Abstract
Objective
Smaller hippocampal volume has been reported in several stress-related psychiatric disorders, including posttraumatic stress disorder (PTSD), borderline personality disorder with early abuse, and depression with early abuse. Patients with borderline personality disorder and early abuse have also been found to have smaller amygdalar volume. The authors examined hippocampal and amygdalar volumes in patients with dissociative identity disorder, a disorder that has been associated with a history of severe childhood trauma.
Method
The authors used magnetic resonance imaging to measure the volumes of the hippocampus and amygdala in 15 female patients with dissociative identity disorder and 23 female subjects without dissociative identity disorder or any other psychiatric disorder. The volumetric measurements for the two groups were compared.
Results
Hippocampal volume was 19.2% smaller and amygdalar volume was 31.6% smaller in the patients with dissociative identity disorder, compared to the healthy subjects. The ratio of hippocampal volume to amygdalar volume was significantly different between groups.
Conclusions
The findings are consistent with the presence of smaller hippocampal and amygdalar volumes in patients with dissociative identity disorder, compared with healthy subjects.
Dissociative identity disorder is characterized by the presence of two or more identities or personality states, each with its own relatively enduring pattern of perceiving, relating to, and thinking about the environment and the self (DSM-IV-TR). At least two of these identities recurrently take control of the person’s behavior. Dissociative identity disorder is frequently accompanied by dissociative amnesia, characterized by an inability to recall important personal information that is too extensive to be explained by ordinary forgetfulness. Patients with dissociative identity disorder also commonly experience a range of other symptoms, including depersonalization, derealization, spontaneous autohypnotic symptoms, pseudopsychotic symptoms such as passive influence from and/or hearing the hallucinated voices of alter identities, and multiple somato-form symptoms (1). In clinical studies, most patients with dissociative identity disorder have also been found to meet the DSM-IV-TR criteria for posttraumatic stress disorder (PTSD) (2). In epidemiological studies of the general population, the prevalence of dissociative identity disorder has been found to range from 1% to 3% (3).
Numerous studies have shown an association between a dissociative identity disorder diagnosis and an antecedent history of childhood trauma, usually multiple, sustained forms of maltreatment beginning in early childhood (4–8). Although the accuracy of recall in patients with dissociative identity disorder has been debated, patients’ accounts of maltreatment have been independently verified in several studies by using corollary history from family informants, childhood medical records, and social service documents (6, 9). Accordingly, the disorder has been conceptualized as a childhood-onset posttraumatic developmental disorder (10, 11). Despite these findings, essentially nothing is known about the neurobiology of dissociative identity disorder.
In both animal and human studies, early stress has been shown to be associated with changes in the structure of the hippocampus, which plays a critical role in learning, memory, and stress regulation (12, 13). Magnetic resonance imaging (MRI) studies have shown that adults with PTSD related to combat or to childhood physical/sexual abuse have smaller hippocampal size, relative to healthy comparison subjects or to comparison subjects who experienced trauma but did not develop PTSD (14–16). Borderline personality disorder is also commonly associated with exposure to childhood trauma (17). Patients with borderline personality disorder and a history of early abuse have been found to have smaller hippocampal and amygdalar volumes, compared to healthy subjects (18–20). Smaller hippocampal volume has been found in patients with major depressive disorder and a history of early childhood trauma, compared to major depressive disorder patients without early life trauma (21). A consistent finding of these studies is smaller hippocampal volume in patients with a history of exposure to traumatic stress and an accompanying stress-related psychiatric disorder (22). The relationship of early stress and accompanying psychiatric disorders to amygdalar volume is less clear.
The purpose of this study was to compare hippocampal and amygdalar volumes in patients with dissociative identity disorder with those in healthy subjects with no psycho-pathology. We hypothesized that smaller hippocampal and amygdalar volumes would be found in patients with dissociative identity disorder, compared with healthy subjects.
Method
Subjects
Fifteen patients with dissociative identity disorder were compared to 23 healthy subjects without psychiatric disorder. All participating subjects were female. Dissociative identity disorder patients were recruited through the outpatient Trauma Disorders Program of Sheppard Pratt Health System in Baltimore. The diagnosis of dissociative identity disorder was established with the Structured Clinical Interview for DSM-IV Dissociative Disorders (SCID-D) (23) and a consensus diagnosis of two psychiatrists using the DSM-IV-TR criteria and the results of the SCID-D interview. Dissociative identity disorder patients were assessed for a diagnosis of PTSD; a consensus diagnosis of PTSD was based on the DSM-IV-TR criteria for PTSD and a score of more than 50 on the Clinician-Administered PTSD Scale (24). Healthy comparison subjects were assessed for the occurrence of any psychiatric disorder with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-NP) (25) and for the presence of dissociative disorders with the SCID-D. History of childhood trauma was assessed with the Early Trauma Inventory, a reliable and valid instrument for measurement of self-reported early trauma (26). Patients and comparison subjects were excluded if they had a serious medical or neurological illness, including hypertension or diabetes, as determined on the basis of the medical history, physical examination, and laboratory test findings; current exposure to oral or intravenous steroids; Cushing’s disease; and/or organic mental or psychotic disorders. Subjects with retained metal (e.g., shrapnel); a history of head trauma, defined as a hit on the head with subsequent loss of consciousness more than 60 seconds or skull fracture; or a history of cerebral infectious disease were also excluded from the study. Comparison subjects with a current psychiatric disorder on the basis of the SCID-NP and SCID-D assessment were also excluded. Dissociative identity disorder subjects were excluded if they met the DSM-IV-TR criteria for schizophrenia or a current episode of bipolar disorder.
All participants gave written informed consent before participation in the study. The study was approved by the Investigational Review Boards of Yale University School of Medicine and the Sheppard Pratt Health System and was prepared in accordance with the ethical standards laid down in the Declaration of Helsinki.
The dissociative identity disorder patients in this study all had a history of multiple hospitalizations and a relatively chronic course. All 15 patients met the criteria for a comorbid diagnosis of PTSD. Their mean score on the Clinician-Administered PTSD Scale was 84.8 (SD=21.1). In addition, they demonstrated the symptoms of other comorbid disorders besides PTSD. Fourteen patients met the criteria for severe recurrent major depression, and one had a prior history of bipolar disorder episodes. Four patients had a past history of alcohol abuse/dependence. Two of these patients also had a history of abuse of other substances besides alcohol. One patient met the criteria for somatization disorder.
None of the comparison group participants met the criteria for a current psychiatric illness. The comparison group included participants with a past history of major depressive disorder (N=6), PTSD (N=2), panic disorder with agoraphobia (N=1), obsessive-compulsive disorder (N=1), generalized anxiety disorder (N=1), mood disorder secondary to a general medical condition (N=1), and alcohol/cocaine/cannabis dependence (N=1/1/1).
All dissociative identity disorder patients were right-handed. In the comparison group, three subjects were left-handed.
All comparison subjects were medication free at the time of the study. All dissociative identity disorder patients were taking medication at the time of the study. Most of the patients received a variety of psychiatric medications, including antidepressants (N= 15), atypical antipsychotics (N=6), benzodiazepines (N=9), anxiolytics (N=1), estrogens (N=2), opiate antagonists (N=3), psycho-stimulants (N=2), and hypnotics (N=2). In addition, nine dissociative identity disorder patients received various combinations of nonpsychiatric medications.
All patients in the dissociative identity disorder group (100%) had a self-reported history of childhood sexual and/or physical abuse on the basis of assessment with the Early Trauma Inventory. Ten women in the comparison group (46%) positively endorsed Early Trauma Inventory items concerning childhood trauma.
The demographic characteristics of the study subjects are summarized in Table 1. The mean age was 42.7 years (SD=8.7) in the dissociative identity disorder group and 34.6 years (SD=7.7) in the comparison group, a significant difference (t=3.00, df=36, p= 0.005). There was no significant difference between groups in the number of years of education. In the dissociative identity disorder group, one patient was African American and 14 were Caucasian. In the comparison group, five subjects were African American, 17 were Caucasian, and one was Hispanic.
TABLE 1.
Demographic Characteristics of Patients With Dissociative Identity Disorder and Healthy Comparison Subjects in an MRI Study of Hippocampal and Amygdalar Volumes
| Characteristic | Patients With Dissociative Identity Disorder (N=15) | Healthy Comparison Subjects (N=23) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
|
| ||||
| Age (years) | 42.8 | 8.7 | 34.6 | 7.7 |
| Years of education | 16.1 | 3.4 | 17.0 | 2.2 |
|
| ||||
| N | % | N | % | |
|
| ||||
| Race | ||||
| Caucasian | 14 | 93 | 17 | 74 |
| African American | 1 | 7 | 5 | 22 |
| Hispanic | 0 | 0 | 1 | 4 |
MRI
Magnetic resonance images were acquired on 1.5-T GE Signa device (General Electric Medical Systems, Milwaukee) by using a protocol previously described (27). Only sagittal images were acquired. T1-weighted images that used a three-dimensional spoiled gradient recall pulse sequence were acquired with the following parameters: TR=24 msec, TE=5 msec, 45° flip angle, acquisition matrix=256×196, field of view=30 cm, number of excitations=2, slice thickness=1.2 isotropic voxels, no skip (total scan time=20 minute). Coronal images with a slice thickness of 1 mm were reconstructed perpendicular to the longitudinal axis of the hippocampus. This method increases the scan-rescan reliability and improves visualization of the hippocampus (28, 29). Images were transferred through a computer network to a Sun Sparc 80 Workstation (Sun Microsystems, Santa Clara, Calif.), on which volumetric measurements were performed. All scans were made with the same MRI settings; no software or hardware upgrades were made during the course of the study.
The boundaries of the hippocampus were traced manually with a mouse-driven cursor by using the ANALYZE program, version 7c (Mayo Foundation, Rochester, Minn.). The boundaries of the hippocampus and the amygdala in both groups were traced by two raters (E.V., C.S.) with extensive training in hippocampal and amygdalar anatomy. Both raters were blind to the subjects’ diagnostic groups. The accuracy of the method was checked with reliability analysis. The intraclass correlation coefficient (alpha) was 0.98 for hippocampal volume and 0.98 for amygdalar volume.
Measurement of whole hippocampal volume was performed by drawing the boundaries from the posterior to the anterior direction in every consecutive slice starting at the slice where the pulvinar of the thalamus interrupts the fornix superior, which was defined as the posterior landmark of the hippocampus. The superior border of the hippocampus only included gray matter and did not include the alveus and the fimbriae. The inferior border included the subiculum. A straight line from the inferior subcortical white matter extending medially was used to distinguish the parahippocampal gyrus from the subiculum. In the posterior to anterior direction, in several slices around the area displaying the basilar artery, both the hippocampus and amygdala were visible, and consequently both were drawn to obtain whole volumes of those structures. The uncal recess of the temporal horn of the lateral ventricle was used as the most reliable landmark for separation of the hippocampal head from the amygdala. If the uncal recess was not prominent, we traced along the alveus or connected the inferior horn of the lateral ventricle to the sulcus at the inferior margin of the semilunar gyrus. The amygdala was also drawn from posterior to anterior until the last slice, where the amygdala was still visually distinguished from the surrounding infraorbital gyrus (30). Whole brain volumes were assessed by using the nonresliced images. Volumes were assessed by tracing the outline of the brain (excluding the cerebellum) in all axial slices in which it was visualized.
Data Analysis
Repeated-measures analyses of covariance (ANCOVA) with side (left or right) as the repeated measure were used to analyze group differences in total volumes. Whole brain volume was used as covariate. T tests for nonpaired samples were used to compare group differences in left and right hippocampal and amygdalar volumes. Significance was defined as p<0.05.
Results
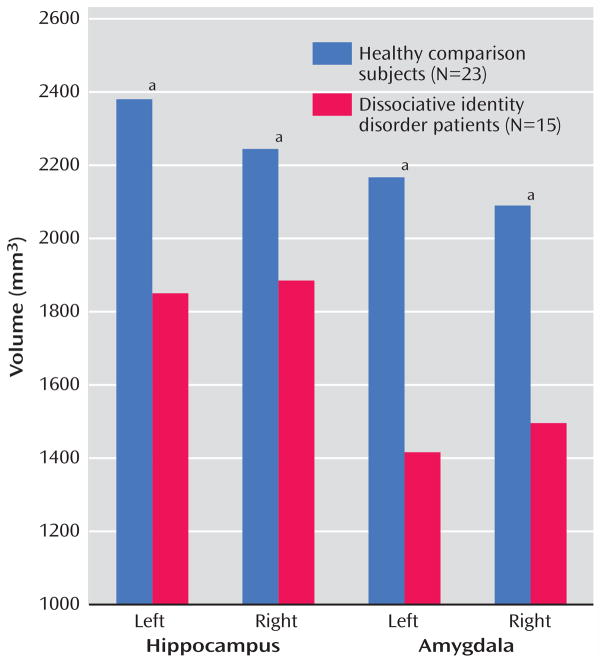
The mean of the left and right hippocampal volumes of the patients with dissociative identity disorder was 19.2% smaller than that of the comparison subjects (patients: mean=1867.58 mm3, SD=305.54; comparison subjects: mean=2312.38 mm3, SD=784.06). Repeated-measures AN-COVA with side as the repeated measure and whole brain volume as covariate showed that this difference was significant (F=4.13, df=1, 35, p=0.05). Both the left and right mean hippocampal volumes were significantly smaller in the patients than in the comparison subjects (Table 2, Figure 1). When we controlled for subjects’ age, the differences were no longer significant. A general linear model analysis with age as a covariate showed that the differences between groups for left and right hippocampal volume were nonsignificant (left: F=1.37, df=1, p=0.25; right: F=0.42, df=1, p=0.53).
TABLE 2.
Hippocampal, Amygdalar, and Whole Brain Volumes in Patients With Dissociative Identity Disorder and Healthy Comparison Subjects
| Region | Volume (mm3)
|
Analysis
|
|||||
|---|---|---|---|---|---|---|---|
| Patients With Dissociative Identity Disorder (N=15)
|
Healthy Comparison Subjects (N=23)
|
||||||
| Mean | SD | Mean | SD | % difference | t (df=36) | p | |
| Hippocampus | |||||||
| Left | 1850.21 | 342.42 | 2380.30 | 811.38 | 22.3 | 2.78 | 0.009 |
| Right | 1884.95 | 274.73 | 2244.46 | 767.77 | 16.0 | 2.05 | <0.05 |
| Amygdala | |||||||
| Left | 1416.18 | 322.00 | 2166.74 | 726.60 | 34.6 | 4.34 | <0.0001 |
| Right | 1495.77 | 347.05 | 2089.94 | 723.19 | 28.4 | 3.39 | 0.002 |
| Whole brain | 343905.30 | 20339.86 | 346796.00 | 28502.45 | 0.8 | 0.36 | 0.72 |
FIGURE 1.
Hippocampal and Amygdalar Volumes in Patients With Dissociative Identity Disorder and Healthy Comparison Subjects
a Significant difference between groups (p<0.05, t test for nonpaired samples).
The mean of the left and right amygdalar volumes of the patients with dissociative identity disorder was 31.6% smaller than that of the comparison subjects (patients: mean=1455.97 mm3, SD=331.42; comparison subjects: mean=2128.34 mm3, SD=717.85). This difference was significant after adjustment for whole brain volume (F=11.41, df=1, p=0.002). Significantly smaller left and right amygdalar volumes were seen in the patients, compared to the healthy subjects (Table 2). There was no alteration in the significance of these findings when we subtracted the volumes for the comparison subjects with a lifetime history of major depressive disorder. Adjustment for age altered the significance: the differences between groups remained significant for the left amygdalar volume (F= 5.05, df=1, p=0.03) but not for the right amygdalar volume (F=2.22, df=1, p=0.15).
The mean ratios of hippocampal volume to amygdalar volume were 1.13 for the left side and 1.10 for the right side in the healthy comparison subjects. In the dissociative identity disorder group, the ratios were 1.34 for the left side and 1.33 for the right side. T tests resulted in significance differences for the left side (t=2.24, df=36, p<0.05) and the right side (t=2.07, df=36, p<0.04). The mean difference in these ratios was 15.8%.
Discussion
Our study is the first to demonstrate smaller hippocampal and amygdalar volumes in female patients with dissociative identity disorder, compared to healthy female subjects. The patients with dissociative identity disorder in our study showed a 19.2% smaller hippocampal volume and a 31.6% smaller amygdalar volume, compared with the healthy subjects. Hippocampal/amygdalar volume ratios were larger in the dissociative identity disorder group than in the healthy comparison group.
All patients with dissociative identity disorder in this study also had a diagnosis of PTSD according to the DSM-IV-TR criteria and the Clinician-Administered PTSD Scale cutoff score. To our knowledge, this is the first study to confirm PTSD diagnoses in dissociative identity disorder patients by using the Clinician-Administered PTSD Scale, generally considered to be the gold-standard psychometric instrument for PTSD diagnosis. These results are consistent with the conceptualization of dissociative identity disorder as an extreme form of early-abuse-related PTSD.
There are virtually no previous empirical studies of the neurobiology of patients with dissociative identity disorder. Some early studies looked at psychophysiologial alterations in different states (31–34). Other studies have shown alterations in memory in dissociative identity disorder (35). In still other studies, researchers attempted to investigate divided attention tasks and memory function in subjects with dissociative identity disorder (36–39).
Apart from single case reports, published brain imaging studies of dissociative disorders are few. In one study, patients with depersonalization disorder had higher activity in somatosensory association areas (40). In another study, functional MRI was used to examine brain activation in PTSD patients in a dissociative state while reexperiencing traumatic memories; greater activation was found in the temporal, inferior, and medial frontal regions and in occipital, parietal, anterior cingulate, and medial prefrontal cortical regions (41). A positron emission tomography study showed different patterns of activation with identity fluctuation in dissociative identity disorder (42). Two studies found a negative correlation between dissociative symptom level (as measured with the Clinician-Administered Dissociative States Scale or the Dissociative Experiences Scale) and hippocampal volume as measured with MRI in women with PTSD related to early childhood sexual abuse (who had elevated levels of dissociative symptoms) (15, 16). Electrical stimulation of the hippocampus and adjacent regions in patients with epilepsy resulted in a number of dissociative-like symptoms, including feelings of déjà vu, depersonalization, derealization, and memory alterations (43, 44). Administration of ketamine, an antagonist of N-methyl-D-aspartic acid (NMDA) receptors, which are highly concentrated in the hippocampus, resulted in dissociative symptoms in healthy subjects, including feelings of being out of body and of time standing still, perceptions of body distortions, and amnesia (45). On the basis of these findings, we have hypothesized that stress, acting through NMDA receptors in the hippocampus, may mediate symptoms of dissociation (46).
Clinical studies have found comorbid PTSD or a lifetime history of PTSD in 80%–100% of dissociative identity disorder patients (2). Davidson and Foa (47), in their summary of work by members of the APA DSM-IV Advisory Committee on PTSD, included multiple personality disorder/dissociative identity disorder among the disorders related to “abnormal stress reaction[s].” They stated that “a concerted research effort is needed to apply common methods to study…those disorders in which the original occurrence of trauma served as a necessary (i.e., etiological) factor” (pp. 234–235). Despite this suggestion, few researchers have attempted to use established psychometric instruments to assess PTSD in patients with dissociative identity disorder (2). Further, no study of dissociative identity disorder patients has included the assessment of neurobiological measures that have been studied in PTSD populations. The study of the psychobiology of dissociative identity disorder patients, compared to that of PTSD patients and other clinical groups, may contribute to the ongoing debate about the validity of the dissociative identity disorder construct and contribute to alternative theories of its etiology. We acknowledge that this study does not imply that dissociative identity disorder is a distinct disorder.
There are several possible explanations for the current study findings. Previous studies have shown that dissociative identity disorder patients essentially universally report high rates of exposure to repeated stressful experiences in early life (5, 9). The hippocampus is a major target organ for glucocorticoids, which are released during stressful experiences. It has been hypothesized that prolonged exposure to glucocorticoids could lead to progressive atrophy of the hippocampus (48, 49). Smaller hippocampal volume in dissociative identity disorder could thus be related to stress exposure and could represent a neurobiological finding that dissociative identity disorder shares with other stress-related psychiatric disorders such as PTSD. The exact mechanism that could lead to smaller amygdalar volume in dissociative identity disorder is unclear. To our knowledge, the amygdala is not a target organ for glucocorticoids. In contrast to the volumetric assessment of the hippocampus, reliable measurement of amygdalar volume with MRI in human subjects has long been thought to be difficult to accomplish (29, 50). However, the use of MR cameras with good resolution, agreement on the structure boundaries, and the use of three-dimensional software packages enabled us to reliably measure both hippocampal and amygdalar volumes.
Smaller hippocampal and amygdalar volumes have been reported in three earlier studies in which patients with borderline personality disorder were compared to healthy subjects (18–20). Driessen et al. (18) found that hippocampal volume was approximately 16% smaller and amygdalar volume 8% smaller in patients with borderline personality disorder, compared to healthy subjects. Schmahl et al. (19) found a 13.1% smaller hippocampal volume and a 21.9% smaller amygdalar volume in borderline personality disorder patients (all of whom had a history of childhood abuse) than in healthy comparison subjects. Tebartz van Elst et al. (20) reported a 20%–21% smaller hippocampal volume and a 23%–25% smaller amygdalar volume at both sides in patients with borderline personality disorder, compared to healthy subjects. Early studies in PTSD patients did not show significantly smaller amygdalar volumes. However, a study of patients with PTSD related to early abuse showed a 15% smaller left amygdalar volume in the patients, compared with matched healthy subjects (14), and a recent study showed smaller amygdalar volumes in police officers with PTSD (51). These findings suggest that early abuse associated with a stress-related psychiatric disorder may be related to smaller amygdalar volume. In addition, these findings are in contrast to findings from studies of depression that have shown no differences or larger amygdalar volume in depressed patients, compared with healthy subjects (52).
Besides glucocorticoids, other neurotransmitters/neuromodulators such as glutamate, serotonin, and endogenous opioids could have an effect on hippocampal and amygdalar volumes (53). Genetic factors that could contribute to the smaller hippocampal and amygdalar volumes that are found in patients with dissociative identity disorder could increase the risk for the development of the disorder (54). In an application of this argument to PTSD, stress may not cause hippocampal damage; rather, individuals who were born with smaller hippocampal volume would be at greater risk for development of PTSD (55). Consistent with this hypothesis, abused subjects without dissociative identity disorder in the current study had larger hippocampal and amygdalar volumes than non-abused subjects without dissociative identity disorder. Larger hippocampal and/or amygdalar volume may be protective in the face of early trauma.
Our finding that the ratio of hippocampal volume to amygdalar volume discriminated the patients from the healthy comparison subjects has not been reported in previous volumetric studies. This finding suggests that dissociative identity disorder is associated with relatively greater volume reductions in the amygdala than in the hippocampus.
Our study had several limitations. As a group, the comparison subjects were significantly younger than the dissociative identity disorder patients. Age-related structural alterations in the hippocampus have been identified (56); however, there are no previous findings of a relationship between age and hippocampal or amygdalar volume in women in the 20–50-year age group (57, 58), the age group represented by the subjects in our study. In a study of the effects on hippocampal volume of age and sex in early adulthood, Pruessner et al. (57) reported a significant negative correlation of age with both left and right hippocampal volumes (a reduction in hippocampal volume of about 1%–1.5% per year) in men only. In women no such relationship was found. No significant effect of age was found for amygdalar volume in either men or women. It is hypothesized that the estrogen level in young adult women protects against loss of volume of the hippocampus. Changes in estrogen level associated with entering menopause could therefore have contributed to hippocampal volumetric alterations, and it is possible that a few of the dissociative identity disorder patients in our study could have entered menopause. Sullivan et al. (58), however, found no difference in hippocampal volume in pre- versus postmenopausal women. These studies add support to an analysis in which dissociative identity disorder patients and healthy subjects are compared without adjustment for effects of age. Accordingly, we believe the significance levels for the between-group comparisons of both hippocampal and amygdalar volumes are valid without correction for this factor. Yet the design would have been stronger if the groups were age-matched.
In addition, we did not perform volumetry of subcortical regions (e.g., the caudate nucleus) to obtain comparison measures. This step would have enabled us to correlate the volume measurements of structures of interest with those of brain regions that are presumed to be unaffected. However, we did use whole brain volume as a covariate. Another limitation was that the dissociative identity disorder subjects were so ill that they could not be safely included in the study as medication-free outpatients, whereas the comparison subjects were medication free during the study. Medication has been shown to influence the volume of the hippocampus. In rodents, antidepressant treatment has been shown to increase neurogenesis in the hippocampus (59). A single study in humans has also demonstrated an increase in hippocampal volume associated with treatment with paroxetine (60). Therefore, it is possible that the differences in hippocampal volume and possibly in amygdalar volume for the dissociative identity disorder group could have been even larger if the patients had not been taking medication. Finally, a potential limitation of this study is that all of the patients with dissociative identity disorder also met the criteria for PTSD, which makes it impossible to establish that the findings are not related to the comorbid PTSD diagnosis. However, patients with true dissociative identity disorder without PTSD essentially do not exist. We hope that the current study will promote a conceptualization of PTSD and dissociative identity disorder as related trauma-spectrum disorders (61).
These findings may have clinical implications for the treatment of dissociative identity disorder patients. For example, an understanding of dissociative identity disorder as a trauma-related disorder that involves neural circuitry alterations in brain areas associated with memory that are also affected in PTSD may help clinicians better understand the symptoms presented by patients in treatment sessions. Neurobiological studies that support the validity of the diagnosis of dissociative identity disorder will help to advance research in this area. Findings that link dissociative identity disorder to other trauma-related disorders may help improve nosological approaches to this disabling disorder.
Acknowledgments
Supported by NIMH grant MH-56120, by the VA National Center for PTSD, and a VA Career Development Award to Dr. Bremner.
The authors thank Nancy Kristen Wilson, M.Sc., Cheryl Zdunek, L.C.S.W., and Heather Douglas Palumberi, M.A., for assistance in patient recruitment and assessment, and Cynthia Payne, M.D., Terry Hickey, R.T.N.M., and Hedy Sarofin, R.T.R., for assistance with magnetic resonance imaging.
References
- 1.Loewenstein RJ, Putnam FW. In: The dissociative disorders, in Comprehensive Textbook of Psychiatry. 8. Sadock BJ, Sadock VA, editors. Vol. 1. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 1844–1901. [Google Scholar]
- 2.Armstrong JG, Loewenstein RJ. Characteristics of patients with multiple personality and dissociative disorders on psychological testing. J Nerv Ment Dis. 1990;178:448–454. doi: 10.1097/00005053-199007000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Loewenstein RJ. Diagnosis, epidemiology, clinical course, treatment, and cost effectiveness of treatment for dissociative disorders and multiple personality disorder: report submitted to the Clinton administration task force on health care financing reform. Dissociation. 1994;7:3–11. [Google Scholar]
- 4.Putnam N, Stein M. Self-inflicted injuries in childhood: a review and diagnostic approach. Clinical Pediatr (Phila) 1985;24:514–518. doi: 10.1177/000992288502400908. [DOI] [PubMed] [Google Scholar]
- 5.Ross CA, Miller SD, Bjornson L, Reagor P, Fraser GA, Anderson G. Abuse histories in 102 cases of multiple personality disorder. Can J Psychiatry. 1991;36:97–101. doi: 10.1177/070674379103600204. [DOI] [PubMed] [Google Scholar]
- 6.Hornstein NL, Putnam FW. Clinical phenomenology of child and adolescent dissociative disorders. J Am Acad Child Adolesc Psychiatry. 1992;31:1077–1085. doi: 10.1097/00004583-199211000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Boon S, Draijer N. Multiple personality disorder in the Netherlands: a clinical investigation of 71 patients. Am J Psychiatry. 1993;150:489–494. doi: 10.1176/ajp.150.3.489. [DOI] [PubMed] [Google Scholar]
- 8.Tutkun H, Sar V, Yargic LI, Ozpulat T, Yanik M, Kiziltan E. Frequency of dissociative disorders among psychiatric inpatients in a Turkish university clinic. Am J Psychiatry. 1998;155:800–805. doi: 10.1176/ajp.155.6.800. [DOI] [PubMed] [Google Scholar]
- 9.Lewis DO, Yeager, Calif, Swica Y, Pincus JH, Lewis M. Objective documentation of child abuse and dissociation in 12 murderers with dissociative identity disorder. Am J Psychiatry. 1997;154:1703–1710. doi: 10.1176/ajp.154.12.1703. [DOI] [PubMed] [Google Scholar]
- 10.Loewenstein RJ. Dissociation, development, and the psychobiology of trauma. J Am Acad Psychoanal. 1993;21:581–603. doi: 10.1521/jaap.1.1993.21.4.581. [DOI] [PubMed] [Google Scholar]
- 11.Spiegel D. Dissociating damage. Am J Clin Hypn. 1986;29:123–131. doi: 10.1080/00029157.1986.10402695. [DOI] [PubMed] [Google Scholar]
- 12.Brunson KL, Chen Y, Avishai-Eliner S, Baram TZ. Stress and the developing hippocampus: a double-edged sword? Mol Neurobiol. 2003;27:121–136. doi: 10.1385/MN:27:2:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- 14.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, Mc-Glashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 16.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 17.Zanarini MC, Williams AA, Lewis RE, Reich RB, Vera SC, Marino MF, Levin A, Yong L, Frankenburg FR. Reported pathological childhood experiences associated with the development of borderline personality disorder. Am J Psychiatry. 1997;154:1101–1106. doi: 10.1176/ajp.154.8.1101. [DOI] [PubMed] [Google Scholar]
- 18.Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, Osterheider M, Petersen D. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 19.Schmahl CG, Vermetten E, Elzinga BM, Douglas Bremner J. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 20.Tebartz van Elst L, Hesslinger B, Thiel T, Geiger E, Haegele K, Lemieux L, Lieb K, Bohus M, Hennig J, Ebert D. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biol Psychiatry. 2003;54:163–171. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- 21.Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. findings in neuropsychiatric disorders. Mol Psychiatry. 2005;10:160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg M, Cicchetti D, Buchanan J, Rakfeldt J, Rounsaville B. Distinguishing between multiple personality disorder (dissociative identity disorder) and schizophrenia using the Structured Clinical Interview for DSM–IV Dissociative Disorders. J Nerv Ment Dis. 1994;182:495–502. doi: 10.1097/00005053-199409000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP) New York: New York State Psychiatric Institute, Biometrics Research; 2002. [Google Scholar]
- 26.Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 27.Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, South-wick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartzokis G, Altshuler LL, Greider T, Curran J, Keen B, Dixon WJ. Reliability of medial temporal lobe volume measurements using reformatted 3D images. Psychiatry Res. 1998;82:11–24. doi: 10.1016/s0925-4927(98)00007-9. [DOI] [PubMed] [Google Scholar]
- 29.Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 1. review of methodologies currently employed. Mol Psychiatry. 2005;10:147–159. doi: 10.1038/sj.mp.4001580. [DOI] [PubMed] [Google Scholar]
- 30.Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurol. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 31.Coons P. Psychophysiologic aspects of multiple personality disorder: a review. Dissociation. 1998;1:47–53. [Google Scholar]
- 32.Putnam FW, Zahn TP, Post RM. Differential autonomic nervous system activity in multiple personality disorder. Psychiatry Res. 1990;31:251–260. doi: 10.1016/0165-1781(90)90094-l. [DOI] [PubMed] [Google Scholar]
- 33.Putnam FW. The psychophysiologic investigation of multiple personality disorder: a review. Psychiatr Clin North Am. 1984;7:31–39. [PubMed] [Google Scholar]
- 34.Miller SD, Triggiano PJ. The psychophysiological investigation of multiple personality disorder: review and update. Am J Clin Hypn. 1992;35:47–61. doi: 10.1080/00029157.1992.10402982. [DOI] [PubMed] [Google Scholar]
- 35.Elzinga BM, Phaf RH, Ardon AM, van dyck R. Directed forgetting between, but not within, dissociative personality states. J Abnorm Psychol. 2003;112:237–243. doi: 10.1037/0021-843x.112.2.237. [DOI] [PubMed] [Google Scholar]
- 36.Eich EM, Macaulay D, Loewenstein RJ, Dihle PH. Memory, amnesia, and dissociative identity disorder. Psychol Science. 1997;8:417–422. [Google Scholar]
- 37.Dorahy MJ, Irwin HJ, Middleton W. Assessing markers of working memory function in dissociative identity disorder using neutral stimuli: a comparison with clinical and general population samples. Aust NZ J Psychiatry. 2004;38:47–55. doi: 10.1177/000486740403800101. [DOI] [PubMed] [Google Scholar]
- 38.Huntjens RJ, Postma A, Peters ML, Woertman L, van der Hart O. Interidentity amnesia for neutral, episodic information in dissociative identity disorder. J Abnorm Psychol. 2003;112:290–297. doi: 10.1037/0021-843x.112.2.290. [DOI] [PubMed] [Google Scholar]
- 39.Huntjens RJC. Apparent Amnesia: Interidentity Memory Functioning in Dissociative Identity Disorder. Ridderkerk, the Netherlands: Ridderprint; 2002. [Google Scholar]
- 40.Simeon D, Guralnik O, Hazlett EA, Spiegel-Cohen J, Hollander E, Buchsbaum MS. Feeling unreal: a PET study of depersonalization disorder. Am J Psychiatry. 2000;157:1782–1788. doi: 10.1176/appi.ajp.157.11.1782. [DOI] [PubMed] [Google Scholar]
- 41.Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Gati JS, Menon RS. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- 42.Reinders AA, Nijenhuis ER, Paans AM, Korf J, Willemsen AT, den Boer JA. One brain, two selves. Neuroimage. 2003;20:2119–2125. doi: 10.1016/j.neuroimage.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 43.Halgren E, Walter RD, Cherlow DG, Crandall PH. Mental phenomena evoked by electrical stimulation of the human hippocampal formation and amygdala. Brain. 1978;101:83–117. doi: 10.1093/brain/101.1.83. [DOI] [PubMed] [Google Scholar]
- 44.Penfield W, Perot P. The brain’s record of auditory and visual experience: a final summary and discussion. Brain. 1963;86:595–696. doi: 10.1093/brain/86.4.595. [DOI] [PubMed] [Google Scholar]
- 45.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 46.Chambers RA, Bremner JD, Moghaddam B, Southwick SM, Charney DS, Krystal JH. Glutamate and post-traumatic stress disorder: toward a psychobiology of dissociation. Semin Clin Neuropsychiatry. 1999;4:274–281. doi: 10.153/SCNP00400274. [DOI] [PubMed] [Google Scholar]
- 47.Davidson JRT, Foa EB, editors. Posttraumatic Stress Disorder: DSM–IV and Beyond. Washington, DC: American Psychiatric Press; 1993. [Google Scholar]
- 48.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D, Gannon MN, Gould E, Mendelson S, Sakai R, Spencer R, Woolley C. Paradoxical effects of adrenal steroids on the brain: protection versus degeneration. Biol Psychiatry. 1992;31:177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- 50.Brierley B, Shaw P, David AS. The human amygdala: a systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain Res Brain Res Rev. 2002;39:84–105. doi: 10.1016/s0165-0173(02)00160-1. [DOI] [PubMed] [Google Scholar]
- 51.Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, den Heeten GJ, Gersons BP. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56:356–363. doi: 10.1016/j.biopsych.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 52.Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med. 2004;34:1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- 53.Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- 54.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pitman RK. Hippocampal diminution in PTSD: more (or less?) than meets the eye. Hippocampus. 2001;11:73–74. doi: 10.1002/hipo.1022. discussion 82–84. [DOI] [PubMed] [Google Scholar]
- 56.Kaye JA, Swihart T, Howieson D, Dame A, Moore MM, Karnos T, Camicioli R, Ball M, Oken B, Sexton G. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurol. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- 57.Pruessner JC, Collins DL, Pruessner M, Evans AC. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J Neurosci. 2001;21:194–200. doi: 10.1523/JNEUROSCI.21-01-00194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol Aging. 2005;26:1093–1098. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 59.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bremner JD. Acute and chronic responses to psychological trauma: where do we go from here? Am J Psychiatry. 1999;156:349–351. doi: 10.1176/ajp.156.3.349. [DOI] [PubMed] [Google Scholar]