Abstract
Background and Purpose
This is the first prospective evaluation of changes in systemic hematologic status following administration of intraventricular rt-PA in patients with intraventricular hemorrhage.
Methods
Laboratory data from subjects enrolled in the Clot Lysis: Evaluating Accelerated Resolution of IVH (CLEAR IVH) Trials were analyzed. We analyzed pre/post rt-PA dosing coagulation parameters. Longer-term changes in hematologic status were studied in subjects that received study agent after blood clot in the 3rd/4th ventricles had resolved radiologically.
Results
Plasma fibrinogen increased significantly in both treatment groups. Dosing did not have a significant impact on any systemic coagulation parameters in either treatment group.
Conclusions
Intraventricular rt-PA is unlikely to impact systemic coagulation or compound the effects of systemic anticoagulation for DVT prophylaxis.
Keywords: coagulation[174], intracerebral hemorrhage[62], thrombolysis[73]
Introduction
Thrombolytic treatment of intraventricular hemorrhage (IVH) with low dose intraventricular rt-PA1 has shown significant improvement in 30-day survival. Intravenous rt-PA produces a transient systemic hypocoagulable state.2 Administering intraventricular thrombolytics to hypertensive IVH patients could contribute to a systemic coagulopathy and increase risk for both intra and extra-cranial bleeding. We performed a retrospective analysis of data from a large placebo-controlled multisite trial to assess the impact of intraventricular rt-PA on systemic coagulation.
Methods
The CLEAR IVH Trial study procedures have been published previously.3 We investigated changes in PT, PTT, platelets, plasma plasminogen, and plasma fibrinogen in patients who had blood coagulation data both prior to and within 36 hours after the first dose of study agent. To explore longer term effects of rt-PA when dosing may have systemic effects after restoration of normal CSF resorption, we studied a smaller subgroup that received study agent after blood clot in the 3rd/4th ventricles had resolved in whom an additional lab draw was performed within 24 hours after a dose was administered following clot resolution in the 3rd/4th ventricles. Adverse events were recorded prospectively.
Rt-PA and placebo groups were compared for demographic and clinical characteristics using chi-square analysis, t-test, and the Kruskal-Wallis one-way analysis of variance as appropriate. Regression analyses were used to compare treatment and placebo groups adjusting for baseline coagulation parameters, baseline IVH volume and gender. All results are presented as mean±standard deviation. P-values less than 0.05 were considered significant.
Results
Laboratory data were available for 78 of 100 enrolled subjects. Demographic and clinical characteristics are shown in Table 1. Coagulation data was similar in both treatment groups at baseline and during dosing (Table 2). Percent increase in plasma fibrinogen was statistically associated with time relative to first dose, but there was no statistically significant difference between placebo and rt-PA groups (P<0.01, Figure 1).
Table 1.
Patient Demographics
| Coagulation Post First Dose(s) | Coagulation Around Ventricle Clearing | |||||
|---|---|---|---|---|---|---|
| Characteristic | rt-PA (n=57)(%) | Placebo (n=21)(%) | P Value | rt-PA (n=20)(%) | Placebo (n=7)(%) | P Value |
| Age (yr) | 54±11 | 56±7 | 0.43 | 53.6±11 | 54.7±5 | 0.85 |
| Gender | ||||||
| Male | 36 (63.16) | 7 (33.33) | 0.02 | 14 (70.00) | 3 (42.9) | 0.20 |
| Female | 21 (36.84) | 14 (66.67) | 6 (30.00) | 4 (57.1) | ||
| Race | ||||||
| African-American | 32 (56.14) | 9 (42.86) | 11(55.0) | 5 (71.4) | ||
| White | 13 (22.81) | 7 (33.33) | 0.33 | 4 (20.0) | 2 (28.6) | 0.54 |
| Asian | 6 (10.53) | 1 (4.76) | 3 (15.0) | 0 (0.00) | ||
| Hispanic | 4 (7.02) | 4 (19.05) | 2 (10.0) | 0 (0.00) | ||
| Other | 2 (3.51) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Diagnostic CTIVH Volume (mL) | 46.0±33 | 50.8±36 | 0.53 | 45.5±23 | 52.3±39 | 0.96 |
| Diagnostic CT ICH Volume (mL) | 9.9±9 | 12.7±13 | 0.81 | 12.3±10 | 12.6±17 | 0.45 |
| Hypertension | 46 (80.70) | 18 (85.71) | 0.61 | 17 (85.0) | 7 (100.0) | 0.28 |
| Prior Antiplatelet Use | 5 (8.77) | 4 (19.05) | 0.21 | 1 (5.0) | 0 (00.0) | 0.55 |
| Total rt-PA Administered | 3.1±2.0 | 0 | <0.00 | 8.33±6.9 | 0 | <0.00 |
| Prior to Lab Draw (mg) | 1 | 1 | ||||
| Time From First Dose to Lab Draw (Hours) | 14.74±8.85 | 11.95±7.35 | 0.20 | |||
| Time From Last Prior Dose to Lab Draw (Hours) | 7.57±4.74 | 5.51±5.18 | 0.44 | |||
| Time From Ventricle Clearing to Dose (Hours) | 5.34±4.4 | 8.87±5.5 | 0.14 | |||
| Time From Ventricle Clearing Dose to Lab Draw (Hours) | 10.20±6.7 | 5.27±3.15 | 0.10 | |||
| Time From First Dose to 3rd/4th Ventricle Clearing (Days) | 2.74±1.1 | 7.04±3.5 | 0.03 | |||
Table 2.
Baseline and Post-First Dose Coagulation Parameters
| Baseline | Post First Dose | % Δ in Coagulation Parameter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation Parameter | rt-PA | Placebo | rt-PA | Placebo | rt-PA | Placebo | ||||
| Mean±SD (n) | Mean±SD (n) | Mean±SD | Mean±SD | Mean±SD | Median | Range | Mean±SD | Median | Range | |
| Platelet Count (plt/mm3) | 236±61 (56) | 238±65 (21) | 211±59 | 204±62 | −9.81±14.6 | −12.4 | −48.6–31.2 | −14.42±13.1 | −13.13 | −43.3–11.3 |
| PTT (Seconds) | 29.4±4.2 (57) | 28.8±6.0 (18) | 30.0±4.2 | 29.0±5.9 | 2.16±8.0 | 0 | −12.4–41.5 | 0.81±2.6 | 0 | 0–10.5 |
| PT (Seconds) | 13.0±2.0 (52) | 12.6±1.7 (20) | 13.2±2.0 | 12.7±1.6 | 0.95±3.4 | 0 | −6.76–13.1 | 0.54±2.4 | 0 | −2.04–10.7 |
| Fibrinogen (mg/dL) | 461±140 (43) | 448±92 (16) | 508±150 | 478±103 | 12.37±22.14 | 2.38 | −33.7–97.24 | 7.24±12. | 2.79 | −6.82–39.7 |
| Plasminoge n (%) | 103±15 (34) | 108±17 (12) | 103±17 | 107±18 | 0.27±8.14 | 0 | −25.56–18.0 | −0.46±8.60 | 0 | −11.11–21.3 |
Figure 1.
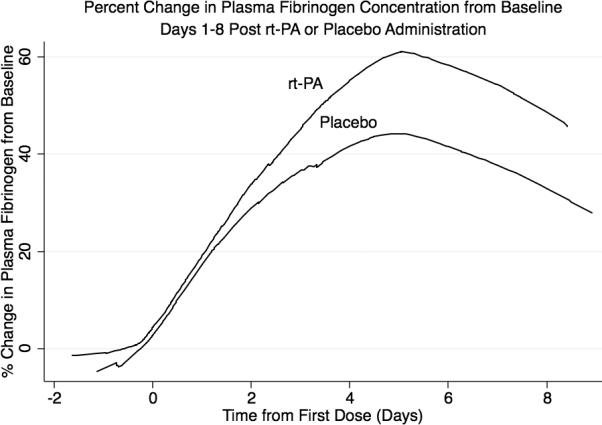
Percent change in plasma fibrinogen concentration from baseline shown by locally weighted scatterplot smoothing.
Demographic and coagulation data were similar in both treatment groups in the 27 subjects who had laboratory data after clearance of 3rd/4th ventricles (Table 1) (please see http://stroke.ahajournals.org), except for time from administration of the first dose to clot resolution in the 3rd/4th ventricles. After re-matching the placebo group to the rt-PA group using the time of clearance of the rt-PA group to account for time related change, there were no significant differences between the two groups' coagulation parameters.
Deep venous thrombosis (DVT) occurred in 2.5% of rt-PA and 4.5% of placebo patients. One patient had a myocardial infarction. Symptomatic brain bleeding occurred in eight rt-PA and one placebo subject. Coagulation data were similar between subjects who had symptomatic brain bleeding and those who did not. The only non-CNS bleeding event reported (GI hemorrhage) was in the placebo group. None of 29 subjects who received subcutaneous heparin or coumadin during intraventricular rt-PA dosing experienced systemic bleeding.
Discussion
In contrast to intravenous thrombolytic administration, patients were exposed to significantly lower doses of intraventricular rt-PA (maximum of 3.0 mg q12h for up to 13 days). Our findings show that administration of low-dose intraventricular rt- PA does not significantly change systemic hematologic status in patients with IVH either before or after radiographic clearance of the 3rd/4th ventricles.
Following administration of 100 mg intravenous rt-PA, there is a decrease (16–36%) in circulating fibrinogen.4 We observed a mean increase in fibrinogen in both groups after the first dose and after ventricle clearance, which we attribute to an acute phase hematologic response to intracranial hemorrhage.5
Systemic hematologic status is an important consideration in the treatment of hemorrhagic stroke, as hemorrhagic stroke is an independent risk factor for DVT.6 The rate of DVT and pulmonary embolism among all rt-PA subjects in the CLEAR IVH trial is consistent with other studies,1,7 suggesting clinically that intraventriclar rt-PA does not initiate systemic fibrinolysis, and would be unlikely to compound the effects of systemic low dose anticoagulation for DVT prophylaxis. The effect of local factors on brain bleeding such as extent of tissue trauma and timing of local t-PA delivery in relation to hemostasis would benefit from further investigation.
Supplementary Material
Acknowledgements
We thank Dr. Thomas Kickler for reviewing this manuscript
Sources of Funding The Intraventricular Hemorrhage Thrombolysis Trials were supported by grants FD-R-001693 and FD-R 002018 from the Office of Orphan Products Development, Food and Drug Administration, National Institute of Neurological Disorders and Stroke grant U01 NS062851 and funding from the American Heart Association.
Footnotes
Conflicts of Interest/Disclosures Dr. Hanley has received research support as Principal Investigator of the CLEAR IVH trial.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Findlay JM, Grace MG, Weir BK. Treatment of intraventricular hemorrhage with tissue plasminogen activator. Neurosurgery. 1993;32:941–947. doi: 10.1227/00006123-199306000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Tanne D, Macko RF, Lin Y, Tilley BC, Levine SR, For the NINDS rtPA Stroke Study Group Hemostatic activation and outcome after recombinant tissue plasminogen activator therapy for acute ischemic stroke. Stroke. 2006;37:1798–1804. doi: 10.1161/01.STR.0000226897.43749.27. [DOI] [PubMed] [Google Scholar]
- 3.Naff N, Williams M, Keyl PM, Tuhrim S, Bullock RM, Mayer S, et al. Low-dose rt-PA enhances clot resolution in brain hemorrhage: The intraventricular hemorrhage thrombolysis trial. Stroke. 2011 doi: 10.1161/STROKEAHA.110.610949. In-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topol EJ, Morriss DC, Smalling RW, Schumacher RR, Taylor CR, Nishikawa A, et al. A multicenter, randomized, placebo-controlled trial of a new form of intravenous recombinant tissue-type plasminogen activator (activase) in acute myocardial infarction. J Am Coll Cariol. 1987;9:1205–1213. doi: 10.1016/s0735-1097(87)80457-6. [DOI] [PubMed] [Google Scholar]
- 5.Marti-Fabregas J, Borrell M, Silva Y, Delgado-Mederos R, Martinez-Ramirez S, de Juan-Delago M, et al. Hemostatic Proteins and Their Association With Hematoma Growth in Patients With Acute Intracerebral Hemorrhage. Stroke. 2010;41:2976–2978. doi: 10.1161/STROKEAHA.110.595868. [DOI] [PubMed] [Google Scholar]
- 6.Gregory PC, Kuhlemeier KV. Prevalence of venous thromboembolism in acute hemorrhagic and thromboembolic stroke. Am J Phys Med Rehabil. 2003;82:364–369. doi: 10.1097/01.PHM.0000064725.62897.A5. [DOI] [PubMed] [Google Scholar]
- 7.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


