Abstract
Preclinical studies show that stress is associated with changes in structure of the hippocampus, a brain area that plays a critical role in memory, inhibition of neurogenesis, and memory deficits. Studies in animals showed that both serotonin reuptake inhibitors (SSRIs) and the epilepsy medication phenytoin (dilantin) block the effects of stress on the hippocampus. Imaging studies in posttraumatic stress disorder (PTSD) have found smaller volume of the hippocampus as measured with magnetic resonance imaging (MRI) in patients with PTSD related to both combat and childhood abuse. These patients were also found to have deficits in memory on neuropsychological testing. Functional imaging studies using positron emission tomography (PET) found decreased hippocampal activation with memory tasks. In an initial study, we found that a year of treatment with paroxetine led to a 5% increase in hippocampal volume and a 35% increase in memory function. A second study showed that phenytoin was efficacious for symptoms of PTSD and led to a significant 6% increase in both right hippocampal and right whole brain volume, with no significant change in memory. These studies suggest that medications may counteract the effects of stress on the brain in patients with PTSD.
Keywords: PTSD, hippocampus, pharmacotherapy, stress, neurogenesis, paroxetine, depression
Patients with posttraumatic stress disorder (PTSD) exhibit a broad range of problems with memory, including gaps in memory, problems with declarative memory, attentional biases to trauma-related information, and intrusive memories. The past two decades of research have seen a convergence of findings from the clinical observations, and clinical and preclinical research.
Studies in animals exposed to stress showed deficits in hippocampal-based memory function1 and alterations in hippocampal morphology.2, 3 Stress interfered with hippocampal-based mechanisms of memory function, including long-term potentiation (LTP).1, 4 Mechanisms proposed for these findings include elevated levels of glucocorticoids released during stress,5, 6 stress-related inhibition of brain-derived neurotrophic factor (BDNF),7, 8 changes in serotonergic function,9 or inhibition of neurogenesis (or the growth of new neurons)10, 11 in the hippocampus. These effects are reversible with treatment with serotonin reuptake inhibitor (SSRI) medications,12–14 tianeptine,15 and phenytoin.16 Antidepressant-induced promotion of neurogenesis may underlie the behavioral effects of these medications,17 although the relationship between the hippocampus and depression and PTSD is still not clear.18
Studies have used neuropsychological testing as a probe of brain function in PTSD. Several studies that have demonstrated verbal declarative memory deficits in PTSD19–22 using a variety of measures (including the Wechsler Memory Scale, the visual and verbal components of the Selective Reminding Test, the Auditory Verbal Learning Test, the California Verbal New Learning Test, and the Rivermead Behavioral Memory Test), found specific deficits in verbal declarative memory function23–36 (although see Refs. 37 and 38). These studies have been conducted in both patients with PTSD related to Vietnam combat,23, 25, 28–33, 35, 36 rape,26 adults with early childhood abuse,24 and traumatized children.27 Studies in women with PTSD showed that verbal declarative memory deficits are specifically associated with PTSD, and are not a non-specific effect of trauma exposure.26, 39 Other types of memory disturbances studies in PTSD include gaps in memory for everyday events (dissociative amnesia),40 deficits in autobiographical memory,41 false recall of material,42, 43 an attentional bias for trauma-related material,44–53 and frontal lobe-related impairments.54 These studies fairly consistently show that PTSD is associated with deficits memory that covers a range of categories.
Neuroimaging studies showed alterations in the hippocampus in PTSD. Magnetic resonance imaging (MRI) showed smaller volume of the hippocampus in PTSD; decreases in right hippocampal volume in the PTSD patients were associated with deficits in short-term memory.55 Findings of smaller hippocampal volume and/or a reduction in NAA in the hippocampus (a marker of neuronal integrity) in adults with chronic, long-standing PTSD have been replicated several times in the published literature.56–63 Studies in childhood64–66 and new onset67, 68 PTSD did not find hippocampal volume reduction, although reduced NAA (indicating loss of neuronal integrity) was found in medial pre-frontal cortex in childhood PTSD.69 In a recent meta-analysis we pooled data from all of the published studies and found smaller hippocampal volume for both the left and the right sides, equally in adult men and women with chronic PTSD, and no change in children.70 PTSD patients showed deficits in hippocampal activation while performing a verbal declarative memory task.63, 71 Both hippocampal atrophy and hippocampal-based memory deficits reversed with treatment with the SSRI, paroxetine, which has been shown to promote neurogenesis (the growth of neurons) in the hippocampus in preclinical studies.72 Treatment with phenytoin resulted in improved PTSD symptoms,73 a 6% increase in right cerebral volume and 5% increase in right hippocampal volume.74 These findings suggested that long-term treatment with paroxetine or phenytoin is associated with changes in brain structure that may underlie improvement in symptoms.72
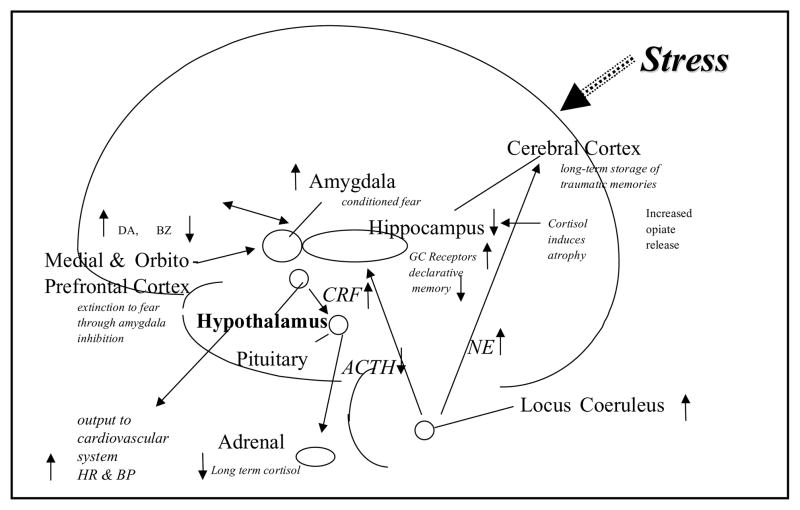
We hypothesize that stress-induced hippocampal dysfunction may mediate many of the symptoms of PTSD that are related to memory dysregulation, including both explicit memory deficits as well as fragmentation of memory in abuse survivors (FIG.1). It is unclear at the current time whether these changes are specific to PTSD, whether certain common environmental events (e.g., stress) in different disorders lead to similar brain changes, or whether common genetic traits lead to similar outcomes.75 Obviously, the increase in hippocampal volume with medication treatments known to promote neurogenesis in preclinical studies is not consistent with a pure genetic contribution to smaller hippocampal volume in PTSD.
FIGURE 1.
Lasting effects of trauma on the brain, showing long-term dysregulation of norepinephrine and cortisol systems, and vulnerable areas of hippocampus, amygdala, and medial prefrontal cortex that are affected by trauma.
Acknowledgments
The research reviewed in this paper was supported by grants from GlaxoSmithKline, a VA Research Career Development Award to Dr. Bremner, NIMH R01 MH56120 to Dr. Bremner, and the Emory Conte Center for Early Life Stress.
References
- 1.Luine V, et al. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 2.Uno H, et al. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sapolsky RM, et al. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond DM, et al. Psychological stress impairs spatial working memory: relevance to electrophysiological studies of hippocampal function. Behav Neurosci. 1996;110:661–672. doi: 10.1037//0735-7044.110.4.661. [DOI] [PubMed] [Google Scholar]
- 5.Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence MS, Sapolsky RM. Glucocorticoids accelerate ATP loss following metabolic insults in cultured hippocampal neurons. Brain Res. 1994;646:303–306. doi: 10.1016/0006-8993(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 7.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MA, et al. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNA in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEwen BS, et al. Paradoxical effects of adrenal steroids on the brain: protection versus degeneration. Biol Psychiatry. 1992;31:177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- 10.Gould E, et al. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler CD, et al. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2001;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- 12.Malberg JE, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duman RS, Malberg JE, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- 14.Lee H, et al. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Mol Psychiatry. 2001;6:725–728. doi: 10.1038/sj.mp.4000954. [DOI] [PubMed] [Google Scholar]
- 15.Czeh B, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe Y, et al. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- 17.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 18.Vollmayr B, et al. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry. 2003;54:1035–1040. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- 19.Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD: a review of the empirical literature. Clin Psychol Rev. 2000;28:1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- 20.Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in PTSD? J Affect Disord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brewin CR. A cognitive neuroscience account of post-traumatic stress disorder and its treatment. Behav Res Therapy. 2001;39:373–393. doi: 10.1016/s0005-7967(00)00087-5. [DOI] [PubMed] [Google Scholar]
- 22.Golier J, Yehuda R. Neuroendocrine activity and memory-related impairments in posttraumatic stress disorder. Dev Psychopathol. 1998;10:857–869. doi: 10.1017/s0954579498001904. [DOI] [PubMed] [Google Scholar]
- 23.Bremner JD, et al. Deficits in short-term memory in post-traumatic stress disorder. Am J Psychiatry. 1993;150:1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- 24.Bremner JD, et al. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res. 1995;59:97–107. doi: 10.1016/0165-1781(95)02800-5. [DOI] [PubMed] [Google Scholar]
- 25.Gilbertson MW, et al. Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J Trauma Stress. 2001;14:413–420. doi: 10.1023/A:1011181305501. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins MA, et al. Learning and memory in rape victims with posttraumatic stress disorder. Am J Psychiatry. 1998;155:278–279. doi: 10.1176/ajp.155.2.278. [DOI] [PubMed] [Google Scholar]
- 27.Moradi AR, et al. Everyday memory deficits in children and adolescents with PTSD: performance on the Rivermead Behavioural Memory Test. J Child Psychol Psychiatry. 1999;40:357–361. [PubMed] [Google Scholar]
- 28.Roca V, Freeman TW. Complaints of impaired memory in veterans with PTSD. Am J Psychiatry. 2001;158:1738. doi: 10.1176/appi.ajp.158.10.1738-a. [DOI] [PubMed] [Google Scholar]
- 29.Uddo M, et al. Memory and attention in posttraumatic stress disorder. J Psychopathol Behav Assess. 1993;15:43–52. [Google Scholar]
- 30.Vasterling JJ, et al. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- 31.Vasterling JJ, et al. Attention, learning, and memory performance and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- 32.Yehuda R, et al. Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1995;152:137–139. doi: 10.1176/ajp.152.1.137. [DOI] [PubMed] [Google Scholar]
- 33.Barrett DH, et al. Cognitive functioning and posttraumatic stress disorder. Am J Psychiatry. 1996;153:1492–1494. doi: 10.1176/ajp.153.11.1492. [DOI] [PubMed] [Google Scholar]
- 34.Gil T, et al. Cognitive functioning in posttraumatic stress disorder. J Trauma Stress. 1990;3:29–45. [Google Scholar]
- 35.Sachinvala N, et al. Memory, attention, function, and mood among patients with chronic posttraumatic stress disorder. J Nerv Ment Dis. 2000;188:818–823. doi: 10.1097/00005053-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Golier J, et al. Sustained attention in combat-related posttraumatic stress disorder. Integr Physiol Behav Sci. 1997;32:52–61. doi: 10.1007/BF02688613. [DOI] [PubMed] [Google Scholar]
- 37.Stein MB, et al. Memory functioning in adult women traumatized by childhood sexual abuse. J Trauma Stress. 1999;12:527–534. doi: 10.1023/A:1024775222098. [DOI] [PubMed] [Google Scholar]
- 38.Zalewski C, Thompson W, Gottesman I. Comparison of neuropsychological test performance in PTSD, generalized anxiety disorder, and control Vietnam veterans. Assessment. 1994;1:133–142. doi: 10.1177/1073191194001002003. [DOI] [PubMed] [Google Scholar]
- 39.Bremner JD, et al. Deficits in verbal declarative memory function in women with childhood sexual abuse-related posttraumatic stress disorder (PTSD) J Nerv Ment Dis. 2004;192:643–649. doi: 10.1097/01.nmd.0000142027.52893.c8. [DOI] [PubMed] [Google Scholar]
- 40.Bremner JD, et al. Use of the structured clinical interview for DSMIV-dissociative disorders for systematic assessment of dissociative symptoms in posttraumatic stress disorder. Am J Psychiatry. 1993;150:1011–1014. doi: 10.1176/ajp.150.7.1011. [DOI] [PubMed] [Google Scholar]
- 41.McNally RJ, et al. Emotional priming of autobiographical memory in posttraumatic stress disorder. Cogn Emotion. 1994;8:351–367. [Google Scholar]
- 42.Bremner JD, Shobe KK, Kihlstrom JF. False memories in women with self-reported childhood sexual abuse: an empirical study. Psychol Sci. 2000;11:333–337. doi: 10.1111/1467-9280.00266. [DOI] [PubMed] [Google Scholar]
- 43.Clancy SA, et al. False recognition in women reporting recovered memories of sexual abuse. Psychol Sci. 2000;11:26–31. doi: 10.1111/1467-9280.00210. [DOI] [PubMed] [Google Scholar]
- 44.Cassiday KL, McNally RJ, Zeitlin SB. Cognitive processing of trauma cues in rape victims with posttraumatic stress disorder. Cogn Ther Res. 1992;16:283–295. [Google Scholar]
- 45.Foa EB, et al. Processing of threat related information in rape victims. J Abnorm Psychol. 1991;100:156–162. doi: 10.1037//0021-843x.100.2.156. [DOI] [PubMed] [Google Scholar]
- 46.McNally RJ, et al. Selective processing of threat cues in posttraumatic stress disorder. J Abnorm Psychol. 1990;99:398–402. doi: 10.1037//0021-843x.99.4.398. [DOI] [PubMed] [Google Scholar]
- 47.McNally RJ, English GE, Lipke HJ. Assessment of intrusive cognition in PTSD: use of the modified Stroop paradigm. J Trauma Stress. 1993;6:33–41. [Google Scholar]
- 48.Moradi AR, et al. Memory bias for emotional information in children and adolescents with posttraumatic stress disorder: a preliminary study. J Anxiety Disord. 2000;14:521–534. doi: 10.1016/s0887-6185(00)00037-2. [DOI] [PubMed] [Google Scholar]
- 49.Bryant RA, Harvey AG. Processing threatening information in post-traumatic stress disorder. J Abnorm Psychol. 1995;104:537–541. doi: 10.1037//0021-843x.104.3.537. [DOI] [PubMed] [Google Scholar]
- 50.Beck JG, et al. Specificity of Stroop interference in patients with pain and PTSD. J Abnorm Psychol. 2001;110:536–543. doi: 10.1037//0021-843x.110.4.536. [DOI] [PubMed] [Google Scholar]
- 51.McNeil DW, et al. Response to depression and anxiety Stroop stimuli in posttraumatic stress disorder, obsessive-compulsive disorder and major depressive disorder. J Nerv Ment Dis. 1999;187:512–516. doi: 10.1097/00005053-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Thrasher SM, Dalgleish T, Yule W. Information processing in post-traumatic stress disorder. Behav Res Ther. 1994;32:247–254. doi: 10.1016/0005-7967(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 53.Constans JI, et al. Suppression of attentional bias in PTSD. J Abnorm Psychol. 2004;113:315–323. doi: 10.1037/0021-843X.113.2.315. [DOI] [PubMed] [Google Scholar]
- 54.Beckham JC, Crawford AL, Feldman ME. Trail making test performance in Vietnam combat veterans with and without posttraumatic stress disorder. J Trauma Stress. 1998;11:811–819. doi: 10.1023/A:1024409903617. [DOI] [PubMed] [Google Scholar]
- 55.Bremner JD, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gurvits TG, et al. Magnetic resonance imaging study of hippocampal volume in chronic combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:192–199. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villareale G, et al. Reduced hippocampal volume and total white matter in posttraumatic stress disorder. Biol Psychiatry. 2002;15:119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- 58.Bremner JD, et al. MRI-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bremner JD, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder (PTSD) Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 60.Stein MB, et al. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 61.Freeman TW, et al. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn Reson Med. 1998;40:66–71. doi: 10.1002/mrm.1910400110. [DOI] [PubMed] [Google Scholar]
- 62.Schuff N, et al. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001;50:952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bremner JD, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder (PTSD) Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 64.De Bellis MD, et al. A.E. Bennett Research Award: developmental traumatology: part II. Brain development Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 65.Carrion VG, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 66.De Bellis MD, et al. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 67.Bonne O, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Notestine CF, et al. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;51:1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 69.De Bellis MD, et al. N-acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am J Psychiatry. 2000;157:1175–1177. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- 70.Kitayama N, et al. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88(1):79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 71.Shin LM, et al. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14:292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- 72.Vermetten E, et al. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bremner JD, et al. Treatment of posttraumatic stress disorder with phenytoin: an open label pilot study. J Clin Psychiatry. 2004;65:1559–1564. doi: 10.4088/jcp.v65n1120. [DOI] [PubMed] [Google Scholar]
- 74.Bremner JD, et al. Effects of phenytoin on memory, cognition and brain structure in posttraumatic stress disorder: a pilot study. J Psychopharmacol. 2005;19(2):159–165. doi: 10.1177/0269881105048996. [DOI] [PubMed] [Google Scholar]
- 75.Gilbertson MW, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]