Abstract
Familial Tumoral calcinosis (TC) is a rare disorder distinguished by the development of ectopic and vascular calcified masses that occur in settings of hyperphosphatemia (hFTC) and normophosphatemia (nFTC). Serum phosphorus concentrations are relatively tightly controlled by interconnected endocrine activity at the level of the intestine, kidney, and skeleton. Discovering the molecular causes for heritable forms of hFTC has shed new light on the regulation of serum phosphate balance. This review will focus upon the genetic basis and clinical approaches for hFTC, due to genes that are related to the phosphaturic hormone Fibroblast growth factor-23 (FGF23). These include FGF23 itself, an FGF23-glycosylating enzyme (GALNT3), and the FGF23 co-receptor α-Klotho (αKL). Our understanding of the molecular basis of hFTC will, in the short term, aid in understanding normal phosphate balance, and in the future, provide potential insight into the design of novel therapeutic strategies for both rare and common disorders of phosphate metabolism.
Keywords: FGF23, FGF-23, phosphate, hyperphosphatemia, GALNT3, α-Klotho, TC, HHS
I. Introduction
Familial Tumoral calcinosis (TC; MIM ID #211900) is a rare autosomal recessive metabolic disorder characterized by the progressive deposition of calcium phosphate crystals in periarticular spaces and soft tissues. TC may manifest under situations of hyperphosphatemia (hyperphosphatemic familial TC; hFTC) or normophosphatemia (normophophatemic familial TC; nFTC). Maintenance of serum phosphate concentrations is required for proper skeletal development and for preservation of bone integrity. In addition, phosphate is required for cellular processes involving energy transfer in the form of ATP, is an integral molecule in DNA and RNA, and is a critical component of multiple intracellular signaling pathways. Recent advances in our understanding of hFTC have shed light on the underlying mechanisms that control phosphate homeostasis in normal and in disordered states. The biochemical hallmark of tumoral calcinosis is hyperphosphatemia caused by increased renal reabsorption of phosphate. This review will summarize the heterogeneous genetic defects and molecular mechanisms that lead to hFTC involving the phosphaturic hormone Fibroblast growth factor-23 (FGF23), and nFTC will be reviewed elsewhere in this issue.
2. Phosphate homeostasis
Maintenance of serum phosphate concentrations involves hormonal regulation at the level of the intestine, skeleton, and kidneys. The skeleton represents the largest stores of phosphate complexed with calcium in hydroxyapatite crystals, which constitute the main inorganic component of the mineralized bone matrix. Normal serum concentrations of phosphate in adults range from 2.5–4.5 mg/dl and are fairly tightly regulated. Serum phosphate concentrations are higher in infancy, decreasing as the child ages to adolescence and adulthood. Absorption of phosphate in the intestine is directly proportional to dietary intake, but is also influenced by the active form of vitamin D, 1,25(OH)2 vitamin D (1,25D). In normal individuals, 25-hydroxy vitamin D is converted to 1,25D by the 25-hydroxy vitamin D 1-alpha hydroxylase enzyme (1α (OH)ase; Cyp27b1) and inactivated by the vitamin D 24-hydroxylase (Cyp24) in the kidney proximal tubule. 1,25D regulates serum phosphate concentrations by increasing calcium and phosphate absorption in the intestines, and at high concentrations, by increasing phosphate release by stimulating skeletal reabsorption [1]. Rising serum calcium concentrations decrease PTH secretion, which in turn releases expressional inhibition of the primary transport protein in the proximal tubule, the type IIa sodium-dependent phosphate co-transporter, NPT2a [2–4]. The abundance of apical NPT2a is the main determinant of percent tubular reabsorption of phosphate (%TRP) as this transporter accounts for 70–80% of reabsorption [5, 6]. Increased abundance of the NPT2a protein confers increased %TRP and consequently raises serum phosphate level and vice versa. Accordingly, in normal individuals the serum phosphate concentration also determines the expression of NPT2a: hypophosphatemia increases the expression of NPT2a, and hyperphosphatemia results in decreased expression of NPT2a, in order to re-establish normal serum phosphate. Although less is known about the type IIc sodium-phosphate co-transporter, NPT2c [7], this protein must also play a role in proximal tubule phosphate reabsorption, as inactivating NPT2c mutations lead to renal phosphate wasting and the autosomal recessive disorder hereditary hypophosphatemic rickets with hypercalciuria (HHRH) [8].
3. Disorders of elevated FGF23
The biochemical abnormalities in hFTC include hyperphosphatemia, increased %TRP and inappropriately normal or elevated 1,25D concentrations. HFTC can be considered as the clinical converse of several diseases characterized by elevation of the phosphaturic hormone Fibroblast growth factor-23 (FGF23). The FGF23 gene, composed of three exons encoding a 251 residue polypeptide, is located on the human chromosome 12p13 [9]. Although FGF23 mRNA can be detected at low levels in many tissues including heart, liver, thyroid/parathyroid, and small intestine, it is predominantly expressed in bone [9] by osteoblasts, osteocytes, flattened bone lining cells, and osteoprogenitor cells [10]. The manifestations of prolonged elevations in FGF23 include reduced serum phosphorus concentrations due to isolated renal phosphate wasting, inappropriately low or normal serum 1,25D concentrations with typically normal serum calcium and PTH concentrations and osteomalacia/rickets.
Autosomal dominant hypophosphatemic rickets (ADHR), is an allelic disorder to hFTC (see below), and caused by gain-of-function mutations in the FGF23 gene [9]. These mutations replace the arginine (R) residues at positions 176 or 179 with glutamine (Q) or tryptophan (W) within a 176RXXR179/S180 subtilisin-like proprotein convertase (SPC) site that separates the conserved FGF-like N-terminal domain from the variable C-terminal tail [9, 11–13], and lead to stabilization of the intact, bioactive FGF23 molecule [11, 13]. Injection of intact FGF23 or purified N- and C-terminal fragments into rodents showed that only the intact FGF23 species caused hypophosphatemia [13], consistent with the idea that the intact form of FGF23 is bioactive. In patients with X-linked hypophosphatemic rickets (XLH; loss of function PHEX mutations), and autosomal recessive hypophosphatemic rickets (ARHR; loss of function DMP1 or ENPP1 mutations) [14–16], wild type FGF23 can be markedly elevated. In addition, the corresponding mouse models of these diseases, Hyp (XLH) and Dmp1-null (ARHR), have increased FGF23 mRNA expression and serum protein concentrations, potentially due to improper osteoblast to osteocyte differentiation [14]. The acquired disorder tumor induced osteomalacia (TIO), is associated with FGF23 over expression by a rare category of neoplasm, classified as phosphaturic mesenchymal tumors of the mixed connective tissue variant (PMTMCT) [17].
4. Bioactivity of FGF23
FGF23 is central to renal phosphate and vitamin D metabolism, and has emerging roles in the regulation of PTH. FGF23-dependent control of renal phosphate reabsorption was demonstrated by the finding that mice transgenic for human FGF23 had markedly increased phosphate excretion secondary to decreased proximal tubule Npt2a and Npt2c expression [18, 19]. In normal individuals, hypophosphatemia is typically a strong stimulator for increased serum 1,25D [20]. However, in patients with syndromes caused by elevated FGF23, there is a lack of appropriate elevation of 1,25D in response to hypophosphatemia. Animal studies demonstrated that in spite of severe hypophosphatemia, FGF23 transgenic mice demonstrated decreased renal levels of Cyp27b1 and increased Cyp24 [18, 19, 21]. Thus, the effects of FGF23 on the renal vitamin D metabolic enzymes accounts for the reductions in serum 1,25D concentrations observed in TIO, ADHR, and XLH patients. More recently, short term treatment with FGF23 has been shown to suppress PTH mRNA and protein production in vitro in cultured bovine parathyroid cells [22], and in vivo in intact rat parathyroid glands [23].
5. TC due to loss of function in FGF23-related genes
hFTC is a genetically heterogeneous syndrome, caused by loss of function mutations in genes relevant to the production of the intact, bioactive form of FGF23 (FGF23 and GALNT3), and to the end-organ effects of FGF23 bioactivity (αKlotho). Human genetic studies, taken together with analyses of corresponding animal models, have provided key insight into the molecular etiology of hFTC.
5A. TC due to FGF23 loss of function mutations
Using a candidate gene approach, several groups demonstrated the hyperphosphatemic TC phenotype can be caused by recessive mutations in the FGF23 gene. These mutations replace conserved residues in the N-terminal FGF-like domain of the mature FGF23 (Table 1) [24–26] and appear to destabilize the bioactive intact form of the hormone. In this regard, the use of two FGF23 serum assays with overlapping specificities has provided critical insight into the molecular mechanisms associated with TC. FGF23 can be measured in the serum using two distinct ELISAs. The `Intact' FGF23 assay (Kainos, Inc.) uses conformation-specific monoclonal antibodies to N- and C-terminal portions of FGF23 that detect the intact, bioactive form of FGF23 [27]. The `C-terminal' FGF23 ELISA (Immutopics, Int'l. Inc.), uses polyclonal antibodies that bind two different peptide epitopes, both `C-terminal' (3′) to the FGF23 176RXXR179/S180proteolytic cleavage site, and thus recognizes both the intact FGF23 and the C-terminal fragments present in the circulation [28].
Table 1.
Mutations in FGF23, GALNT3 and αKL
| Syndrome | Gene | Mutation | Protein | Reference |
|---|---|---|---|---|
| hFTC | FGF23 | 123c>a | H41Q | Masi 2005 |
| hFTC | FGF23 | 160c>a | Q54K | Garringer 2008 |
| hFTC | FGF23 | 287t>c | M96T | Chefetz 2005 |
| hFTC | FGF23 | 211a>g | S71G | Larsson 2005, Benet-Pages 2005 |
| hFTC | FGF23 | 367g>t | G123W | Lammoglia 2008 |
| hFTC | FGF23 | 385t>c | S129P | Bergwitz 2005 |
| hFTC | FGF23 | 386c>t | S129F | Araya 2005 |
| HHS | GALNT3 | 2t>a | M1K | Gok 2009 |
| hFTC | GALNT3 | 41–58del | R14fsX21 | Garringer 2006 |
| hFTC | GALNT3 | 484c>t | R162X | Topaz 2004, Ichikawa 2005 |
| hFTC | GALNT3 | 485g>a | R162Q | Ichikawa 2010 |
| hFTC | GALNT3 | 516-2a>t | (Splice Site) | Ichikawa 2005 |
| hFTC | GALNT3 | 677delC | A226VfsX3 | Ichikawa 2010 |
| hFTC | GALNT3 | 815c>a | T272K | Ichikawa 2006 |
| HHS | GALNT3 | 803_804insC | A268fsX271 | Ichikawa 2007 |
| HHS | GALNT3 | 839g>a | C280Y | Gok 2009 |
| hFTC | GALNT3 | 842a>g | E281G | Joseph 2010 |
| hFTC | GALNT3 | 966t>a | Y322X | Barbieri 2007 |
| hFTC | GALNT3 | 1076c>a | T359K | Ichikawa 2006 |
| hFTC | GALNT3 | 1097t>g | L366R | Joseph 2010 |
| hFTC | GALNT3 | 1102_1103inst | E375X | Garringer 2007 |
| hFTC | GLANT3 | 1245t>a | H415Q | Yancovitch 2011 |
| hFTC | GALNT3 | 1312c>t | R438C | Dumitrescu 2008 Yancovitch 2011 |
| HHS | GALNT3 | 1313g>a | R438H | Olauson 2008 |
| hFTC | GALNT3 | 1387a>t | K463X | Campagnoli 2006 |
| HHS | GALNT3 | 1392+1g>a | (Splice Site) | Ichikawa 2010 |
| hFTC | GALNT3 | 1441ct | Q481X | Barbieri 2007 |
| hFTC | GALNT3 | 1460 g>a | W487X | Garringer 2007 |
| hFTC /HHS | GALNT3 | 1524+1g>a | K465_Y508del | Topaz 2004, Fishburg 2005 |
| hFTC | GALNT3 | 1524+5g>a | (Splice Site) | Topaz 2004 |
| HHS | GALNT3 | 1626+1g>a | (Splice Site) | Ichikawa 2007 |
| hFTC | GALNT3 | 1720t>g | C574G | Ichikawa 2010 |
| hFTC | GALNT3 | 1774c>t | Q592X | Specktor 2006 |
| hFTC | α Klotho | 578a>g | H193R | Ichikawa 2007 |
The results of the the two ELISAs generally correlate well with regard to the relative range of FGF23 concentrations in XLH and in TIO patients, and in normal individuals [29]. In contrast, the serum from hFTC patients with S71G and S129F FGF23 mutations revealed low-normal intact FGF23 levels [25, 30], whereas C-terminal FGF23 concentrations were markedly elevated [25, 26, 30]. Taken together, these observations suggested a differential proteolytic cleavage of hFTC-mutant FGF23 to that of wild-type protein. Indeed, it has been demonstrated that the hFTC mutations lead to increased intra-cellular proteolytic degradation [25, 26, 30]. This activity is likely mediated, at least in part, by furin-like proteases [31] which reside in the Trans-Golgi network (TGN) and recognize the FGF23 176RXXR179/S180 SPC site. The apparent loss of secretion of intact bioactive FGF23 in patients with hFTC explains the biochemical phenotype. With impaired intact FGF23 secretion, there is continued renal reabsorption of phosphate and a lack of inhibition of 1,25D production (Figure 1) despite hyperphosphatemia. These alterations likely lead to further increased intestinal calcium and phosphate absorption, creating a biochemical environment favorable for precipitation of calcium-phosphate crystals in the vasculature and soft tissues. In accord with the human findings, the human hFTC phenotype closely resembles that of Fgf23-null mice [32, 33], which manifest hyperphosphatemia, elevated 1,25D, and ectopic and vascular calcifications.
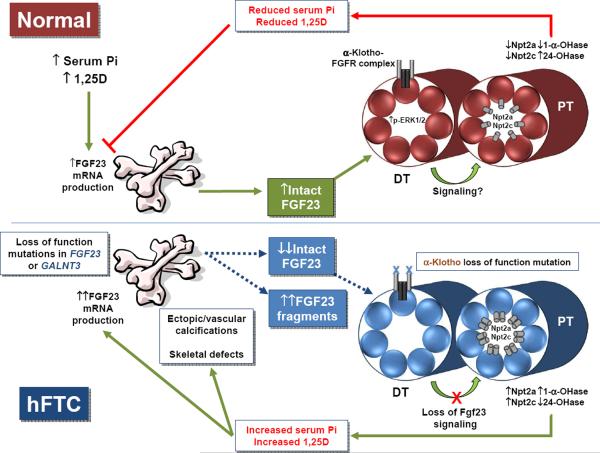
Figure 1.
(top panel) Under normal physiological conditions, increased serum phosphate and 1,25D stimulate FGF23 production in bone, increasing bioactive, intact serum FGF23. FGF23 circulates to the kidney where it binds to a receptor complex comprised of αKlotho and an FGFR in the distal tubule (DT), which then through an unknown mechanism causes a decrease in expression of the sodium phosphate co-transporters, Npt2a and Npt2c, in the proximal tubule (PT) apical membrane. Simultaneously, FGF23 increases expression of the vitamin D catabolic enzyme, 24-hydroxylase, and decreases 1-α-hydroxylase expression. Together, the decrease in Npt2a, Npt2c, and 1,25D leads to a decrease in serum phosphate, which in a negative feedback loop, decreases FGF23 production in the bone. (lower panel) In hFTC, loss of function mutations in either FGF23 or GALNT3 result in decreased intact serum FGF23, and an increase in inactive C-terminal FGF23 fragments. The inactive fragments likely do not signal in the kidney. The αKlotho loss of function mutation leads to a reduction in the activity of the FGF23 receptor signaling complex in the distal tubule. All of the TC mutations likely lead to a release of suppression of Npt2a and Npt2c, a decrease in 24-hydroxylase, and an increase in 1-α-hydroxylase expression, ultimately increasing serum phosphate concentrations, resulting in ectopic and vascular calcifications, and a further stimulus of FGF23 in bone. However, in contrast to FGF23 and GALNT3 mutations, the αKlotho loss of function mutation leads to an increase in serum intact FGF23, as FGF23 protein processing in bone is not directly affected by this mutation.
5B. TC due to loss of function GALNT3 mutations
Using homozygosity mapping in two extended kindreds, hFTC was determined to be caused by inactivating mutations in the GalNAc-transferase 3 (GALNT3) gene, a TGN enzyme which initiates O-linked glycosylation [34] in a wide spectrum of protein substrates. The GALNT3 nucleotide changes represent a variety of missense, splice-site and non-sense mutations, which are predicted to result in loss of GALNT3 function (Table 1).
When inactivating GALNT3 mutations are present in hFTC patients, limited amounts of intact FGF23 are produced, leading to hyperphosphatemia [35] (Figure 1). Mature, bioactive FGF23 protein is O-glycosylated in three regions, with the most critical being within the 176RH177T178R179/S180 SPC-like domain, at position T178 [36]. This key glycosylation site may prevent intracellular degradation of FGF23 at the RXXR motif by SPC-like enzymes in the trans-Golgi network [36]. HFTC patients with loss of function mutations in GALNT3 have a remarkably similar serum FGF23 profile to those with FGF23 mutations, manifesting low serum intact FGF23, but significantly elevated C-terminal FGF23 concentrations [37]. In vitro, deleting GALNT3 causes increased proteolytic cleavage and the appearance of FGF23 immunoreactive polypeptide fragments with reduced intact FGF23 secretion [38]. The biochemical profile of elevated plasma C-terminal FGF23 in conjunction with low intact FGF23 in the setting of hyperphosphatemia appears to be virtually diagnostic for GALNT3- and FGF23-TC (hFTC due to αKL mutations is described below).
The fact that GALNT3- and FGF23-hFTC patients have high C-terminal FGF23 levels likely represents an attempt to increase FGF23 production as a compensatory response to the prevailing hyperphosphatemia. Indeed, the Galnt3-null mouse has increased FGF23 mRNA expression, and resulting high C-terminal FGF23, but low intact FGF23 concentrations [39]. In a small study, some investigators did not find a change in FGF23 in human subjects due to low or high Pi diets [40], but others have since demonstrated changes in FGF23 in response to changes in dietary phosphate load [41, 42]. Cross-sectional studies have not consistently demonstrated an inverse correlation between serum FGF23 and serum phosphorus in healthy humans [41]. This latter study used dietary calcium supplementation to maintain constant serum calcium levels, to blunt the possible confounding regulation of serum phosphate by PTH [41]. The Fgf23 response to serum phosphate in animal studies has been far more consistent than in the human studies. Of note, mice receiving high and low phosphate diets demonstrated appropriate correlations between Fgf23 and phosphate intake [43, 44]. Whether a true `phosphate sensor' molecule (analogous to the calcium-sensing receptor; CaSR) exists, or whether the biological response of FGF23 is a combination of direct and indirect (through other hormones) monitoring of plasma phosphate is currently unknown.
The effects of vitamin D on Fgf23 expression have also been examined. Injections of 1,25D into mice led to dose-dependent increases in serum Fgf23 [45]. At low doses of vitamin D, increases in serum Fgf23 occurred before detectable changes in serum phosphate, indicating that Fgf23 may be directly regulated by 1,25D concentrations. These findings would be consistent with the role of Fgf23 in vitamin D metabolism. Fgf23 down-regulates Cyp27b1 mRNA [18, 45], thus as 1,25D concentrations rise in the blood as a product of Cyp27b1, vitamin D would then stimulate Fgf23 production, which would complete the feedback loop and down-regulate Cyp27b1. In agreement with these observations, increases in FGF23 promoter activity following 1,25D treatment were observed in cell culture experiments, despite the absence of a classical vitamin D response element within the FGF23 promoter [44]. These studies were also substantiated by treatment of the UMR-106 cell line with 1,25D, which showed dramatic elevation of Fgf23 mRNA, which was sensitive to actinomycin D and cyclohexamide [46]. More recently, the Galnt3-null mouse model with inappropriately reduced intact Fgf23 (considering the degree of hyperphosphatemia) as tested by ELISA, had elevated bone Fgf23 mRNA and increased Fgf23 fragments by Western blotting [39]. These studies indicate that the mice display the expected physiological response to increased circulating phosphate and 1,25D by increasing Fgf23 transcription, but the defective O-glycosylation due to the ablation of Galnt3 reduces intact, bioactive Fgf23, with the serum biochemistries of patients with GALNT3-hFTC. Importantly, it has been shown in vitro, that recombinant FGF23 carrying FGF23-hFTC mutations has defective O-glycosylation, thus making this species of FGF23 more prone to intracellular degradation [47]. These observations explain the identical serum FGF23 profile of patients with FGF23- and GALNT3-mutant hFTC [47].
The discovery that mutations in GALNT3 may alter FGF23 production and processing, has substantial impact on the understanding of the molecular pathophysiology of other disorders of phosphate homeostasis. In particular, the ADHR FGF23 mutants are mis-glycosylated when compared to wild type FGF23 [13]. However, even though abnormally glycosylated, these mutants are resistant to endoproteolytic cleavage due to the R176 or R179 mutations interrupting the RXXR motif, which then allows secretion as active, full-length polypeptides [31]. Thus normal glycosylation of wild type FGF23, but not ADHR mutant FGF23, may be necessary for secretion, but not for FGF23 activity at its receptor.
5C. TC due to an αKlotho missense mutation
FGF23 is a member of a class of unique FGFs including FGF19 and FGF21 that can be detected in the circulation [48]. Unlike FGF23, FGF19 and FGF21 are systemic regulators of lipid and glucose metabolism [49]. These three `endocrine' FGFs are predicted to have related structures, but rely on tissue-specific expression of specific co-receptors, α-Klotho (αKL; interacts with FGF23) and β-Klotho (βKL; interacts with FGF19 and FGF21 in concert with cellor tissue-specific FGF receptors (FGFRs) to elicit bioactivity.
αKL is produced as two isoforms due to alternative splicing of a 5-exon gene, and its expression is restricted to kidney distal convoluted tubule (DCT) cells [50], parathyroid gland, the gonads, and brain [51]. Membrane bound αKL (mKL) is a 130 kD single-pass transmembrane protein encoded by all 5 exons, and is comprised of a large extracellular domain with a short intracellular region that lacks signaling motifs [52, 53]. A secreted form of KL (sKL) is approximately 80 kD and is alternatively spliced within exon 3 to result in an αKL protein that contains the extracellular region but lacks the transmembrane domain [52, 53]. Of note, the transmembrane mKL isoform undergoes cleavage near the cell surface to result in another circulating isoform (`cleaved KL' or `cKL'). In parallel with Fgf23-null mice, αKL-null mice have increased levels of Npt2a in the apical membrane of the proximal tubule [50], indicating that the hyperphosphatemia in this animal is secondary to increased renal reabsorption of phosphate.
In vitro studies show that FGF23 interacts with the mKL and cKL isoforms [54], however the roles of the circulating αKL species are not known. The apparent mechanism for FGF23 activity through mKL is the recruitment of canonical FGF receptors (FGFRs) which signal through the mitogen activated protein kinase (MAPK) [54] and potentially Wnt [55] cascades as heteromeric complexes. One group has identified a specific complex between FGFR1c and αKL [56]. In contrast, others have demonstrated that multiple FGFRs (FGFR1c, FGFR3c and FGFR4) may interact with αKL and FGF23 [54], making the specific FGFR associated with FGF23 activity in vivo unclear. More recent data has suggested that FGF23-αKlotho interactions with individual FGFR subtypes may be responsible for different components of the biochemical effects of FGF23 (phosphate transport versus 1,25D regulation) [57]. Within the kidney, αKL localizes to the DCT, however it is well established that FGF23 mediates its effects on NPT2a, NPT2c, and the vitamin D metabolizing enzymes located in the proximal tubule (PT) [18, 58] (Figure 1). Thus, the molecular mechanisms and potential signaling pathways underlying a local FGF23-dependent DCT-PT axis in the kidney are unclear.
A homozygous mutation in αKL, H193R, was shown to be a third cause of hFTC in a single case [59]. This patient presented with hyperphosphatemia, hypercalcemia, elevated PTH, and ectopic calcifications in the heel and brain [59]. The H193R missense mutation is localized within a highly conserved region in exon 1, present in all isoforms of αKL. In vitro analysis of the mutant protein illustrated decreased mature, glycosylated αKL expression, as well as reduced FGF23-dependent signaling through MAPK, thus this mutation is assumed to prevent end-organ FGF23 bioactivity in vivo. In contrast to hFTC patients with mutations in GALNT3 and FGF23, which show disparate results between intact and C-terminal FGF23 ELISAs, the αKL-hFTC patient manifested significant increases for both FGF23 ELISAs (>150-fold control). The intact FGF23 is elevated in this patient due to the up-regulation of FGF23 in response to hyperphosphatemia and increased 1,25D, coupled with the fact that the FGF23 gene itself and its O-glycosylation are not genetically altered to destabilize the intact hormone. These findings provide important support for direct interactions between FGF23 and αKL. Further, the αKL-null mice present phenotypically with severe calcifications [60] as well as markedly elevated serum phosphate and Fgf23 concentrations [56, 61], which parallels the phenotype of hFTC patients and Fgf23-null mice [32]. The fact that the αKL- and Fgf23-null mice are biochemical phenocopies further supports the interactions between these gene products in phosphate and 1,25D metabolism.
5D. Hyperostosis-hyperphosphatemia syndrome (HHS)
The term `hyperostosis-hyperphosphatemia syndrome' is used to describe a disorder of elevated serum phosphate characterized by involvement of the long bones with radiographic findings of periosteal reaction and cortical hyperostosis [62]. Although HHS had been distinguished from hFTC by the presence of bone involvement and the absence of ectopic calcifications, following GALNT3 mutation detection several groups have now demonstrated that the HHS and hFTC clearly represent different manifestations of the same genotype [63, 64] (see Table 1). Thus, several mutations in GALNT3 that were known to result in hFTC are identical to those found in patients with HHS (Table 1). The molecular reasons for the enhanced skeletal involvement in HHS are currently unknown, but could potentially be due to modifier gene interactions.
6. Therapeutic avenues
Tumoral calcinosis lesions can become very large and frequently require surgical resection due to pain, deformity and limitation of joint movements. Surgery may be curative, but unfortunately, these lesions typically recur [64–66] due to persistence of the underlying metabolic defect.
Medical treatment for tumoral calcinosis is based on limited clinical evidence. Due to the rarity of hFTC, controlled trials are lacking and all available treatment information is based on case reports or case series. Assessment of treatment options is complicated by the extreme variability between subjects in terms of the severity of biochemical abnormality and the number and size of TC lesions. Furthermore, the measure of effect varies between reports, including increasing renal phosphate excretion, decreasing serum phosphate concentration and resolution or reduction in size of TC lesions. From the patient's perspective, the most important outcome is probably resolution of TC lesions, as these can be both painful and disabling.
However, in both healthy adults and patients with chronic kidney disease, serum phosphate concentrations are associated with cardiovascular disease and mortality, even within the normal phosphate range [67–69]. Furthermore, vascular calcifications are reported in patients with FTC [25, 59]. Consequently, even without disabling effects of TC lesions, aggressive management of the biochemical profile may be important for patients with hFTC.
Most hFTC treatment regimens involve altering the abnormal biochemical profile to counteract the consequences of insufficient FGF23 action, allowing gradual resolution of the lesions. Thus treatment is targeted at decreasing the intestinal absorption of phosphate, using both dietary phosphate restriction and oral phosphate binding agents, and decreasing tubular reabsorption of phosphate. The FGF23 knockout mouse develops vascular calcifications and is biochemically similar to humans with hFTC. In these mice, while restricting dietary phosphate does decrease serum phosphate and vascular calcifications, vitamin D restriction did not alter these parameters. However, these mice have a shortened lifespan, and although not applicable to human treatment, vitamin D restriction did prolong survival [70].
Phosphate restriction is challenging because phosphate is ubiquitous in the western diet, and is generally very well absorbed. Detailed nutritional education is necessary, and may require dietary restriction as low as 400 mg daily [71]. Classically, dietary phosphate deprivation is combined with aluminum and magnesium based phosphate binders often using high doses. Although several case reports have demonstrated successful decrease in serum phosphate concentrations [71–75] and regression of hFTC lesions [71, 73, 74, 76, 77] with a low phosphate diet combined with phosphate binders (mostly aluminum hydroxide), the results have been inconsistent. However, in many cases these measures have still failed to resolve hFTC lesions, and the response in terms of serum phosphate is highly variable [64, 75, 78–80]. Although hypercalcemia is not typical in TC, calcium based phosphate binders are likely to increase the absorbed calcium and may increase the calcium-phosphorus product which could adversely affect hFTC lesions. Treatment with newer phosphate binders, such as sevalemer has also been reported, with similarly mixed results [35, 64, 80]. However, phosphate binders can be combined with therapies to increase renal phosphate excretion to augment the effect on phosphate balance.
Acetazolamide is a carbonic anhydrase inhibitor that has phosphaturic effect [73, 81, 82], which is independent of PTH as emphasized by both experiments in thyroparathyroidectomized animals [81] and hypoparathyroid or pseudohypoparathyroid humans [82]. This effect has also been demonstrated in hFTC patients [73]. After a single dose of acetazolamide, there is a mild decrease in serum phosphate coinciding with increased urinary phosphate excretion [73]. Long term treatment with acetazolamide combined with sevalemer as a phosphate binding agent has resulted in resolution of a very large hFTC lesion [35], and another case reported lack of recurrence after excision during treatment with acetazolamide [64]. In contrast, other investigators have noted no lesional improvements after acetazolamide [78].
Calcitonin also increases phosphaturia independently of PTH. Calcitonin was demonstrated to increase fractional excretion of phosphate and decrease serum phosphate in hFTC patients [83–85]. However the published response of TC lesions to this treatment is limited. In one patient the TC lesion did not progress during treatment [83], but in two other patients, there was an apparent increase in size of the TC lesions [85].
In fact, data from XLH (a disorder biochemically opposite to hFTC) suggest that calcitonin might actually be harmful as a treatment of TC. At least two studies have demonstrated that administering calcitonin increases serum 1,25D concentration and increases serum phosphate in XLH patients [86, 87]. Furthermore, in one study after infusion of calcitonin, the serum FGF23 concentrations were decreased [87]. These effects on FGF23 and 1,25D would be the opposite of the desired goals for biochemical management of TC. However, although short term calcitonin did increase serum 1,25D in one TC patient [83], the authors noted lack of new lesions during long term treatment.
Other treatments have been tried based on analogy to ectopic calcifications occurring in the setting of inflammatory disorders. Although case reports suggest a possible benefit of bisphosphonates on other forms of calcinosis [88–90], limited reports suggest no benefit for FTC lesions [91, 92]. Other reports have noted lack of benefit with methotrexate or steroids [64, 77]. Since these treatments did not address the primary biochemical problem, it is not surprising that they were ineffective.
From a pathophysiologic sense the ideal treatment for FTC due to GALNT3 or FGF23 mutations would address the deficiency of FGF23, either by administering FGF23 itself or an agonist. Certainly in animal models, administration of FGF23 increases renal phosphate excretion, and lowers serum phosphate [45]. Thus the ability to treat with FGF23 could prevent or eliminate the tumoral calcinosis lesions in subjects with GALNT3 or FGF23 mutations through effects on the biochemical profile. Delivery of αKL protein is unlikely to be an effective treatment for these mutations, as αKL expression is already up-regulated in the Galnt3-null mouse [39] and αKL's role in renal phosphate regulation is largely dependent on the presence of active FGF23.
Conversely, since αKL is necessary for FGF23 action at the renal tubule, FGF23 would likely be an ineffective therapy for FTC due to αKlotho mutations. Although parathyroid hormone decreases renal phosphate reabsorption, PTH also stimulates 1,25D production and increases serum calcium concentrations; thus, despite its phosphaturic effect, the increases in 1,25D and in calcium-phosphate product would likely be harmful rather than beneficial in hFTC.
7. Summary
Familial hyperphosphatemic tumoral calcinosis is a genetically heterogeneous disorder due to mutations in genes that affect FGF23 bioactivity, and result in either reduced intact FGF23 (FGF23 and GALNT3 loss of function mutations) or end organ resistance to serum FGF23 (αKlotho loss of function mutation). Although the field is still challenged with developing reliable therapeutics targeting hFTC, investigating the molecular nature of this syndrome has been invaluable in providing insight into normal and disturbed phosphate homeostasis and could provide novel treatments for more common diseases of phosphate handling and biomineralization.
Table 2.
Medical management of hyperphosphatemic tumoral calcinosis.
| Treatment | Mechanism | Urinary phosphate | Serum phosphate | Serum calcium | Serum 1,25D | Tumoral calcinosis lesions |
|---|---|---|---|---|---|---|
| Dietary phosphate restriction | Decrease phosphate load | ↓ | ↓ | ↔ | ↑ ↔ | ↓ ↔ |
| Phosphate binders | Decrease phosphate load | ↓ | ↓ | ↔ | ↑ ↔ | ↓ ↔ |
| Acetazolamide | Increase phosphate excretion | ↑ | ↓ | ↔ | ↔ | ↓ ↔ |
| Calcitonin | Increase phosphate excretion | ↑ | ↓in TC (but↑ in XLH) | ↔ ↓ | ↑ | ↓ ↔ |
ACKNOWLEDGEMENTS
The authors would like to acknowledge the funding support of NIH through grants R01-DK063934 and R21-AR059278 (KEW), and K23AR057096 (EAI), Genzyme GRIP award (KEW), as well as the Indiana Genomic Initiative (INGEN) of Indiana University, supported in part by Lilly Endowment, Inc. EGF is supported by a fellowship from the National Kidney Foundation (NKF). The sponsors had no role in the collection, analysis and interpretation of data and in the writing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT KEW receives royalties for licensing the FGF23 gene to Kyowa Hakko Kirin Co. Ltd., and EAI is involved in a clinical trial with Kyowa Hakko Kirin Co. Ltd. EGF has no conflicts to declare.
REFERENCES
- 1.Portale AA, Halloran BP, Morris RC., Jr. Physiologic regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men. J Clin Invest. 1989;83:1494–1499. doi: 10.1172/JCI114043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berndt TJ, Knox FG. In: The Kidney: Physiology and Pathophysiology. Seldin DW, Giebisch G, editors. Raven; New York: 1992. pp. 2511–2532. [Google Scholar]
- 3.Gmaj P, Murer H. Cellular mechanisms of inorganic phosphate transport in kidney. Physiol Rev. 1986;66:36–70. doi: 10.1152/physrev.1986.66.1.36. [DOI] [PubMed] [Google Scholar]
- 4.Mizgala CL, Quamme GA. Renal handling of phosphate. Physiol Rev. 1985;65:431–466. doi: 10.1152/physrev.1985.65.2.431. [DOI] [PubMed] [Google Scholar]
- 5.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A. 1998;95:5372–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenenhouse HS, Roy S, Martel J, Gauthier C. Differential expression, abundance, and regulation of Na+-phosphate cotransporter genes in murine kidney. Am J Physiol. 1998;275:F527–534. doi: 10.1152/ajprenal.1998.275.4.F527. [DOI] [PubMed] [Google Scholar]
- 7.Segawa H, Onitsuka A, Kuwahata M, Hanabusa E, Furutani J, Kaneko I, Tomoe Y, Aranami F, Matsumoto N, Ito M, Matsumoto M, Li M, Amizuka N, Miyamoto K. Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J Am Soc Nephrol. 2009;20:104–113. doi: 10.1681/ASN.2008020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006;78:179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ADHR-Consortium Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 10.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 12.Gribaa M, Younes M, Bouyacoub Y, Korbaa W, Ben Charfeddine I, Touzi M, Adala L, Mamay O, Bergaoui N, Saad A. An autosomal dominant hypophosphatemic rickets phenotype in a Tunisian family caused by a new FGF23 missense mutation. J Bone Miner Metab. 2010;28:111–115. doi: 10.1007/s00774-009-0111-5. [DOI] [PubMed] [Google Scholar]
- 13.Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–3182. doi: 10.1210/endo.143.8.8795. [DOI] [PubMed] [Google Scholar]
- 14.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Juppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito T, Shimizu Y, Hori M, Taguchi M, Igarashi T, Fukumoto S, Fujitab T. A patient with hypophosphatemic rickets and ossification of posterior longitudinal ligament caused by a novel homozygous mutation in ENPP1 gene. Bone. 2011;49:913–916. doi: 10.1016/j.bone.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Weidner N, Santa Cruz D. Phosphaturic mesenchymal tumors. A polymorphous group causing osteomalacia or rickets. Cancer. 1987;59:1442–1454. doi: 10.1002/1097-0142(19870415)59:8<1442::aid-cncr2820590810>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 19.Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–414. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 20.Zhang MY, Wang X, Wang JT, Compagnone NA, Mellon SH, Olson JL, Tenenhouse HS, Miller WL, Portale AA. Dietary phosphorus transcriptionally regulates 25-hydroxyvitamin D-1alpha-hydroxylase gene expression in the proximal renal tubule. Endocrinology. 2002;143:587–595. doi: 10.1210/endo.143.2.8627. [DOI] [PubMed] [Google Scholar]
- 21.Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic Mice Overexpressing Human Fibroblast Growth Factor 23(R176Q) Delineate a Putative Role for Parathyroid Hormone in Renal Phosphate Wasting Disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- 22.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garringer HJ, Mortazavi SM, Esteghamat F, Malekpour M, Boztepe H, Tanakol R, Davis SI, White KE. Two novel GALNT3 mutations in familial tumoral calcinosis. Am J Med Genet A. 2007;143:2390–2396. doi: 10.1002/ajmg.a.31947. [DOI] [PubMed] [Google Scholar]
- 25.Larsson T, Yu X, Davis SI, Draman MS, Mooney SD, Cullen MJ, White KE. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab. 2005;90:2424–2427. doi: 10.1210/jc.2004-2238. [DOI] [PubMed] [Google Scholar]
- 26.Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 28.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 29.Imel EA, Peacock M, Pitukcheewanont P, Heller HJ, Ward LM, Shulman D, Kassem M, Rackoff P, Zimering M, Dalkin A, Drobny E, Colussi G, Shaker JL, Hoogendoorn EH, Hui SL, Econs MJ. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2006;91:2055–2061. doi: 10.1210/jc.2005-2105. [DOI] [PubMed] [Google Scholar]
- 30.Araya K, Fukumoto S, Backenroth R, Takeuchi Y, Nakayama K, Ito N, Yoshii N, Yamazaki Y, Yamashita T, Silver J, Igarashi T, Fujita T. A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J Clin Endocrinol Metab. 2005;90:5523–5527. doi: 10.1210/jc.2005-0301. [DOI] [PubMed] [Google Scholar]
- 31.Larsson T, Davis SI, Garringer HJ, Mooney SD, Draman MS, Cullen MJ, White KE. Fibroblast growth factor-23 mutants causing familial tumoral calcinosis are differentially processed. Endocrinology. 2005;146:3883–3891. doi: 10.1210/en.2005-0431. [DOI] [PubMed] [Google Scholar]
- 32.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, H JA-P, Lanske B. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 35.Garringer HJ, Fisher C, Larsson TE, Davis SI, Koller DL, Cullen MJ, Draman MS, Conlon N, Jain A, Fedarko NS, Dasgupta B, White KE. The role of mutant UDP-N-acetyl-alpha-D-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 in regulating serum intact fibroblast growth factor 23 and matrix extracellular phosphoglycoprotein in heritable tumoral calcinosis. J Clin Endocrinol Metab. 2006;91:4037–4042. doi: 10.1210/jc.2006-0305. [DOI] [PubMed] [Google Scholar]
- 36.Kato K, Jeanneau C, Tarp MA, Benet-Pages A, Lorenz-Depiereux B, Bennett EP, Mandel U, Strom TM, Clausen H. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281:18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa S, Imel EA, Sorenson AH, Severe R, Knudson P, Harris GJ, Shaker JL, Econs MJ. Tumoral calcinosis presenting with eyelid calcifications due to novel missense mutations in the glycosyl transferase domain of the GALNT3 gene. J Clin Endocrinol Metab. 2006;91:4472–4475. doi: 10.1210/jc.2006-1247. [DOI] [PubMed] [Google Scholar]
- 38.Frishberg Y, Ito N, Rinat C, Yamazaki Y, Feinstein S, Urakawa I, Navon-Elkan P, Becker-Cohen R, Yamashita T, Araya K, Igarashi T, Fujita T, Fukumoto S. Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res. 2007;22:235–242. doi: 10.1359/jbmr.061105. [DOI] [PubMed] [Google Scholar]
- 39.Ichikawa S, Sorenson AH, Austin AM, Mackenzie DS, Fritz TA, Moh A, Hui SL, Econs MJ. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology. 2009;150:2543–2550. doi: 10.1210/en.2008-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 41.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 42.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 43.Yu X, Sabbagh Y, Davis SI, Demay MB, White KE. Genetic dissection of phosphate-and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone. 2005;36:971–977. doi: 10.1016/j.bone.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Ito M, Sakai Y, Furumoto M, Segawa H, Haito S, Yamanaka S, Nakamura R, Kuwahata M, Miyamoto K. Vitamin D and phosphate regulate fibroblast growth factor-23 in K-562 cells. Am J Physiol Endocrinol Metab. 2005;288:E1101–1109. doi: 10.1152/ajpendo.00502.2004. [DOI] [PubMed] [Google Scholar]
- 45.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 46.Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036–1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 47.Bergwitz C, Banerjee S, Abu-Zahra H, Kaji H, Miyauchi A, Sugimoto T, Juppner H. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis. J Clin Endocrinol Metab. 2009;94:4267–4274. doi: 10.1210/jc.2009-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:1–12. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 51.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 52.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 53.Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 54.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farrow EG, Summers LJ, Schiavi SC, McCormick JA, Ellison DH, White KE. Altered renal FGF23-mediated activity involving MAPK and Wnt: effects of the Hyp mutation. J Endocrinol. 2010;207:67–75. doi: 10.1677/JOE-10-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 57.Li H, Martin A, David V, Quarles LD. Compound deletion of Fgfr3 and Fgfr4 partially rescues the Hyp mouse phenotype. Am J Physiol Endocrinol Metab. 2011;300:E508–517. doi: 10.1152/ajpendo.00499.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nabeshima Y. Ectopic calcification in Klotho mice. Clin Calcium. 2002;12:1114–1117. [PubMed] [Google Scholar]
- 61.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Molecular endocrinology (Baltimore, Md. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 62.Mikati MA, Melhem RE, Najjar SS. The syndrome of hyperostosis and hyperphosphatemia. J Pediatr. 1981;99:900–904. doi: 10.1016/s0022-3476(81)80013-3. [DOI] [PubMed] [Google Scholar]
- 63.Frishberg Y, Topaz O, Bergman R, Behar D, Fisher D, Gordon D, Richard G, Sprecher E. Identification of a recurrent mutation in GALNT3 demonstrates that hyperostosis-hyperphosphatemia syndrome and familial tumoral calcinosis are allelic disorders. J Mol Med. 2005;83:33–38. doi: 10.1007/s00109-004-0610-8. [DOI] [PubMed] [Google Scholar]
- 64.Ichikawa S, Baujat G, Seyahi A, Garoufali AG, Imel EA, Padgett LR, Austin AM, Sorenson AH, Pejin Z, Topouchian V, Quartier P, Cormier-Daire V, Dechaux M, Malandrinou F, Singhellakis PN, Le Merrer M, Econs MJ. Clinical variability of familial tumoral calcinosis caused by novel GALNT3 mutations. Am J Med Genet A. 2010;152A:896–903. doi: 10.1002/ajmg.a.33337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lafferty FW, Reynolds ES, Pearson OH. Tumoral Calcinosis: A Metabolic Disease of Obscure Etiology. Am J Med. 1965;38:105–118. doi: 10.1016/0002-9343(65)90164-6. [DOI] [PubMed] [Google Scholar]
- 66.Yancovitch A, Hershkovitz D, Indelman M, Galloway P, Whiteford M, Sprecher E, Kilic E. Novel mutations in GALNT3 causing hyperphosphatemic familial tumoral calcinosis. J Bone Miner Metab. 2011;29:621–625. doi: 10.1007/s00774-011-0260-1. [DOI] [PubMed] [Google Scholar]
- 67.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB, Sr., Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Archives of internal medicine. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 68.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 70.Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007;18:2116–2124. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 71.Gregosiewicz A, Warda E. Tumoral calcinosis: successful medical treatment. A case report. J Bone Joint Surg Am. 1989;71:1244–1249. [PubMed] [Google Scholar]
- 72.Lufkin EG, Kumar R, Heath H., 3rd Hyperphosphatemic tumoral calcinosis: effects of phosphate depletion on vitamin D metabolism, and of acute hypocalcemia on parathyroid hormone secretion and action. J Clin Endocrinol Metab. 1983;56:1319–1322. doi: 10.1210/jcem-56-6-1319. [DOI] [PubMed] [Google Scholar]
- 73.Lufkin EG, Wilson DM, Smith LH, Bill NJ, DeLuca HF, Dousa TP, Knox FG. Phosphorus excretion in tumoral calcinosis: response to parathyroid hormone and acetazolamide. J Clin Endocrinol Metab. 1980;50:648–653. doi: 10.1210/jcem-50-4-648. [DOI] [PubMed] [Google Scholar]
- 74.Mozaffarian G, Nakhjavani MK, Hedayati MH, Shamekh S. Phosphorus deprivation treatment of tumoral calcinosis. Ann Intern Med. 1977;86:120. doi: 10.7326/0003-4819-86-1-120_2. [DOI] [PubMed] [Google Scholar]
- 75.Alkhooly AZ. Medical treatment for tumoral calcinosis with eight years of follow-up: a report of four cases. J Orthop Surg (Hong Kong) 2009;17:379–382. doi: 10.1177/230949900901700328. [DOI] [PubMed] [Google Scholar]
- 76.Janssen MC, de Sevaux RG. Tumoral calcinosis. J Inherit Metab Dis. 2010;33:91–92. doi: 10.1007/s10545-009-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mozaffarian G, Lafferty FW, Pearson OH. Treatment of tumoral calcinosis with phosphorus deprivation. Ann Intern Med. 1972;77:741–745. doi: 10.7326/0003-4819-77-5-741. [DOI] [PubMed] [Google Scholar]
- 78.Yamaguchi T, Sugimoto T, Imai Y, Fukase M, Fujita T, Chihara K. Successful treatment of hyperphosphatemic tumoral calcinosis with long-term acetazolamide. Bone. 1995;16:247S–250S. doi: 10.1016/8756-3282(95)00019-a. [DOI] [PubMed] [Google Scholar]
- 79.Alves C, Lima R. Hyperphosphatemic tumoral calcinosis: a 10-year follow-up. J Pediatr Endocrinol Metab. 2011;24:25–27. doi: 10.1515/jpem.2011.106. [DOI] [PubMed] [Google Scholar]
- 80.Campagnoli MF, Pucci A, Garelli E, Carando A, Defilippi C, Lala R, Ingrosso G, Dianzani I, Forni M, Ramenghi U. Familial tumoral calcinosis and testicular microlithiasis associated with a new mutation of GALNT3 in a white family. Journal of clinical pathology. 2006;59:440–442. doi: 10.1136/jcp.2005.026369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knox FG, Haas JA, Lechene CP. Effect of parathyroid hormone on phosphate reabsorption in the presence of acetazolamide. Kidney Int. 1976;10:216–220. doi: 10.1038/ki.1976.100. [DOI] [PubMed] [Google Scholar]
- 82.Sinha TK, Allen DO, Queener SF, Bell NH, Larson S, McClintock R. Effects of acetazolamide on the renal excretion of phosphate in hypoparathyroidism and pseudohypoparathyroidism. J Lab Clin Med. 1977;89:1188–1197. [PubMed] [Google Scholar]
- 83.Candrina R, Cerudelli B, Braga V, Salvi A. Effects of the acute subcutaneous administration of synthetic salmon calcitonin in tumoral calcinosis. J Endocrinol Invest. 1989;12:55–57. doi: 10.1007/BF03349921. [DOI] [PubMed] [Google Scholar]
- 84.Salvi A, Cerudelli B, Cimino A, Zuccato F, Giustina G. Phosphaturic action of calcitonin in pseudotumoral calcinosis. Horm Metab Res. 1983;15:260. doi: 10.1055/s-2007-1018689. [DOI] [PubMed] [Google Scholar]
- 85.Kallmeyer JC, Seimon LP, MacSearraigh ET. The effect of thyrocalcitonin therapy and phosphate deprivation on tumoral calcinosis. S Afr Med J. 1978;54:963–966. [PubMed] [Google Scholar]
- 86.Econs MJ, Lobaugh B, Drezner MK. Normal Calcitonin Stimulation of Serum Calcitriol in Patients with X-linked Hypophosphatemic Rickets. Journal of Clinical Endocrinology and Metabolism. 1992;75:408–411. doi: 10.1210/jcem.75.2.1639943. [DOI] [PubMed] [Google Scholar]
- 87.Liu ES, Carpenter TO, Gundberg CM, Simpson CA, Insogna KL. Calcitonin administration in X-linked hypophosphatemia. N Engl J Med. 2011;364:1678–1680. doi: 10.1056/NEJMc1010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ambler GR, Chaitow J, Rogers M, McDonald DW, Ouvrier RA. Rapid improvement of calcinosis in juvenile dermatomyositis with alendronate therapy. J Rheumatol. 2005;32:1837–1839. [PubMed] [Google Scholar]
- 89.Marco Puche A, Calvo Penades I, Lopez Montesinos B. Effectiveness of the treatment with intravenous pamidronate in calcinosis in juvenile dermatomyositis. Clin Exp Rheumatol. 2010;28:135–140. [PubMed] [Google Scholar]
- 90.Slimani S, Abdessemed A, Haddouche A, Ladjouze-Rezig A. Complete resolution of universal calcinosis in a patient with juvenile dermatomyositis using pamidronate. Joint Bone Spine. 2010;77:70–72. doi: 10.1016/j.jbspin.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 91.Hug I, Guncaga J. Tumoral calcinosis with sedimentation sign. Br J Radiol. 1974;47:734–736. doi: 10.1259/0007-1285-47-562-734. [DOI] [PubMed] [Google Scholar]
- 92.Leicht E, Tkocz HJ, Seeliger H, Lauffenburger T, Haas HG. Tumoral calcinosis. Observations during six years. Horm Metab Res. 1980;12:269–273. doi: 10.1055/s-2007-996264. [DOI] [PubMed] [Google Scholar]