphic populations do males show a preference for blotched females. Therefore, this colour pattern may be a mechanism of rapid sympatric speciation by sexual selection [53–55] and represents a simple genetic basis of behavioural phenotypes.
Laboratory mapping crosses followed by association mapping of populations in nature pinpointed the causative locus of OB in Lake Malawi cichlids [56]. A single origin of the mutation in the Lake Malawi OB species was proposed, but there appears to have been an independent origin in Lake Victoria OB species. A single gene was found to be associated with the OB phenotype: Pax7 expression is increased in tailfins of OB individuals in all three Lake Malawi species examined though no sequence differences were found in the Pax7 coding regions [56].
Closely linked to coloration, the evolution of vision-related genes has been a focus of investigation in African cichlid fishes [5,46–48,57]. These studies of sequence and expression variation in opsin genes use a candidate gene approach (see [45] for an exception) rather than being broadly ‘genomic’, and therefore will not be discussed here (but see [3,58] for recent reviews). Our present analyses (table 1) that compare expressed opsin sequence patterns in African and Neoptropical sister species identify few molecular parallelisms across lineages, but this remains to be investigated further.
(c). Sex determination
Because coloration and colour patterns are often sex-linked in cichlids, the link between sex ratios, genomic incompatibility and colour assortative mating means that speciation may be promoted by sex-determining genomic regions [55,56,59]. For example, sexually antagonistic selection can promote the evolution of a novel sex determiner if genetic conflict (locus-specific alleles that increase fitness of one sex but decrease fitness in the other) is thereby resolved [55,60]. It is proposed that, across lineages in African lakes, OB coloration may increase fitness by improving body camouflage [56] (although OB individuals are arguably more conspicuous, further testing is necessary [61]; A. Meyer, personal observation) but OB males may suffer reduced mating success because the species' typical nuptial coloration is lost [56]. It has been proposed that the genetic conflict inherent in the OB phenotype was resolved by the evolution of a dominant female determiner that is tightly linked with Pax7, making OB almost exclusively female and therefore no detriment to males [56].
Recent research on the genetic basis of sex identified that at least two sex chromosomes evolved during the radiation of Malawi cichlids [59]. Moreover, these two sex chromosomes are not overlapping with the sex chromosomes of the Nile tilapia (O. niloticus), which are located at different chromosomal regions [59]. Given that sex determination is so variable in such a species-rich group as cichlid fishes suggests that they may provide an excellent model for studying the initial stages of sex chromosome evolution and its role in speciation [62].
(d). Social behaviour and breeding systems
There is a great variability and diversity of social and breeding behaviours in cichlids, and this may contribute to rapid speciation. The genetic basis of this behavioural variability and plasticity has recently been a focus of research using genomic and transcriptomic methods. There may be considerable sex-specific and species-specific gene regulation associated with breeding systems such as monogamy or polygyny [63]. In the polygynous mating system of A. burtoni, social dominance and therefore reproductive potential is associated with differences in gonad size, growth, hormone levels and coloration. Social dominance is highly plastic and an individual male may switch between dominant and subordinate phenotypes and back (reviewed in [10]). By using a microarray approach, it was shown that dominant and subordinate males differ significantly in their expression levels of almost 5 per cent of the tested genes, including co-regulated gene sets of neuroendocrine pathways [56]. Female A. burtoni also differ in gene-expression levels depending on social context: those who witness their preferred male win a fight against another male had dramatically different expression of the ‘immediate early genes’ c-fos and egr-1 in key social and reproductively relevant areas of the brain [64]. In a cooperatively breeding cichlid with helpers-at-the-nest, Neolamprologus pulcher (figure 1c), it was found that the expression of arginine vasotocin was higher in breeding fishes independent of their sex [65]. Three different genes were upregulated exclusively in helpers, and gene expression of breeding females was more similar to that of males than to helper females, suggesting that hierarchy rather than sex was the key modulating factor [65].
(e). Summary and suggested directions for future research
Research on speciation in cichlids has to date primarily focused on the species-rich flocks of the African Rift lakes. While abundant and rapid speciation makes African cichlids excellent models for evolutionary biology, research on the origins of species in these lakes is complicated by three factors. First, these lakes are old, and conditions such as water levels have fluctuated dramatically over time and impacted diversification rates and population connectivity [66–68], with the effect of clouding the geography of speciation. Second, given considerable time since common ancestry for many of these species-rich groups [66,67,69], it is difficult to know the environmental and ecological conditions that originally promoted speciation. Third, it has proved difficult or impossible to reconstruct the phylogenetic relationships for some of these young and serially hybridizing adaptive radiations [69–71], which impedes hypothesis testing.
A better context for testing the ecological conditions and genomic patterns of speciation are isolated and homogeneous environments with recently diverged sister species [62]. For this reason, the Neotropical adaptive radiation of Midas cichlid fishes (A. citrinellus species complex) is an ideal geographical, ecological and biological system in which to study the genomics of adaptation and speciation (reviewed in [15]). The crater lakes were seeded by Midas cichlids from the great lakes, Nicaragua and Managua, and then diversified rapidly in ecology and body shape [4,38,72–74].
2. Transcriptome diversification in cichlids
Several interesting and important questions remain to be addressed in genomic studies of cichlid radiations. For example, do genes that evolve under positive selection in adaptive radiations of African cichlids also contribute to the speciation of Neotropical cichlids, or vice versa? Genomic research has focused heavily on the speciation of African cichlids (table 1) and we know of only one study on Neotropical cichlids [75].
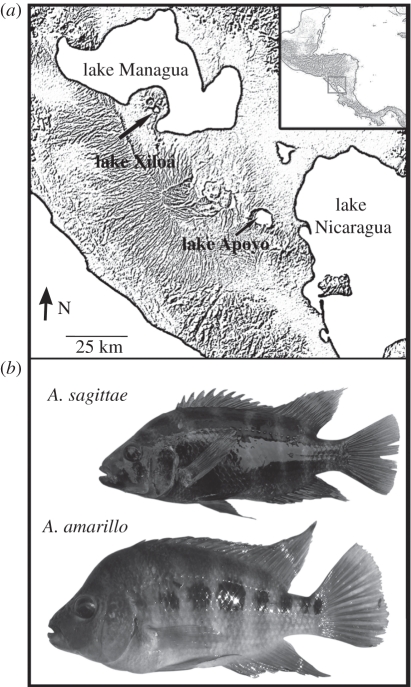
To address such questions of intra- and inter-adaptive radiation genome evolution, we analysed newly generated ESTs for two sympatric, endemic, ecologically divergent, very young species of Midas cichlid from the Nicaraguan crater lake Xiloá (ca 6 kyr old; figure 2). This species pair, Amphilophus amarillo and Amphilophus sagittae, diverged along a benthic–limnetic phenotypic axis [15,76], similar to the Midas species pair from the older crater lake Apoyo [4,75]. With the overall goal of assessing patterns of genetic parallelism in transcriptome evolution across cichlid lineages, we compared signals of divergent selection in the Neotropical adaptive radiation from Lake Xiloá with candidate gene sequences under divergent selection from the African intralacustrine adaptive radiations.
Figure 2.
(a) Lake Xiloá is an isolated crater lake in western Nicaragua. Lake Apoyo is a larger and older crater lake to the south. (b) Amphilophus amarillo is a benthic and Amphilophus sagittae a limnetic Midas cichlid species.
(a). Methods
We generated EST libraries following previous published methods [75]. Briefly, wild-caught A. amarillo and A. sagittae were bred in the laboratory (University of Konstanz) and sibs from a single brood of each species were sampled for RNA at 1 day (n = 6), one week (n = 10) and one month (n = 2) post-hatch. After pooling RNA equimolar for each stage per species, cDNA was generated by random-priming, and normalized EST libraries were commercially prepared by Vertis Biotechnologie (Freising, Germany). Libraries were normalized in order to maximize the total length and number of ESTs sequenced, though this comes at the cost of gene-expression inference. Sequencing was carried out at the Genomics Centre of the University of Konstanz using Roche 454 FLX Titanium technology.
We adopted a previously implemented analysis pipeline [75] in which the ambiguous, low-quality sites and contigs less than 200 bp long were excluded. Putatively orthologous genes between species were determined by the bi-directional blast hit method. Coding regions of the putative orthologous genes were annotated by comparison with currently available vertebrate proteins. High-quality ESTs were defined as those in which sequences from both species contained coding regions with E-value ⩽1E − 5. These candidate genes were functionally annotated according to the latest version of the Uniprot database [77] and gene ontology was annotated by Blast2GO [78]. Amphilophus EST sequences were compared with all publicly available African cichlid ESTs. The ratio of non-synonymous to synonymous substitutions (Ka/Ks) was estimated with maximum likelihood in PAML v. 4 [79]; pairs with Ka/Ks > 1 were confirmed by outgroup comparison with African cichlids.
(b). Results and discussion
(i). Genome-wide estimates of selection in Neotropical cichlids
Sequencing generated a total of 780 104 and 1 000 805 raw reads for A. amarillo and A. sagittae, respectively, which assembled into a working dataset of 75 687 and 102 360 A. amarillo and A. sagittae EST contigs (average n = 50 size of 540 bp). We identified 39 466 putatively orthologous gene fragments between the two species. Given our stringent annotation criterion, this was reduced to 1612 pairs of high-quality ESTs.
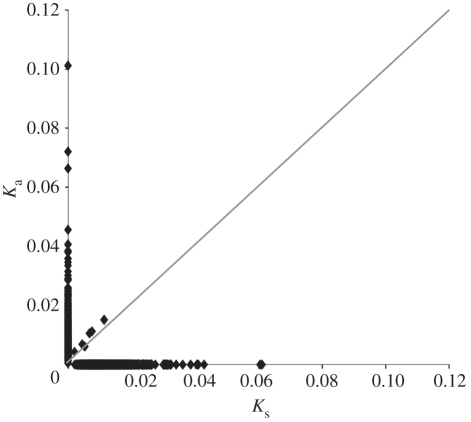
Nine of these EST pairs showed a strong signal of positive selection (Ka/Ks > 1; 75–100% of the full-length gene; figure 3). Functional annotation indicated that most of these genes were related to cellular, metabolic and biological regulation processes (see electronic supplementary material, table S1). Several of these genes are reasonable candidate genes that might contribute to the biological differences between cichlid species. For example, the protein product of CLEC3B, tetranectin, is involved in the skeletal system development process and its deletion causes deformity in mice [80]. Also, the growth arrest and DNA-damage-inducible, gamma (GADD45G) gene is an important growth regulator and environmental response gene in humans [81,82]. These and others will be interesting candidates for future research on genomic patterns of diversification in the Midas cichlid complex.
Figure 3.
The distribution of Ka/Ks for high-quality ESTs between Lake Xiloá A. amarillo and A. sagittae. Expressed sequence tags with Ka/Ks <1 fall above the grey line.
The low number of genes under positive selection between A. amarillo and A. sagittae (nine out of 1612 ESTs, or 0.6%) agrees with the previous findings from Lake Apoyo Midas cichlids, wherein 0.8 per cent (14 of 1721) of shared ESTs were found to be under selection [75]. This also agrees with theoretical predictions and empirical data that very few genes will diverge under positive selection in the early stage of speciation, whereas the remaining regions of the genome are indistinguishable ([30,83,84] and reviewed in Nosil et al. [85]). Identifying positive selection between very recently diverged species (less than 6 kya) can be limited by various factors and also our family-based samples. First, the positive selection signals between two young species, A. amarillo and A. sagittae, may be elevated by segregating polymorphisms in the ancestral species [86]. Adding samples from the ancestral species, A. citrinellus, will clarify the origin of the variation between the two species, such as lineage-specific mutations or standing variations [87]. Second, most genes we identify as being under positive selection have accumulated relatively few single nucleotide polymorphisms (SNPs) owing to the short divergence time between species [15]. As a result, it is difficult to distinguish whether selection on these genes has significance for evolution. Thus, functional tests of these genes or more rigorous statistical methods based on population samples [88] are needed in future studies.
(ii). Parallel molecular evolution between African and Neotropical cichlids
These Midas cichlid sister species differ in ecomorphological traits such as body shape [15], coloration, breeding depth and habitat [38,76], and diet and lower pharyngeal jaw shape [76]. Therefore, we tested for interspecific signals of selection at 11 previously published candidate genes associated with these aspects of diversification in the adaptive radiation of African cichlid fishes (table 1). We found that the EST coding region sequences of all these candidate genes either had no nucleotide substitutions between A. amarillo and A. sagittae or showed no signal of positive selection (Ka/Ks ≪ 1; table 1).
The candidate genes from African cichlids that we tested in the Midas cichlids can be grouped into two basic categories: genes related primarily to communication and sexual selection (e.g. colour and light perception) and genes related to morphology (e.g. body shape and trophic apparatus). The observation that none of these candidate genes shown previously to be involved in the adaptive radiation of African cichlid fishes exhibit a signal of positive selection in the orthologous genes between Midas cichlid species (table 1) suggests a non-parallel genetic basis across the New and Old World lineages of cichlids, at least specifically the flocks under comparison. We suggest four possible reasons for this finding.
First, it may be that the phenotypic targets of selection during Midas cichlid diversification are entirely different from those of African cichlids (for which ESTs have been sequenced to date), making the different genetic basis or non-parallel molecular signals of selection unsurprising. In this case, future contrasts of additional Neotropical and African species could potentially identify shared genetic signals of selection in ESTs if the contrasted species had experienced similar phenotypic selection. For example, sexual dimorphism in coloration is a hallmark of most African cichlid fishes and known to be a target of selection [5], whereas Midas cichlid fishes are not sexually dimorphic in colour. However, for phenotypic variation more under natural (rather than sexual) selection, such as in trophic apparatus [34], immunity (e.g. MHC complexes [44]) and vision [48], one might expect that the phenotypic targets of selection and their genetic bases may be the same between African and Neotropical lineages. This requires further investigation.
Alternatively, it may even be that equivalent ecomorphological differentiation and adaptation occurs by different genetic routes in African cichlids compared with Neotropical cichlids. In this case, even comparing parallel adaptive phenotypes across lineages would still identify non-overlap of genes under selection because the underlying genetic processes and architecture of parallel phenotypes might be entirely different.
Third, there might be genetic parallelism underlying phenotypic parallelism in Neotropical versus African cichlids that cannot be identified by examining the coding region of ESTs (e.g. from cis-regulatory elements). Other transcriptomic approaches that can infer abundance (e.g. RNA-Seq) and location (e.g. in situ staining) of variable gene expression would be required to identify this type of genomic parallelism.
Finally, there are limitations in the strength of our approach to infer positive selection between A. amarillo and A. sagittae. Family-based sampling with normalized libraries cannot quantify intraspecific polymorphism or lineage-specific fixation and could either over- or underestimate shared mutations. Thus, even if coding region mutations are informative about selection between species, our approach may lack power to detect it. This and previous studies [75] therefore act as a launch pad for future gene-specific and population-level approaches to transcriptome evolution in the Midas cichlid species complex (in preparation).
There are only a handful of examples in which the genetic bases of adaptations are known; therefore, it is too early to draw conclusions about the generalities of particular mechanisms. It is clear, however, that adaptive changes may not always involve the same genes, even between closely related species (reviewed in earlier studies [3,89]). Only further genomic and transcriptomic comparisons between Neotropical and African cichlid adaptive radiations, ideally aided by mapping and whole genome comparisons, can discern these differences. All comparisons gain tremendously in power if made in an explicit phylogenetic framework.
3. Conclusions and future directions
Cichlid fishes have long been an ecological and evolutionary model system for studying the formation of adaptive radiation and rapid speciation. In the age of genomics, cichlids are proving themselves even more to be an informative and accessible research system. Recent research on traits under ecological and/or sexual selection—such as ecological niche, jaws and teeth, coloration, reproductive behaviour, and sex determination—has been successful at exposing and quantifying underlying evolutionarily relevant genomic and transcriptomic variation. Candidate gene approaches, or traditional population genetic work, could not have identified the selected loci owing to their low frequency, though these loci appear to be important elements of genomic or transcriptomic differentiation. Recent insights highlight the advances that ‘next-generation’ technologies promise to yield. Soon, complete genomic sequences will permit better annotation, synteny analyses and information on the relevance of structural variation in the explosive rates of diversification of cichlid fishes. The advent of the genomic era in cichlid fish biology will therefore likely yield profound insights into fundamental questions in evolutionary biology.
Acknowledgements
This work was financially supported by a fellowship from University of Konstanz to S.F., an NSERC postdoctoral fellowship and a Young Scholars award (University of Konstanz) to K.R.E., and grants of the Deutsche Forschungsgemeinschaft to A.M. Fishes were collected with the authorization of MARENA, Nicaragua. We thank S. Selent and E. Hespeler for assistance with library preparation and sequencing and J. Sieling for assistance in the aquarium. Thanks to M. Pierotti and three anonymous reviewers for comments improving the manuscript, and P. Eriksson, S. Balshine and A. Konings for contributing images.
References
- 1.Kuraku S., Meyer A. 2008. Genomic analysis of cichlid fish 'natural mutants'. Curr. Opin. Genet. Dev. 18, 551–558 10.1016/j.gde.2008.11.002 (doi:10.1016/j.gde.2008.11.002) [DOI] [PubMed] [Google Scholar]
- 2.Salzburger W. 2009. The interaction of sexually and naturally selected traits in the adaptive radiations of cichlid fishes. Mol. Ecol. 18, 169–185 10.1111/j.1365-294X.2008.03981.x (doi:10.1111/j.1365-294X.2008.03981.x) [DOI] [PubMed] [Google Scholar]
- 3.Elmer K. R., Meyer A. 2011. Adaptation in the age of ecological genomics: insights from parallelism and convergence. Trends Ecol. Evol. 26, 298–306 10.1016/j.tree.2011.02.008 (doi:10.1016/j.tree.2011.02.008) [DOI] [PubMed] [Google Scholar]
- 4.Barluenga M., Stolting K. N., Salzburger W., Muschick M., Meyer A. 2006. Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 439, 719–723 10.1038/Nature04325 (doi:10.1038/Nature04325) [DOI] [PubMed] [Google Scholar]
- 5.Seehausen O., et al. 2008. Speciation through sensory drive in cichlid fish. Nature 455, 620–626 10.1038/Nature07285 (doi:10.1038/Nature07285) [DOI] [PubMed] [Google Scholar]
- 6.International Cichlid Genome Consortium. 2006. Genetic basis of vertebrate diversity: the cichlid fish model. See http://www.genome.gov/Pages/Research/Sequencing/SeqProposals/CichlidGenomeSea.pdf .
- 7.Nosil P., Feder J. L. 2012. Genomic divergence during speciation: causes and consequences. Phil. Trans. R. Soc. B 367, 332–342 10.1098/rstb.2011.0263 (doi:10.1098/rstb.2011.0263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang M., Miyake T., Braasch I., Tinnemore D., Siegel N., Salzburger W., Amemiya C. T., Meyer A. 2006. A BAC library of the East African haplochromine cichlid fish Astatotilapia burtoni. J. Exp. Zool. B Mol. Dev. Evol. 306, 35–44 10.1002/jez.b.21068 (doi:10.1002/jez.b.21068) [DOI] [PubMed] [Google Scholar]
- 9.Sanetra M., Henning F., Fukamachi S., Meyer A. 2009. A microsatellite-based genetic linkage map of the cichlid fish, Astatotilapia burtoni (Teleostei): a comparison of genomic architectures among rapidly speciating cichlids. Genetics 182, 387–397 10.1534/genetics.108.089367 (doi:10.1534/genetics.108.089367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renn S. C. P., Aubin-Horth N., Hofmann H. A. 2008. Fish and chips: functional genomics of social plasticity in an African cichlid fish. J. Exp. Biol. 211, 3041–3056 10.1242/Jeb.018242 (doi:10.1242/Jeb.018242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salzburger W., Renn S. C., Steinke D., Braasch I., Hofmann H. A., Meyer A. 2008. Annotation of expressed sequence tags for the East African cichlid fish Astatotilapia burtoni and evolutionary analyses of cichlid ORFs. BMC Genomics 9, 96. 10.1186/1471-2164-9-96 (doi:10.1186/1471-2164-9-96) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinke D., Salzburger W., Meyer A. 2006. Novel relationships among ten fish model species revealed based on a phylogenomic analysis using ESTs. J. Mol. Evol. 62, 772–784 10.1007/s00239-005-0170-8 (doi:10.1007/s00239-005-0170-8) [DOI] [PubMed] [Google Scholar]
- 13.Barlow G. W. 2000. The cichlid fishes: nature's grand experiment in evolution. Cambridge, UK: Perseus Press [Google Scholar]
- 14.Clabaut C., Bunje P. M. E., Salzburger W., Meyer A. 2007. Geometric morphometric analyses provide evidence for the adaptive character of the Tanganyikan cichlid fish radiations. Evolution 61, 560–578 10.1111/j.1558-5646.2007.00045.x (doi:10.1111/j.1558-5646.2007.00045.x) [DOI] [PubMed] [Google Scholar]
- 15.Elmer K. R., Kusche H., Lehtonen T. K., Meyer A. 2010. Local variation and parallel evolution: morphological and genetic diversity across a species complex of neotropical crater lake cichlid fishes. Phil. Trans. R. Soc. B 365, 1763–1782 10.1098/rstb.2009.0271 (doi:10.1098/rstb.2009.0271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kocher T. D., Conroy J. A., McKaye K. R., Stauffer J. R. 1993. Similar morphologies of cichlid fish in Lakes Tanganyika and Malawi are due to convergence. Mol. Phylogenet. Evol. 2, 158–165 10.1006/mpev.1993.1016 (doi:10.1006/mpev.1993.1016) [DOI] [PubMed] [Google Scholar]
- 17.Fan S. H., Elmer K. R., Meyer A. 2011. Positive Darwinian selection drives the evolution of the morphology-related gene, EPCAM, in particularly species-rich lineages of African cichlid fishes. J. Mol. Evol. 73, 1–9 10.1007/s00239-011-9452-5 (doi:10.1007/s00239-011-9452-5) [DOI] [PubMed] [Google Scholar]
- 18.Slanchev K., Carney T. J., Stemmler M. P., Koschorz B., Amsterdam A., Schwarz H., Hammerschmidt M. 2009. The epithelial cell adhesion molecule EPCAM is required for epithelial morphogenesis and integrity during zebrafish epiboly and skin development. PLoS Genet. 5, e1000563. 10.1371/journal.pgen.1000563 (doi:10.1371/journal.pgen.1000563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouton N., Seehausen O., van Alphen J. J. M. 1997. Resource partitioning among rock-dwelling haplochromines (Pisces: Cichlidae) from Lake Victoria. Ecol. Freshw. Fish. 6, 225–240 10.1111/j.1600-0633.1997.tb00165.x (doi:10.1111/j.1600-0633.1997.tb00165.x) [DOI] [Google Scholar]
- 20.Elmer K. R., Lehtonen T. K., Kautt A. F., Harrod C., Meyer A. 2010. Rapid sympatric ecological differentiation of crater lake cichlid fishes within historic times. BMC Biol. 8, 60. 10.1186/1741-7007-8-60 (doi:10.1186/1741-7007-8-60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liem K. F. 1973. Evolutionary strategies and morphological innovations: cichlid pharyngeal jaws. Syst. Zool. 22, 425–441 10.2307/2412950 (doi:10.2307/2412950) [DOI] [Google Scholar]
- 22.Hulsey C. D. 2006. Function of a key morphological innovation: fusion of the cichlid pharyngeal jaw. Proc. R. Soc. B 273, 669–675 10.1098/rspb.2005.3375 (doi:10.1098/rspb.2005.3375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albertson R. C., Streelman J. T., Kocher T. D., Yelick P. C. 2005. Integration and evolution of the cichlid mandible: the molecular basis of alternate feeding strategies. Proc. Natl Acad. Sci. USA 102, 16 287–16 292 10.1073/pnas.0506649102 (doi:10.1073/pnas.0506649102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albertson R. C., Streelman J. T., Kocher T. D. 2003. Genetic basis of adaptive shape differences in the cichlid head. J. Hered. 94, 291–301 10.1093/jhered/esg071 (doi:10.1093/jhered/esg071) [DOI] [PubMed] [Google Scholar]
- 25.Albertson R. C., Kocher T. D. 2006. Genetic and developmental basis of cichlid trophic diversity. Heredity 97, 211–221 10.1038/sj.hdy.6800864 (doi:10.1038/sj.hdy.6800864) [DOI] [PubMed] [Google Scholar]
- 26.Albertson R. C., Streelman J. T., Kocher T. D. 2003. Directional selection has shaped the oral jaws of Lake Malawi cichlid fishes. Proc. Natl Acad. Sci. USA 100, 5252–5257 10.1073/pnas.0930235100 (doi:10.1073/pnas.0930235100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terai Y., Morikawa N., Okada N. 2002. The evolution of the pro-domain of bone morphogenetic protein 4 (Bmp4) in an explosively speciated lineage of East African cichlid fishes. Mol. Biol. Evol. 19, 1628–1632 [DOI] [PubMed] [Google Scholar]
- 28.Kijimoto T., Watanabe M., Fujimura K., Nakazawa M., Murakami Y., Kuratani S., Kohara Y., Gojobori T., Okada N. 2005. cimp1, a novel astacin family metalloproteinase gene from East African cichlids, is differentially expressed between species during growth. Mol. Biol. Evol. 22, 1649–1660 10.1093/molbev/msi159 (doi:10.1093/molbev/msi159) [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi N., Watanabe M., Kijimoto T., Fujimura K., Nakazawa M., Ikeo K., Kohara Y., Gojobori T., Okada N. 2006. magp4 gene may contribute to the diversification of cichlid morphs and their speciation. Gene 373, 126–133 10.1016/j.gene.2006.01.016 (doi:10.1016/j.gene.2006.01.016) [DOI] [PubMed] [Google Scholar]
- 30.Renaut S., Maillet N., Normandeau E., Sauvage C., Derome N., Rogers S. M., Bernatchez L. 2012. Genome-wide patterns of divergence during speciation: the lake whitefish case study. Phil. Trans. R. Soc. B 367, 354–363 10.1098/rstb.2011.0197 (doi:10.1098/rstb.2011.0197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Streelman J. T., Webb J. F., Albertson R. C., Kocher T. D. 2003. The cusp of evolution and development: a model of cichlid tooth shape diversity. Evol. Dev. 5, 600–608 10.1046/j.1525-142X.2003.03065.x (doi:10.1046/j.1525-142X.2003.03065.x) [DOI] [PubMed] [Google Scholar]
- 32.Streelman J. T., Albertson R. C. 2006. Evolution of novelty in the cichlid dentition. J. Exp. Zool. B Mol. Dev. Evol. 306, 216–226 10.1002/jez.b.21101 (doi:10.1002/jez.b.21101) [DOI] [PubMed] [Google Scholar]
- 33.Fraser G. J., Bloomquist R. F., Streelman J. T. 2008. A periodic pattern generator for dental diversity. BMC Biol. 6, 32. 10.1186/1741-7007-6-32 (doi:10.1186/1741-7007-6-32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraser G. J., Hulsey C. D., Bloomquist R. F., Uyesugi K., Manley N. R., Streelman J. T. 2009. An ancient gene network is co-opted for teeth on old and new jaws. PLoS Biol. 7, 233–247 10.1371/journal.pbio.1000031 (doi:10.1371/journal.pbio.1000031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hubbard J. K., Uy J. A. C., Hauber M. E., Hoekstra H. E., Safran R. J. 2010. Vertebrate pigmentation: from underlying genes to adaptive function. Trends Genet. 26, 231–239 10.1016/j.tig.2010.02.002 (doi:10.1016/j.tig.2010.02.002) [DOI] [PubMed] [Google Scholar]
- 36.McKinnon J. S., Pierotti M. E. R. 2010. Colour polymorphism and correlated characters: genetic mechanisms and evolution. Mol. Ecol. 19, 5101–5125 10.1111/j.1365-294X.2010.04846.x (doi:10.1111/j.1365-294X.2010.04846.x) [DOI] [PubMed] [Google Scholar]
- 37.Henning F., Renz A. J., Fukamachi S., Meyer A. 2010. Genetic, comparative genomic, and expression analyses of the Mc1r locus in the polychromatic Midas cichlid fish (Teleostei, Cichlidae Amphilophus sp.) species group. J. Mol. Evol. 70, 405–412 10.1007/s00239-010-9340-4 (doi:10.1007/s00239-010-9340-4) [DOI] [PubMed] [Google Scholar]
- 38.Elmer K. R., Lehtonen T. K., Meyer A. 2009. Color assortative mating contributes to sympatric divergence of neotropical cichlid fish. Evolution 63, 2750–2757 10.1111/j.1558-5646.2009.00736.x (doi:10.1111/j.1558-5646.2009.00736.x) [DOI] [PubMed] [Google Scholar]
- 39.Barson N. J., Knight M. E., Turner G. F. 2007. The genetic architecture of male colour differences between a sympatric Lake Malawi cichlid species pair. J. Evol. Biol. 20, 45–53 10.1111/j.1420-9101.2006.01228.x (doi:10.1111/j.1420-9101.2006.01228.x) [DOI] [PubMed] [Google Scholar]
- 40.Gunter H. M., Clabaut C., Salzburger W., Meyer A. 2011. Identification and characterization of gene expression involved in the coloration of cichlid fish using microarray and qRT-PCR approaches. J. Mol. Evol. 72, 127–137 10.1007/s00239-011-9431-x (doi:10.1007/s00239-011-9431-x) [DOI] [PubMed] [Google Scholar]
- 41.Salzburger W., Braasch I., Meyer A. 2007. Adaptive sequence evolution in a color gene involved in the formation of the characteristic egg-dummies of male haplochromine cichlid fishes. BMC Biol. 5, 51. 10.1186/1741-7007-5-51 (doi:10.1186/1741-7007-5-51) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kocher T. D. 2004. Adaptive evolution and explosive speciation: the cichlid fish model. Nat. Rev. Genet. 5, 288–298 10.1038/nrg1316 (doi:10.1038/nrg1316) [DOI] [PubMed] [Google Scholar]
- 43.Terai Y., Morikawa N., Kawakami K., Okada N. 2002. Accelerated evolution of the surface amino acids in the WD-repeat domain encoded by the hagoromo gene in an explosively speciated lineage of east African cichlid fishes. Mol. Biol. Evol. 19, 574–578 [DOI] [PubMed] [Google Scholar]
- 44.Blais J., Rico C., van Oosterhout C., Cable J., Turner G. F., Bernatchez L. 2007. MHC adaptive divergence between closely related and sympatric African cichlids. PLoS ONE 2, e734. 10.1371/journal.pone.0000734 (doi:10.1371/journal.pone.0000734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerrard D. T., Meyer A. 2007. Positive selection and gene conversion in SPP120, a fertilization-related gene, during the east African cichlid fish radiation. Mol. Biol. Evol. 24, 2286–2297 10.1093/molbev/msm159 (doi:10.1093/molbev/msm159) [DOI] [PubMed] [Google Scholar]
- 46.Spady T. C., Seehausen O., Loew E. R., Jordan R. C., Kocher T. D., Carleton K. L. 2005. Adaptive molecular evolution in the opsin genes of rapidly speciating cichlid species. Mol. Biol. Evol. 22, 1412–1422 10.1093/molbev/msi137 (doi:10.1093/molbev/msi137) [DOI] [PubMed] [Google Scholar]
- 47.Terai Y., Mayer W. E., Klein J., Tichy H., Okada N. 2002. The effect of selection on a long wavelength-sensitive (LWS) opsin gene of Lake Victoria cichlid fishes. Proc. Natl Acad. Sci. USA 99, 15 501–15 506 10.1073/pnas.232561099 (doi:10.1073/pnas.232561099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofmann C. M., O'Quin K. E., Marshall N. J., Cronin T. W., Seehausen O., Carleton K. L. 2009. The eyes have it: regulatory and structural changes both underlie cichlid visual pigment diversity. PLoS Biol. 7, e1000266. 10.1371/journal.pbio.1000266 (doi:10.1371/journal.pbio.1000266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terai Y., Morikawa N., Kawakami K., Okada N. 2003. The complexity of alternative splicing of hagoromo mRNAs is increased in an explosively speciated lineage in East African cichlids. Proc. Natl Acad. Sci. USA 100, 12 798–12 803 10.1073/pnas.2132833100 (doi:10.1073/pnas.2132833100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugie A., Terai Y., Ota R., Okada N. 2004. The evolution of genes for pigmentation in African cichlid fishes. Gene 343, 337–346 10.1016/j.gene.2004.09.019 (doi:10.1016/j.gene.2004.09.019) [DOI] [PubMed] [Google Scholar]
- 51.Seehausen O., van Alphen J. J. M., Lande R. 1999. Color polymorphism and sex ratio distortion in a cichlid fish as an incipient stage in sympatric speciation by sexual selection. Ecol. Lett. 2, 367–378 10.1046/j.1461-0248.1999.00098.x (doi:10.1046/j.1461-0248.1999.00098.x) [DOI] [Google Scholar]
- 52.Dijkstra P. D., van Dijk S., Groothuis T. G. G., Pierotti M. E. R., Seehausen O. 2009. Behavioral dominance between female color morphs of a Lake Victoria cichlid fish. Behav. Ecol. 20, 593–600 10.1093/beheco/arp036 (doi:10.1093/beheco/arp036) [DOI] [Google Scholar]
- 53.Pierotti M. E. R., Martin-Fernandez J. A., Seehausen O. 2009. Mapping individual variation in male mating preference space: multiple choice in a color polymorphic cichlid fish. Evolution 63, 2372–2388 10.1111/j.1558-5646.2009.00716.x (doi:10.1111/j.1558-5646.2009.00716.x) [DOI] [PubMed] [Google Scholar]
- 54.Pierotti M. E. R., Knight M. E., Immler S., Barson N. J., Turner G. F., Seehausen O. 2008. Individual variation in male mating preferences for female coloration in a polymorphic cichlid fish. Behav. Ecol. 19, 483–488 10.1093/beheco/arm154 (doi:10.1093/beheco/arm154) [DOI] [Google Scholar]
- 55.Lande R., Seehausen O., van Alphen J. J. M. 2001. Mechanisms of rapid sympatric speciation by sex reversal and sexual selection in cichlid fish. Genetica 112, 435–443 10.1023/A:1013379521338 (doi:10.1023/A:1013379521338) [DOI] [PubMed] [Google Scholar]
- 56.Roberts R. B., Ser J. R., Kocher T. D. 2009. Sexual conflict resolved by invasion of a novel sex determiner in Lake Malawi cichlid fishes. Science 326, 998–1001 10.1126/science.1174705 (doi:10.1126/science.1174705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Quin K. E., Hofmann C. M., Hofmann H. A., Carleton K. L. 2010. Parallel evolution of opsin gene expression in African cichlid fishes. Mol. Biol. Evol. 27, 2839–2854 10.1093/molbev/msq171 (doi:10.1093/molbev/msq171) [DOI] [PubMed] [Google Scholar]
- 58.Carleton K. 2009. Cichlid fish visual systems: mechanisms of spectral tuning. Integr. Zool. 4, 75–86 10.1111/j.1749-4877.2008.00137.x (doi:10.1111/j.1749-4877.2008.00137.x) [DOI] [PubMed] [Google Scholar]
- 59.Ser J. R., Roberts R. B., Kocher T. D. 2010. Multiple interacting loci control sex determination in Lake Malawi cichlid fish. Evolution 64, 486–501 10.1111/j.1558-5646.2009.00871.x (doi:10.1111/j.1558-5646.2009.00871.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Doorn G. S., Kirkpatrick M. 2007. Turnover of sex chromosomes induced by sexual conflict. Nature 449, 909–912 10.1038/Nature06178 (doi:10.1038/Nature06178) [DOI] [PubMed] [Google Scholar]
- 61.Maan M. E., Eshuis B., Haesler M. P., Schneider M. V., van Alphen J. J. M., Seehausen O. 2008. Color polymorphism and predation in a Lake Victoria cichlid fish. Copeia 3, 621–629 10.1643/Ce-07-114 (doi:10.1643/Ce-07-114) [DOI] [Google Scholar]
- 62.Coyne J. A., Orr H. A. 2004. Speciation. Sunderland, MA: Sinauer Associates [Google Scholar]
- 63.Machado H. E., Pollen A. A., Hofmann H. A., Renn S. C. 2009. Interspecific profiling of gene expression informed by comparative genomic hybridization: a review and a novel approach in African cichlid fishes. Integr. Comp. Biol. 49, 644–659 10.1093/Icb/Icp080 (doi:10.1093/Icb/Icp080) [DOI] [PubMed] [Google Scholar]
- 64.Desjardins J. K., Klausner J. Q., Fernald R. D. 2010. Female genomic response to mate information. Proc. Natl Acad. Sci. USA 107, 21 176–21 180 10.1073/pnas.1010442107 (doi:10.1073/pnas.1010442107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aubin-Horth N., Desjardins J. K., Martei Y. M., Balshine S., Hofmann H. A. 2007. Masculinized dominant females in a cooperatively breeding species. Mol. Ecol. 16, 1349–1358 10.1111/j.1365-294X.2007.03249.x (doi:10.1111/j.1365-294X.2007.03249.x) [DOI] [PubMed] [Google Scholar]
- 66.Elmer K. R., Reggio C., Wirth T., Verheyen E., Salzburger W., Meyer A. 2009. Pleistocene desiccation in East Africa bottlenecked but did not extirpate the adaptive radiation of Lake Victoria haplochromine cichlid fishes. Proc. Natl Acad. Sci. USA 106, 13 404–13 409 10.1073/pnas.0902299106 (doi:10.1073/pnas.0902299106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salzburger W., Mack T., Verheyen E., Meyer A. 2005. Out of Tanganyika: genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evol. Biol. 5, 17. 10.1186/1471-2148-5-17 (doi:10.1186/1471-2148-5-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haberyan K. A., Hecky R. E. 1987. The late Pleistocene and Holocene stratigraphy and paleolimnology of Lakes Kivu and Tanganyika. Palaeogeogr. Palaeoclimatol. Palaeoecol. 61, 169–197 10.1016/0031-0182(87)90048-4 (doi:10.1016/0031-0182(87)90048-4) [DOI] [Google Scholar]
- 69.Joyce D. A., Lunt D. H., Genner M. J., Turner G. F., Bills R., Seehausen O. 2011. Repeated colonization and hybridization in Lake Malawi cichlids. Curr. Biol. 21, 526. 10.1016/j.cub.2011.02.044 (doi:10.1016/j.cub.2011.02.044) [DOI] [PubMed] [Google Scholar]
- 70.Seehausen O. 2004. Hybridization and adaptive radiation. Trends Ecol. Evol. 19, 198–207 10.1016/j.tree.2004.01.003 (doi:10.1016/j.tree.2004.01.003) [DOI] [PubMed] [Google Scholar]
- 71.Verheyen E., Salzburger W., Snoeks J., Meyer A. 2003. Origin of the superflock of cichlid fishes from Lake Victoria, East Africa. Science 300, 325–329 10.1126/science.1080699 (doi:10.1126/science.1080699) [DOI] [PubMed] [Google Scholar]
- 72.Barluenga M., Meyer A. 2004. The Midas cichlid species complex: incipient sympatric speciation in Nicaraguan cichlid fishes? Mol. Ecol. 13, 2061–2076 10.1111/j.1365-294X.2004.02211.x (doi:10.1111/j.1365-294X.2004.02211.x) [DOI] [PubMed] [Google Scholar]
- 73.Barlow G. W. 1976. The Midas cichlid in Nicaragua. In Investigations of the Ichthyofauna of Nicaraguan lakes (ed. Thorson T. B.), pp. 333–358 Lincoln, NE: University of Nebraska [Google Scholar]
- 74.Barluenga M., Meyer A. 2010. Phylogeography, colonization and population history of the Midas cichlid species complex (Amphilophus spp.) in the Nicaraguan crater lakes. BMC Evol. Biol. 10, 326. 10.1186/1471-2148-10-326 (doi:10.1186/1471-2148-10-326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elmer K. R., Fan S., Gunter H. M., Jones J. C., Boekhoff S., Kuraku S., Meyer A. 2010. Rapid evolution and selection inferred from the transcriptomes of sympatric crater lake cichlid fishes. Mol. Ecol. 19, 197–211 10.1111/j.1365-294X.2009.04488.x (doi:10.1111/j.1365-294X.2009.04488.x) [DOI] [PubMed] [Google Scholar]
- 76.Vivas R., McKaye K. R. 2001. Habitat selection, feeding ecology, and fry survivorship in the Amphilophus citrinellus species complex in Lake Xiloa. J. Aquacult. Aq. Sci. IX, 32–48 [Google Scholar]
- 77.Apweiler R., et al. 2010. The universal protein resource (Uniprot) in 2010. Nucleic Acids Res. 38, D142–D148 10.1093/Nar/Gkp846 (doi:10.1093/Nar/Gkp846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conesa A., Gotz S., Garcia-Gomez J. M., Terol J., Talon M., Robles M. 2005. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21, 3674–3676 10.1093/bioinformatics/bti610 (doi:10.1093/bioinformatics/bti610) [DOI] [PubMed] [Google Scholar]
- 79.Yang Z. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 10.1093/molbev/msm088 (doi:10.1093/molbev/msm088) [DOI] [PubMed] [Google Scholar]
- 80.Iba K., Durkin M. E., Johnsen L., Hunziker E., Damgaard-Pedersen K., Zhang H., Engvall E., Albrechtsen R., Wewer U. M. 2001. Mice with a targeted deletion of the tetranectin gene exhibit a spinal deformity. Mol. Cell. Biol. 21, 7817–7825 10.1128/MCB.21.22.7817-7825.2001 (doi:10.1128/MCB.21.22.7817-7825.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Azam N., Vairapandi M., Zhang W., Hoffman B., Liebermann D. A. 2001. Interaction of CR6 (GADD45 gamma) with proliferating cell nuclear antigen impedes negative growth control. J. Biol. Chem. 276, 2766–2774 10.1074/jbc.M005626200 (doi:10.1074/jbc.M005626200) [DOI] [PubMed] [Google Scholar]
- 82.Ying J. M., Srivastava G., Hsieh W. S., Gao Z. F., Murray P., Liao S. K., Ambinder R., Tao Q. 2005. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin. Cancer Res. 11, 6442–6449 10.1158/1078-0432.Ccr-05-0267 (doi:10.1158/1078-0432.Ccr-05-0267) [DOI] [PubMed] [Google Scholar]
- 83.Turner T. L., Hahn M. W., Nuzhdin S. V. 2005. Genomic islands of speciation in Anopheles gambiae. PLoS Biol. 3, e285. 10.1371/journal.pbio.0030285 (doi:10.1371/journal.pbio.0030285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harr B. 2006. Genomic islands of differentiation between house mouse subspecies. Genome Res. 16, 730–737 10.1101/Gr.5045006 (doi:10.1101/Gr.5045006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nosil P., Funk D. J., Ortiz-Barrientos D. 2009. Divergent selection and heterogeneous genomic divergence. Mol. Ecol. 18, 375–402 10.1111/j.1365-294X.2008.03946.x (doi:10.1111/j.1365-294X.2008.03946.x) [DOI] [PubMed] [Google Scholar]
- 86.Peterson G. I., Masel J. 2009. Quantitative prediction of molecular clock and Ka/Ks at short timescales. Mol. Biol. Evol. 26, 2595–2603 10.1093/molbev/msp175 (doi:10.1093/molbev/msp175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barrett R. D. H., Schluter D. 2008. Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44 10.1016/j.tree.2007.09.008 (doi:10.1016/j.tree.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 88.Nielsen R., Hellmann I., Hubisz M., Bustamante C., Clark A. G. 2007. Recent and ongoing selection in the human genome. Nat. Rev. Genet. 8, 857–868 10.1038/nrg2187 (doi:10.1038/nrg2187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Manceau M., Domingues V. S., Linnen C. R., Rosenblum E. B., Hoekstra H. E. 2010. Convergence in pigmentation at multiple levels: mutations, genes and function. Phil. Trans. R. Soc. B 365, 2439–2450 10.1098/rstb.2010.0104 (doi:10.1098/rstb.2010.0104) [DOI] [PMC free article] [PubMed] [Google Scholar]