Abstract
CD4+Vβ5+ peripheral T cells in B6 mice respond to encounter with a peripherally-expressed endogenous superantigen by undergoing either deletion or TCR revision. In this latter process, cells lose surface Vβ5 expression and undergo RAG-dependent rearrangement of endogenous TCRβ genes, driving surface expression of novel TCRs. While post-revision CD4+Vβ5−TCRβ+ T cells accumulate with age in Vβ5 transgenic mice and bear a diverse TCR Vβ repertoire, it is unknown whether they respond to homeostatic and antigenic stimuli, and thus may benefit the host. We now demonstrate that post-revision cells are functional. These cells have a high rate of steady-state homeostatic proliferation in situ and they undergo extensive MHC class II-dependent lymphopenia-induced proliferation. Importantly, post-revision cells do not proliferate in response to the tolerizing superantigen, implicating TCR revision as a mechanism of tolerance induction and demonstrating that TCR-dependent activation of post-revision cells is not driven by the transgene-encoded receptor. Post-revision cells proliferate extensively to commensal bacterial Ags and can generate I-Ab-restricted responses to Ag by producing IFNγ following Listeria monocytogenes challenge. These data show that rescued post-revision T cells are responsive to homeostatic signals and recognize self and foreign peptides in the context of self MHC, and are thus useful to the host.
Keywords: TCR revision, tolerance, T cell homeostasis
Introduction
Developing T cells within the thymus undergo rigorous selection to ensure the usefulness and safety of mature T cells bearing rearranged αβ TCRs. Thymocytes at the CD4−CD8− double-negative stage undergo RAG-mediated TCRβ gene rearrangement, and generation of a functional TCRβ chain induces proliferation and CD4 and CD8 coreceptor expression. At the CD4+CD8+ double-positive stage, a second wave of RAG-dependent rearrangement, this time at the TCRα locus, generates rearrangements that encode TCRα chains that pair with the already available TCRβ chains and these TCRαβ pairs are tested for interactions with self-MHC molecules. Useful affinity for self-MHC class II and class I molecules drives positive selection, upregulation of TCR surface expression, and commitment to the CD4 and CD8 T cell lineages, respectively. Insufficient interactions with MHC result in death by neglect. Positively-selected CD4 single-positive (SP) and CD8 SP thymocytes test their TCRs against a broad array of self-MHC:self-peptide ligands for self-reactivity, and those cells that recognize self are removed from the repertoire by apoptosis (reviewed in Ref. 1). After thymic exit, basal TCR signaling in the lymphoid periphery promotes survival and homeostatic proliferation, and is an additional selective force that maintains useful components of the peripheral T cell repertoire (reviewed in Ref. 2).
Mechanisms in the lymphoid periphery exist to keep in check self-reactive peripheral T cells that elude negative selection in the thymus. In Vβ5 transgenic (Tg) mice, Vβ5+CD4+ T cells in the lymphoid periphery encounter an endogenous superantigen encoded by a defective mouse mammary tumor virus (Mtv), and are tolerized to this self Ag by several means. Mtv superantigen drives deletion of most CD4+ T cells, resulting in a severe age-dependent decline in the CD4:CD8 ratio (3, 4). Mtv drives some cells to undergo TCR revision, through which CD4+ T cells lose surface Vβ5 expression, re-express RAG and undergo TCRβ gene rearrangement, resulting in the age-dependent accumulation of a rescued population of post-revision CD4+Vβ5−TCRβ+ T cells (5, 6). The expression of RAG (5, 7–18) and the presence of TCR Vβ- (DJ)β or Vα-Dα recombination intermediates (5, 7, 11, 13–17) in peripheral T cells, as well as a blockade in the generation of post-revision CD4+Vβ5−TCRβ+ T cells when Rag2 is conditionally deleted in post-thymic mature Vβ5+ T cells (19), unequivocally identify TCR revision as a process that targets mature CD4+Vβ5+ cells in the lymphoid periphery for RAG-dependent generation of new TCRs (reviewed in Ref. 20). Post-revision T cells express a diverse TCRβ repertoire with TCRs characterized by shortened CDR3 loops resulting from fewer N nucleotides (18).
Given the specialized selecting environment of the thymus, it is unknown whether cells generated through TCR revision in the lymphoid periphery have been selected for appropriate T cell function that could benefit the host. Although post-revision Vβ5− T cells proliferate in response to TCR crosslinking (5) and contain a population of IL-17 producing cells following PMA + ionomycin stimulation (21), their homeostatic potential and immunocompetence remain untested. Here, we assessed the ability of post-revision cells to respond to homeostatic factors, including self-MHC and foreign Ag following bacterial challenge of Vβ5 Tg mice. Our data indicate that post-revision T cells require TCR-MHC interactions for maximal lymphopenia-induced proliferation (LIP) and the generation of Ag-specific self-MHC restricted effector T cell responses to bacterial Ag. Additionally, post-revision cells possess no residual reactivity to Mtv superantigen, showing that TCR signaling occurs through the revised TCR and not the transgene-encoded receptor, further defining TCR revision as a mechanism of peripheral T cell tolerance.
Materials and Methods
Mice
Vβ5 Tg and non-transgenic (nonTg) littermates on the C57BL/6 (B6) background were bred under specific-pathogen free conditions at the University of Washington. Mice on the B6 background carry the endogenous proviral genes encoding Mtv-8, -9, -17, and -30. B6 Ly5.2+ and B6.SJL (B6.SJL-PtprcaPepcb/BoyJ) Ly5.1+ mice and their F1 generation (Ly5.1+Ly5.2+) were purchased from The Jackson Laboratory or bred in-house. B6.PL-Thy1a/CyJ (Thy-1.1+) mice were kindly provided by M.K. Kaja (University of Washington, Seattle, WA). CD4−/− (22) and I-Aβ−/− (23) mice on the B6 background were originally purchased from The Jackson Laboratory. Rag2−/− (hereafter Rag−/−) mice on the B6 background were provided by D. Stetson (University of Washington, Seattle, WA). To induce lymphopenia in recipient mice, whole body sublethal irradiation of 650 rads was administered 1 day before cell transfer. For antibiotic treatment of Rag−/− mice, drinking water containing 1 g/mL neomycin trisulfate, 1g/mL ampicillin (both from Sigma-Aldrich), 1 g/mL polymixin B sulfate (Gibco), and 50 mg/mL Ciprofloxacin (Claris Lifesciences Inc.) was administered beginning 6 days before cell transfer and made fresh every 6 days. All experiments were conducted in accordance with the University of Washington Institutional Animal Care and Use Committee.
Cell preparation and flow cytometry
Single-cell suspensions were prepared from spleen and mesenteric lymph nodes (MLNs), and RBC were removed from spleen by water lysis. For flow cytometry, cells were surface stained in HBSS containing 1% BSA. Fc receptors were blocked using anti-CD16/32 (2.4G2; BD Pharmingen) followed by staining with FITC, phycoerythrin, Peridinin-Chlorophyll-Protein-Cy5.5, phycoerythrin-Cy7, allophycocyanin, and allophycocyanin -AlexaFluor 750 or - AlexaFluor 780 fluorochrome-conjugated antibodies and, in some cases, biotinylated antibodies followed by allophycocyanin-conjugated streptavidin. Antibodies were purchased from BD Biosciences or eBioscience and included monoclonal antibodies recognizing mouse CD4 (RM4-5), CD44 (IM7), CD45.1/Ly5.1 (A20), CD45.2/Ly5.2 (104), CD62L (MEL-14), Thy-1.2 (53- 2.1), panTCRβ (H57-597.13), and Vβ5 (MR9-4). Flow cytometry data were collected using a FACSCanto (Becton Dickinson) and analyzed with FlowJo software (Treestar; Ashland, OR). Cell sorting was done using a FACSAria (Becton Dickinson).
Cell enrichments, CFSE labeling, and cell transfers
“Untouched” CD4 T cell enrichments were performed using an EasySep Negative Selection Mouse CD4+ T cell Enrichment Kit (Stem Cell Technologies). For cell transfers, enriched or sorted cells were CFSE labeled at a concentration of 50×106 cells/mL in 5μM CFSE for 10 minutes at 37°C. CFSE labeling was arrested by adding cold HBSS containing 1% BSA. Labeled cells were washed and injected into the lateral tail vein in a 200 μl volume of HBSS.
In situ BrdU labeling
Mice were injected i.p. with 0.8 mg of BrdU (Sigma-Aldrich) in a 200 μL volume, followed by administration of sterile drinking water containing 0.8 mg/mL BrdU, made fresh and changed daily for 5 days. For flow cytometric detection of BrdU, cells were surface stained, followed by intranuclear staining with FITC-conjugated anti-BrdU (BD Biosciences) using the BrdU Flow Kit and protocol (BD Biosciences).
In vitro T cell activation
A mix containing an equal number of congenically marked nonTg and Vβ5 Tg splenocytes were CFSE labeled. 2×106 CFSE-labeled mixed splenocytes were plated in a 24 well plate either in the absence or presence of 1–3×105 irradiated (4300 rads) PN53HI B lymphoma cells that express high levels of Mtv superantigen and drive measurable proliferation of Vβ5+ T cells in vitro. PN53HI cells were isolated from tumor-prone P53+/− perforin null mice and were kindly provided by Mark J. Smyth (Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia). Alternatively, CFSE labeled splenocytes were stimulated with 5 ng/ml anti- CD3ε (clone 145-C11) + 1 μg/mL anti-CD28 (clone 37.51), both from BD Pharmingen, in a total volume of 2 mL RP10 (RPMI with 10% FBS, 10 mM HEPES, 4 mM L-glutamine, 100 U/ml penicillin, 100 μg/mL streptomycin, 50 μM β-mercaptoethanol, and 50 μg/mL gentamycin). Cells were cultured for 3 days at 37°C and 7% CO2, followed by surface staining and flow cytometric analysis.
L. monocytogenes infections
Wild-type Listeria monocytogenes was grown in brain-heart infusion broth and measured by OD (A600) at mid-log growth phase. The culture was diluted in sterile PBS and 200 μL containing 2000 CFU injected into the lateral tail vein.
Intracellular cytokine staining
To detect IFNγ-producing T cells from uninfected mice, 2–3×106 splenocytes were incubated in 96 well plates for 6 hours at 37° C in RP10 in the presence of monensin (GolgiStop, BD Pharmingen) either in the absence or presence of 0.7 μM ionomycin + 50 ng/mL PMA (both from Sigma-Aldrich). For detection of L. monocytogenes-specific CD4+ T cells, splenocytes from mice infected 7 days previously were incubated in 96 well plates for 5 hours at 37° C in RP10 in the presence of monensin, and either in the absence or presence of 1 μg/mL of the I-Ab- restricted peptide LLO190–201 (NEKYAQAPNVS) from Invitrogen. After incubation, cells were surface stained, followed by intracellular staining with allophycocyanin-conjugated anti-IFNγ using the Cytofix/Cytoperm kit and protocol (BD Biosciences).
Results
Elevated steady-state proliferation by post-revision T cells
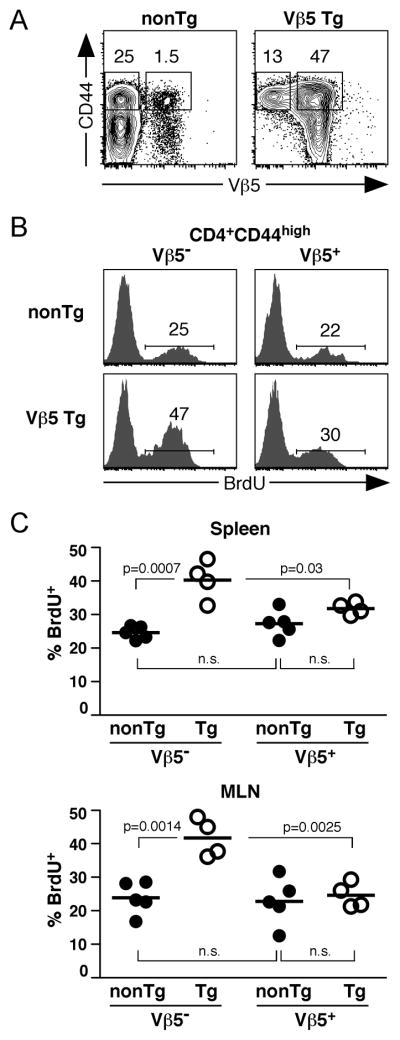
To determine whether post-revision CD4+Vβ5−TCRβ+ T cells are functional, we first examined their steady-state proliferation. Aged Vβ5 Tg and nonTg mice were administered BrdU for 5 days and CD4+ T cells analyzed for BrdU incorporation. Post-revision CD4+Vβ5− T cells in Vβ5 Tg mice express high levels of surface CD44 (Fig. 1A). Therefore, we compared the basal proliferation of post-revision CD4+Vβ5− cells to that of CD4+Vβ5+CD44high CD4+ T cells from Vβ5Tg mice and to that of Vβ5−CD44high and Vβ5+CD44high CD4+ T cells from nonTg mice (Fig. 1). Post-revision Vβ5− cells underwent a significantly higher rate of steady-state proliferation, with 40–50% incorporating BrdU during the 5-day labeling period, compared to 25–30% of Vβ5−CD44high CD4+ T cells in nonTg mice and Vβ5+CD44high cells in Vβ5 Tg mice (Fig 1B and 1C). This more extensive proliferation of post-revision T cells was observed in both spleen and MLN (Fig. 1C).
Figure 1. Post-revision T cells show an elevated steady-state proliferation in situ.
Vβ5 Tg and nonTg mice 25–30 weeks of age were given BrdU by i.p. injection, followed by a 5 day administration of BrdU in the drinking water. Spleen and MLN cells were isolated and stained for surface markers and BrdU incorporation. A. Vβ5 and CD44 analysis of CD4+ gated splenocytes, showing further gating of CD44high Vβ5− and Vβ5+ T cells from nonTg and Vβ5 Tg mice. B. Representative BrdU incorporation in CD4+CD44high Vβ5− and Vβ5+ splenocytes from nonTg and Vβ5 Tg mice. C. Charts indicate the percent BrdU+ for CD4+CD44high gated populations from spleen and MLN of all nonTg (filled circles) and Vβ5 Tg (open circles) mice analyzed. Data are compiled from 3 independent experiments (Vβ5 Tg mice: N=4, nonTg mice: N=5). Bars represent the mean percent of BrdU+ cells of the CD4+CD44high populations. P values were calculated using a two-tailed nonparametric Student’s t test. Significant p values are shown; n.s. indicates non-significant p values of >0.05.
Post-revision cells undergo extensive MHC class II-dependent LIP
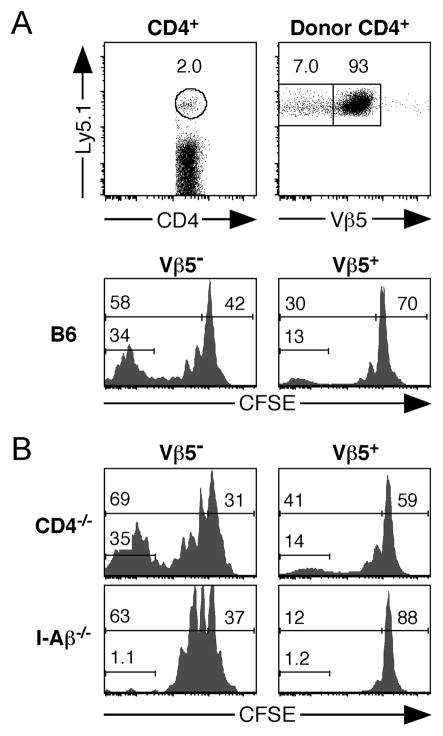
Homeostatic proliferation of CD4+ T cells is regulated by several factors, including TCR-MHC/self-peptide interactions and cytokines. Transfer of T cells into recipient mice made acutely lymphopenic through irradiation provides a sensitive model to assess the requirements for homeostasis of T cell subsets (24). In acutely lymphopenic recipients, transferred CD4+ T cells undergo LIP, typically revealing a subpopulation that undergoes rapid cell proliferation driven by contact with self-MHC/peptide complexes, and a subpopulation that undergoes slower cell division that is IL-7 dependent (24). To further characterize the functionality of post-revision T cells in vivo, we first tested their ability to respond to homeostatic signals by assessing their capacity to undergo LIP following transfer of CFSE-labeled CD4+ T cells from Vβ5 Tg mice into sublethally irradiated syngeneic recipient mice. Donor CD4+ T cells were easily identifiable within recipient spleens by congenic marker staining and were further gated into CD4+Vβ5− and CD4+Vβ5+ donor populations (Fig. 2A). Post-revision CD4+Vβ5− cells underwent substantial LIP, with approximately 60% of cells dividing during this 8 day time period. In addition to a more slowly dividing subset of cells, >30% of CD4+Vβ5− cells were rapid dividers, having undergone more than 5 cell divisions (Fig. 2A). Thus post-revision CD4+ T cells respond to homeostatic factors and proliferate in a lymphopenic environment to a greater extent than do transferred CD4+Vβ5+ cells.
Figure 2. MHC class II and other factors drive extensive LIP of post-revision CD4+Vβ5− T cells.
3×106 CFSE-labeled CD4+ T cells enriched from pooled spleen and lymph nodes of aged Vβ5 Tg mice were transferred into the indicated congenic recipients that had been sublethally irradiated (650 rads) 1 day previously to induce lymphopenia. Donor CD4+ T cells were analyzed 8 days later for the extent of proliferation as measured by CFSE dilution. For CFSE histograms, numbers represent the percent of gated cells that are undivided (upper right), have undergone at least one division (upper left), or have divided more than 5 times (lower left). A. Analysis of B6 recipient spleen with gate indicating Ly5.1+ donor CD4+ T cells (top left) and Vβ5 expression of gated donor CD4+Ly5.1+ T cells (top right). CFSE analysis of donor CD4+Vβ5− (bottom left) and donor CD4+Vβ5+ (bottom right) T cells. Data are representative of 2 independent experiments analyzing a total of 4 recipients. B. Analysis of donor CD4+Vβ5− (left) and CD4+Vβ5+ (right) cells after transfer into CD4−/− (MHC II+ lymphopenic control) and I-Aβ−/− recipients. Data are representative of 3 independent experiments, and a total of 5–6 recipients of each genotype. The percent of donor Vβ5− post-revision cells that had fully diluted CFSE was 36.1±3.8 in CD4−/− recipients and 7.1±1.6 in I-Aβ−/− recipients; p=0.0001 as determined using a two-tailed nonparametric Student’s t test.
To determine whether MHC-dependent TCR signaling contributes to the extensive LIP of post-revision T cells, CFSE labeled Vβ5 Tg CD4+ T cells were transferred to sublethally irradiated recipient I-Aβ−/− or similarly lymphopenic control CD4−/− B6 mice. Substantial proliferation of post-revision cells occurred in both types of recipients in 8 days; however, the rapidly dividing population of post-revision cells was absent in I-Aβ−/− recipients compared to control CD4−/− hosts (Fig. 2B). As expected, the smaller, rapidly proliferating population of CD4+Vβ5+ cells was also dependent on the presence of MHC II molecules (Fig. 2B). These experiments define MHC II as a critical factor for maximal homeostatic proliferation of post- revision CD4+ T cells under lymphopenic conditions.
Post-revision cells undergo LIP in competition with nonTg memory phenotype cells
To determine whether post-revision cells can undergo LIP even in competition with nonTg CD4+ T cells with a similar CD44 phenotype, an equal number of congenically marked CD44high CD4+ T cells from aged nonTg and Vβ5 Tg mice were co-transferred into acutely lymphopenic hosts. CD4+ T cells from donor nonTg and Vβ5 Tg mice that were sorted to obtain CD62L− CD4+ T cells had indistinguishably high surface CD44 expression (Fig. 3A). An equal number of sorted CD4+CD44high nonTg and Vβ5 Tg cells were mixed, CFSE labeled, and transferred into congenically-marked irradiated recipient mice. A similar proportion of post-revision cells from Vβ5 Tg donors underwent cell division compared to CD4+Vβ5−CD44high cells from nonTg mice, and were not outcompeted by them for proliferation (Fig. 3B) or recovery (data not shown), although a reduced population of rapidly dividing cells was observed among the post-revision population (Fig. 3B).
Figure 3. Post-revision T cells undergo LIP when competing against TCR nonTg effector/memory phenotype cells.
CD4+ T cells from 25–30-week-old Thy-1.2+ nonTg (Ly5.2+) and Vβ5 Tg (Ly5.1+) mice were enriched by negative selection, stained with CD62L, and sorted to isolate untouched CD62L− CD4+CD44high T cells. An equal number of sorted CD4+ Tg and nonTg cells were mixed, labeled with CFSE, and a total of 2×106 CD4+ T cells adoptively transferred into sublethally irradiated Thy-1.1+Thy-1.2− B6 recipient mice. Thy-1.2+ donor CD4+CD44high splenocyte populations were analyzed for the extent of proliferation as measured by CFSE dilution. A. CD62L and CD44 analysis of pre- and post-sort CD4+ T cells from nonTg and Vβ5 Tg mice. B. CFSE analysis of donor CD44high nonTg and Vβ5 Tg CD4+Vβ5− (left) and CD4+Vβ5+ (right) cells 8 days after transfer into sublethally irradiated recipients. Numbers in CFSE histograms represent the percent of gated cells that are undivided (upper right), have undergone at least one division (upper left), or have divided more than 5 times (lower left). Data are representative of 2 independent experiments, for a total of 4 recipients. The percent of donor Vβ5− cells that had undergone at least 1 cell division was 62.3±4.0 for nonTg cells and 72.1±4.2 for Vβ5− post-revision cells; p=0.1391 as determined using a two-tailed nonparametric Student’s t test.
Post-revision T cells are tolerant to Mtv superantigen
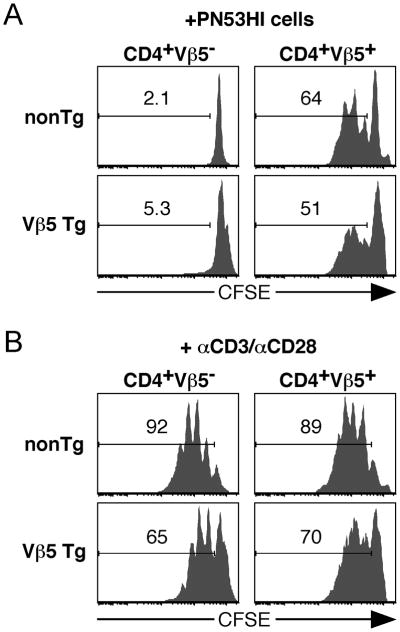
Previous attempts to use cytokine secretion or proliferation to measure Vβ5+ T cell reactivity to Mtv superantigens on normal antigen presenting cells have been unsuccessful, possibly due to the poor expression of the relevant Mtvs and the weak nature of this ligand for TCR Vβ5 (25, 26). To measure the Mtv-reactivity of post-revision T cells, we made use of PN53HI B lymphoma cells that express very high levels of Mtv, driving proliferation of Vβ5+ CD4+ and CD8+ T cells in vitro and in vivo (Fig. 4A and data not shown). To determine whether post-revision T cells are Mtv-tolerant, CFSE-labeled splenocytes mixed from nonTg and Vβ5 Tg mice were cultured in the presence of irradiated PN53HI tumor cells and the extent of their proliferation measured 3 days later. Despite driving proliferation of CD4+Vβ5+ nonTg and Vβ5 Tg T cells, PN53HI tumor cells induced no proliferative response in either post-revision CD4+Vβ5− cells or CD4+Vβ5− cells from nonTg mice (Fig. 4A). Thus, post-revision cells no longer recognize the Mtv tolerogen that directed them into the TCR revision pathway. These cells are functional, however, as judged by their rapid proliferation in response to αCD3 +αCD28 stimulation (Fig. 4B). These data designate TCR revision as a tolerance pathway, generating functional post-revision cells. In addition, TCR-dependent activation/function of post-revision T cells likely comes through the newly generated receptor, and not through any residual expression and signaling via the transgene-encoded Vβ5 TCR.
Figure 4. Post-revision T cells are tolerant to Mtv superantigen.
Splenocytes from aged Ly5.1+ nonTg and Ly5.2+ Vβ5 Tg mice were mixed, CFSE labeled, and cultured for 3 days in the presence of either 3×105 irradiated Mtv-overexpressing PN53HI tumor cells (A) or solubleαCD3 + αCD28 (B). 3 days later, cells were analyzed for proliferation as measured by CFSE dilution. Numbers in CFSE histograms represent the percent of gated cells that have undergone at least one division. Data are representative of 4 independent experiments.
Post-revision T cells respond to commensal bacteria in chronically lymphopenic mice
To determine whether post-revision T cells can proliferate in response to microbial Ags, we adoptively transferred CFSE-labeled CD4+ T cells from aged Vβ5 Tg mice into chronically lymphopenic Rag−/− recipient mice. These mice support extensive proliferation of transferred T cells driven by an exaggerated burden of microbial Ags that results from their immunodeficient state (27). To assess the relative contribution of bacterial Ags to the proliferation of the transferred T cells, a cohort of Rag−/− recipients treated with a cocktail of antibiotics was included. Six days following transfer, donor CD4+ T cells comprised 2–3% of Rag−/− recipient spleen cells and 50% of cells in the MLN; the donor CD4+ T cell frequency was dramatically reduced in antibiotic treated recipient mice (Fig. 5A). Post-revision Vβ5− cells accumulated preferentially compared to Vβ5+ cells in Rag−/− recipients, while antibiotic treatment resulted in a Vβ5− to Vβ5+ cell ratio similar to that of the inoculum (Fig. 5B and Fig. 5 legend). In untreated recipients, donor Vβ5− and Vβ5+ cells divided extensively, with >80% of cells having completely diluted their CFSE, while proliferation was greatly retarded in antibiotic-treated recipients (Fig. 5C). Donor Vβ5− post-revision T cells were recovered at high frequencies in the MLN and to a lesser degree in the spleen of untreated recipients. The recovery of post-revision cells compared to that of Vβ5+ T cells was approximately 5–7-fold higher in the spleen, and 11– 15-fold higher in the MLN of Rag−/− recipients (Fig. 5D). Antibiotic treatment of recipient mice resulted in a 700-fold reduction in donor CD4+Vβ5− T cell recovery in the MLN and a 40-fold reduction in the spleen (Fig. 5D). Together, these data demonstrate that post-revision cells, and to a lesser degree CD4+Vβ5+ cells, proliferate and accumulate extensively in response to microbial Ags in Rag−/− recipient mice.
Figure 5. Post-revision T cells undergo rapid proliferation to bacterial Ag following transfer into chronically lymphopenic recipients.
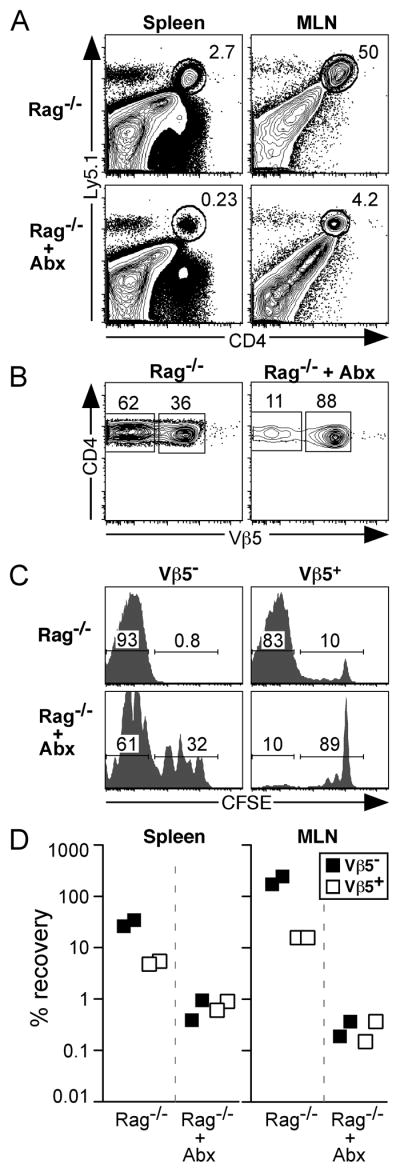
2.6×106 CFSE-labeled Ly5.1+ CD4+ T cells (comprised of 12% Vβ5− post-revision cells and 88% Vβ5+ cells) enriched from pooled spleen and lymph nodes of aged Vβ5 Tg mice were transferred into chronically lymphopenic Rag−/− recipient mice that were either untreated or treated (+ Abx) with a cocktail of antibiotics in their drinking water beginning 6 days prior to adoptive transfer. Donor CD4+ T cells were analyzed 6 days post-transfer for the extent of proliferation as measured by CFSE dilution and the percent recovery of each population. A. Analysis of live gated splenocytes and MLN cells with gate indicating Ly5.1+ donor CD4+ T cells. B. CD4 and Vβ5 analysis of donor Ly5.1+CD4+ gated cells from the MLN. C. CFSE analysis of donor CD4+Vβ5− (left) and CD4+Vβ5+ (right) T cells from the MLN. Numbers within CFSE histograms represent the percent of gated cells that have fully diluted CFSE (left), or have undergone 0–4 cell divisions (right). D. Charts indicate the percent recovery of donor Vβ5− and Vβ5+ CD4+ T cells in spleen and MLN.
Effector function of post-revision CD4+ T cells
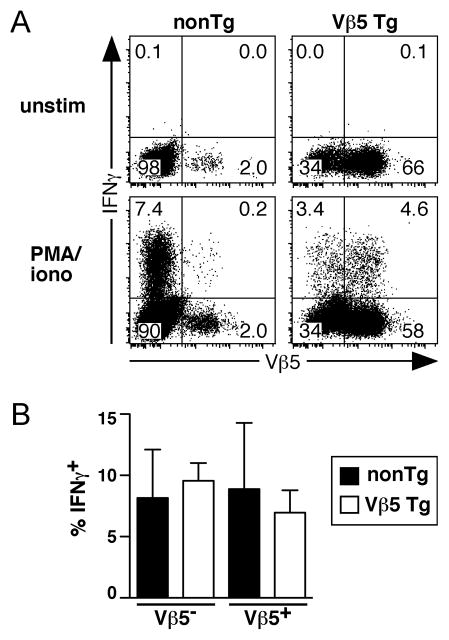
To assess the immunocompetence of post-revision T cells, we first determined whether they produce IFNγ. CD4+ T cells from aged nonTg and Vβ5 Tg mice were stimulated with PMA + ionomycin for 5 hours and found to include similar frequencies of IFNγ-producing cells (Fig. 6A). Comparison of Vβ5− and Vβ5+ CD4+ T cells from nonTg and Vβ5 Tg mice indicated that post-revision cells and Vβ5+ and Vβ5− CD4+ T cells from age-matched nonTg mice have similar frequencies of IFNγ+ cells (Fig. 6A and 6B). Thus, the post-revision T cell pool contains functional cytokine-producing cells that may contribute to host immunity.
Figure 6. Post-revision T cells include IFNγ-producing cells.
Splenocytes from 25–34-week-old nonTg and Vβ5 Tg mice were stimulated with PMA + ionomycin (iono) for 6 hours or left untreated (unstim), followed by surface CD4 and Vβ5 and intracellular IFNγ staining. A. Representative Vβ5 and IFNγ analysis of CD4+ T cells. B. Chart indicates the percent of stimulated Vβ5− and Vβ5+ CD4+ T cells from nonTg and Vβ5 Tg mice that produce IFNγ. Bars represent the mean percent of each indicated cell population with error bars indicating the SD (Vβ5 Tg: N=3; nonTg: N=3). No significant differences were obtained using a two-tailed nonparametric Student’s t test.
To test whether post-revision cells are self-MHC restricted, aged Vβ5 Tg mice were infected with Listeria monocytogenes. Seven days post-infection, Ag-specific CD4+IFNγ+ T cells were identified by culturing splenocytes from infected mice with the immunodominant I-Ab restricted Listeria epitope (LLO190–201 peptide), followed by intracellular cytokine staining (Fig. 7A). Post- revision Vβ5− cells comprised >95% of the LLO190–201-specific CD4+IFNγ+ population in Vβ5 Tg mice (Fig. 7B). Similar to Ag-specific CD4+ T cells from infected nonTg mice, IFNγ+ LLO190–201-specific post-revision cells exhibited decreased levels of surface TCRβ, resulting from contact with specific peptide during the 5 hour incubation (Fig. 7C). Thus, post-revision cells can recognize Ag in a self-MHC restricted manner, and become functional cytokine-producing effector cells in response to a bacterial challenge.
Figure 7. Post-revision Vβ5− T cells produce IFNγ in a primary response to Listeria infection.
Aged nonTg and Vβ5 Tg mice were infected with Listeria monocytogenes and 7 days later, CD8-depleted splenocytes were cultured for 5 hours with or without LLO190–201 peptide, followed by surface staining for CD4, Vβ5, and TCRβ, and intracellular staining for IFNγ. A. Intracellular IFNγ staining of CD4+ gated splenocytes. B. Vβ5 expression by total CD4+ and Ag-specific CD4+IFNγ+ gated splenocytes. C. Surface panTCRβ expression by CD4+ gated and Ag-specific CD4+IFNγ+ gated splenocytes.
Discussion
In this study, we show that post-revision CD4+Vβ5− T cells generated through peripheral TCR revision are capable of undergoing homeostatic proliferation. Previous studies defining the homeostatic requirements of memory phenotype CD4+CD44highCD25− T cells (which may or may not contain memory cells formed through encounter with specific foreign Ag) upon transfer into acutely lymphopenic recipients have revealed a heterogeneous population of rapidly dividing cells that require MHC class II for their extensive proliferation, and a population that does not require MHC class II and relies on IL-7 to drive slow cell division (28). In contrast, Ag-specific CD4+CD44high memory cells generated through encounter with a known viral Ag only underwent the slower, IL-7-dependent LIP (28). Here we show that post-revision CD4+Vβ5− T cells undergo more extensive LIP in irradiated recipients than their Vβ5+ counterparts. This difference in proliferation may have resulted from the Mtv-dependent anergy that characterizes the CD4+Vβ5+ T cell population in B6 mice (4). Importantly, the most extensive LIP of post-revision cells requires the presence of MHC class II molecules, because the rapidly dividing population was absent following transfer to irradiated I-Aβ−/− mice. In addition to the rapidly proliferating population, we also noted a slower dividing population of post-revision T cells that is not dependent on the presence of MHC, and may instead be driven by IL-7. Our comparison of co-transferred post-revision and effector/memory nonTg CD44highCD62L− cells demonstrates that post-revision cells compete effectively with effector/memory CD4+ T cells from nonTg mice for homeostatic factors. In summary, similar to CD4+CD44high memory phenotype cells from nonTg B6 mice, post-revision cells transferred into acutely lymphopenic irradiated hosts require the presence of MHC II for the most rapid proliferation, and other factors (likely IL-7) for slower proliferation.
Unlike memory phenotype cells from nonTg mice, the in situ steady-state proliferation by post-revision T cells (as measured by BrdU labeling) appears to be more rapid than that of the other CD44high cell populations analyzed (Fig. 1). Several possibilities could explain such rapid basal proliferation. First, post-revision cells exist in a CD4-lymphopenic setting, and thus their high basal proliferation may be driven by the increased abundance of homeostatic cytokines and self-MHC ligand compared to that available to CD44high cells in lymphoreplete nonTg B6 mice. However, the steady-state proliferation of post-revision cells is also increased compared to that of Tg CD44highVβ5+ cells sharing the same environment, suggesting that lymphopenia is not the only factor that determines their increased proliferation. A second possibility is that extrathymic selection of post-revision T cells generates a more self-reactive TCR repertoire. The decreased number of N nucleotides and resultant shortened TCRβ CDR3 regions of revised TCRs (18) may endow post-revision TCRs with a more promiscuous peptide recognition and an increased affinity for MHC II α helices, similar to that characterizing TCRs of neonatal T cells (29–31). An increased affinity for MHC could drive the more rapid basal homeostatic proliferation of post-revision T cells.
It remains to be elucidated whether this potential self-reactivity is beneficial for arming post-revision T cells with a promiscuous/cross-reactive TCR repertoire (31), or harmful, by giving post-revision cells self-reactive TCR specificities capable of self-tissue destruction. TdT−/− mice on autoimmune-prone genetic backgrounds are protected from autoimmunity, suggesting that the decreased N nucleotides of neonatal TCRs protects neonates from generating autoaggressive TCR specificities (32, 33), while ensuring functional recognition of foreign Ag with the limited available TCR repertoire (31, 34). Similarly, the low number of N nucleotides in revised TCRs (18) may allow receptors generated through TCR revision to protect the host, absent the functional avidity for self-Ags that can mediate autoimmunity. The absence of overt autoimmunity in Vβ5 Tg mice suggests that post-revision T cells do not actively mediate autoimmune responses, although self-reactive cells may be controlled in these mice by the relatively high proportion of Foxp3+ Treg (21).
Previously, our lab has shown that the Foxp3+ Treg lineage is excluded from the post-revision population, and that TCR revision likely skews the post-revision T cell compartment in favor of IL-17 producing Th17 cells (21). In this study, we observed that the post-revision compartment in aged Vβ5 Tg mice includes a population that readily produces IFNγ when stimulated directly ex vivo with PMA + ionomycin; however, the frequency of IFNγ+ cells is similar to that found in the Vβ5+ population and in the CD4+ T cell population in age-matched nonTg mice. Thus, TCR revision does not preferentially skew the post-revision T cell compartment toward IFNγ production.
Post-revision T cells proliferate extensively to bacterial Ags following transfer to Rag−/− mice and produce IFNγ in response to Listeria monocytogenes challenge of Vβ5 Tg mice. Thus, post-revision T cells are selected, possessing the ability to recognize foreign Ag in the context of self-MHC. It is unknown whether the revised TCRβ chains pair with the TCRα chains originally generated and selected within the thymus of the Vβ5 Tg mouse, or whether TCR revision at the TCRβ locus is accompanied by TCRα locus rearrangement to form a functional, revised TCR that can generate these Ag-specific responses. In either case, the shortened CDR3 loops of revised TCRβ chains may be critical for endowing the revised TCRαβ pair with the ability to interact with MHC molecules in a useful way, allowing for more efficient selection, an interpretation suggested by the increased efficiency of positive selection of TdT−/− thymocytes (35). Our findings suggest that MHC class II+ cells in the lymphoid periphery, perhaps under specific conditions, possess the ability to select the TCRs generated through TCR revision, allowing post-revision T cells to see both MHC-self-peptide and MHC-foreign peptide ligands. The germinal center location of RAG+ revising T cells may provide a unique microenvironment that allows proper selection (negative and/or positive) of the post-revision TCR repertoire (36).
It is unclear why CD4+Vβ5+ cells from both nonTg and Vβ5 Tg mice respond poorly to Listeria challenge. It is possible that the Mtv-driven anergy of Vβ5+ cells impairs their response. Alternatively, Vβ5 TCR chains may lack the ability to recognize the LLO190–201 epitope. We can not distinguish between these possibilities experimentally, because H-2b Mtv− mice derived from a wild mouse genetic background (6) display unusual susceptibility to Listeria infection (data not shown).
The proliferation of donor CD4+ Vβ5+ and post-revision Vβ5− T cells in Rag−/− recipients was clearly dependent on the presence of bacterial microflora, because antibiotic treatment reduced the rate of proliferation and cell recovery for both cell populations (Fig. 5). The blunted response to microbial Ags and decreased accumulation of donor Vβ5+ cells compared to post-revision cells likely stems from the anergic state of Vβ5+ cells, driven by chronic encounter with the Mtv tolerogen previous to cell transfer. This well characterized anergy (4), combined with the weak interaction between Vβ5+ T cells and the Mtv ligand (25, 26), also likely explains the lack of a proliferative response to Mtv by Vβ5+ cells transferred to Rag−/− mice treated with antibiotics. Although some donor T cell proliferation occurred even in antibiotic treated Rag−/− mice, particularly among the Vβ5− population (Fig. 5C), this likely resulted from the presence of microbes that are insensitive to the antibiotic regimen used. Interestingly, despite the continued (although decreased) proliferation of post-revision cells in antibiotic treated Rag−/− mice (Fig. 5C), their frequency is not increased compared to that of Vβ5+ cells (Fig. 5B), which are proliferating less extensively. This suggests that the post-revision cells in the antibiotic treated mice may possibly leave secondary lymphoid organs and migrate to peripheral tissues, in agreement with the higher frequency of post-revision cells found in the peritoneal lavage compared to the spleens of aged Vβ5 Tg mice (data not shown).
Post-revision T cells display TCR-dependent activity for their maximal proliferation in acutely lymphopenic hosts, proliferation to commensal bacterial Ags following transfer to Rag−/− mice, and IFNγ production in response to Listeria infection. It is theoretically possible that a low level of Vβ5 TCR expression on post-revision T cells is responsible for the TCR signaling in these cells. However, this seems unlikely, given that post-revision cells do not proliferate when stimulated with Mtv-overexpressing B lymphoma cells. We conclude therefore, that the TCR-dependent activation/function of post-revision CD4+Vβ5−TCRβ+ cells is mediated through the newly generated receptor formed by TCR revision in the lymphoid periphery. This lack of reactivity to the original Mtv tolerogen further establishes TCR revision as a means of tolerance induction for peripheral T cells that recognize self-Ag. We conclude from these cumulative findings that TCR revision acts as a tolerance mechanism to rescue self-reactive CD4+ T cells, generating a post-revision T cell population that responds to homeostatic factors and possesses immune potential to protect the host against foreign antigenic challenge.
Acknowledgments
The authors would like to thank Dr. Mark J. Smyth who generously provided the PN53HI tumor cells, and Drs. Murali-Krishna Kaja and Daniel Stetson for providing mice.
Non-standard abbreviations
- Tg
transgenic
- nonTg
non-transgenic
- MLN
mesenteric lymph node
- Mtv
mammary tumor virus
- LIP
lymphopenia-induced proliferation
- WT
wild-type
- SP
single-positive
- B6
C57BL/6
Footnotes
This work was supported by the National Institutes of Health (grant RO1 AG 13078 to P.J.F), the Cancer Research Institute’s Predoctoral Emphasis Pathway in Tumor Immunology Program (to J.S.H.), and the National Cancer Institute Basic and Cancer Immunology Grant (grant T32CA09537 to J.S.H). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the CRI, or the NCI.
References
- 1.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 2.Seddon B, Zamoyska R. Regulation of peripheral T cell homeostasis by receptor signalling. Curr Opin Immunol. 2003;15:321–324. doi: 10.1016/s0952-7915(03)00049-9. [DOI] [PubMed] [Google Scholar]
- 3.Fink PJ, Swan K, Turk G, Moore MW, Carbone FR. Both intrathymic and peripheral selection modulate the differential expression of Vβ5 among CD4+ and CD8+ T cells. J Exp Med. 1992;176:1733–1738. doi: 10.1084/jem.176.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fink PJ, Fang CA, Turk GL. The induction of peripheral tolerance by the chronic activation and deletion of CD4+Vβ5+ cells. J Immunol. 1994;152:4270–4281. [PubMed] [Google Scholar]
- 5.McMahan CJ, Fink PJ. RAG reexpression and DNA recombination at T cell receptor loci in peripheral CD4+ T cells. Immunity. 1998;9:637–647. doi: 10.1016/s1074-7613(00)80661-5. [DOI] [PubMed] [Google Scholar]
- 6.Blish CA, Gallay BJ, Turk GL, Kline KM, Wheat W, Fink PJ. Chronic modulation of the T cell receptor repertoire in the lymphoid periphery. J Immunol. 1999;162:3131–3140. [PubMed] [Google Scholar]
- 7.Cooper CJ, Orr MT, McMahan CJ, Fink PJ. T cell receptor revision does not solely target recent thymic emigrants. J Immunol. 2003;171:226–233. doi: 10.4049/jimmunol.171.1.226. [DOI] [PubMed] [Google Scholar]
- 8.Takase M, Kanagawa EM, Kanagawa O. Age-dependent TCR revision mediated by interaction between αβ TCR and self-antigens. J Immunol. 2007;179:2163–2169. doi: 10.4049/jimmunol.179.4.2163. [DOI] [PubMed] [Google Scholar]
- 9.Bynoe MS, Viret C, Flavell RA, Janeway CA., Jr T cells from epicutaneously immunized mice are prone to T cell receptor revision. Proc Natl Acad Sci U S A. 2005;102:2898–2903. doi: 10.1073/pnas.0409880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serra P, Amrani A, Han B, Yamanouchi J, Thiessen SJ, Santamaria P. RAG-dependent peripheral T cell receptor diversification in CD8+ T lymphocytes. Proc Natl Acad Sci USA. 2002;99:15566–15571. doi: 10.1073/pnas.242321099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang CY, Golub R, Wu GE, Kanagawa O. Superantigen-induced TCRα locus secondary rearrangement: role in tolerance induction. J Immunol. 2002;168:3259–3265. doi: 10.4049/jimmunol.168.7.3259. [DOI] [PubMed] [Google Scholar]
- 12.Vaitaitis GM, Poulin M, Sanderson FJ, Haskins K, Wagner DH., Jr Cutting Edge: CD40-induced expression of recombination activating gene (RAG)1 and RAG2: a mechanism for the generation of autoaggressive T cells in the periphery. J Immunol. 2003;170:3455–3459. doi: 10.4049/jimmunol.170.7.3455. [DOI] [PubMed] [Google Scholar]
- 13.Lantelme E, Palermo B, Granziero L, Mantovani S, Campanelli R, Monafo V, Lanzavecchia A, Giachino C. Cutting Edge: Recombinase-activating gene expression and V(D)J recombination in CD4+CD3low mature T lymphocytes. J Immunol. 2000;164:3455–3459. doi: 10.4049/jimmunol.164.7.3455. [DOI] [PubMed] [Google Scholar]
- 14.Li TT, Han S, Cubbage M, Zheng B. Continued expression of recombination-activating genes and TCR gene recombination in human peripheral T cells. Eur J Immunol. 2002;32:2792–2799. doi: 10.1002/1521-4141(2002010)32:10<2792::AID-IMMU2792>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 15.Lantelme E, Mantovani S, Palermo B, Campanelli R, Granziero L, Monafo V, Giachino C. Increased frequency of RAG-expressing, CD4+CD3low peripheral T lymphocytes in patients with defective responses to DNA damage. Eur J Immunol. 2000;30:1520–1525. doi: 10.1002/(SICI)1521-4141(200005)30:5<1520::AID-IMMU1520>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 16.Lantelme E, Turinetto V, Mantovani S, Marchi A, Regazzoni S, Porcedda P, De Marchi M, Giachino C. Analysis of secondary V(D)J rearrangements in mature, peripheral T cells of ataxia-telangiectasia heterozygotes. Lab Invest. 2003;83:1467–1475. doi: 10.1097/01.lab.0000092228.51605.6a. [DOI] [PubMed] [Google Scholar]
- 17.Lantelme E, Orlando L, Porcedda P, Turinetto V, De Marchi M, Amoroso A, Mantovani S, Giachino C. An in vitro model of T cell receptor revision in mature human CD8+ T cells. Mol Immunol. 2008;45:328–337. doi: 10.1016/j.molimm.2007.06.153. [DOI] [PubMed] [Google Scholar]
- 18.McMahan CJ, Fink PJ. Receptor revision in peripheral T cells creates a diverse Vβ repertoire. J Immunol. 2000;165:6902–6907. doi: 10.4049/jimmunol.165.12.6902. [DOI] [PubMed] [Google Scholar]
- 19.Hale JS, Ames KT, Boursalian TE, Fink PJ. Cutting Edge: Rag deletion in peripheral T cells blocks TCR revision. J Immunol. 2010;184:5964–5968. doi: 10.4049/jimmunol.1000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hale JS, Fink PJ. T-cell receptor revision: friend or foe? Immunology. 2010;129:467–473. doi: 10.1111/j.1365-2567.2010.03250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zehn D, Bevan MJ, Fink PJ. Cutting Edge: TCR revision affects predominantly Foxp3 cells and skews them toward the Th17 lineage. J Immunol. 2007;179:5653–5657. doi: 10.4049/jimmunol.179.9.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, Miller RG, Mak TW. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;253:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 23.Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 24.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Foo-Phillips M, Kozak CA, Principato MAC, Abe R. Characterization of the Mlsf system. II. Identification of mouse mammary tumor virus proviruses involved in the clonal deletion of self-Mlsf-reactive T cells. J Immunol. 1992;149:3440–3447. [PubMed] [Google Scholar]
- 26.Waanders GA, Lees RK, Held W, MacDonald HR. Quantitation of endogenous mouse mammary tumor virus superantigen expression by lymphocyte subsets. Eur J Immunol. 1995;25:2632–2637. doi: 10.1002/eji.1830250934. [DOI] [PubMed] [Google Scholar]
- 27.Kieper WC, Troy A, Burghardt JT, Ramsey C, Lee JY, Jiang H, Dummer W, Shen H, Cebra JJ, Surh CD. Cutting Edge: Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol. 2005;174:3158–3163. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 28.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feeney AJ. Junctional sequences of fetal T cell receptor β chains have few N regions. J Exp Med. 1991;174:115–124. doi: 10.1084/jem.174.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogue M, Candeias S, Benoist C, Mathis D. A special repertoire of α:β T cells in neonatal mice. EMBO Journal. 1991;10:3647–3654. doi: 10.1002/j.1460-2075.1991.tb04931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavin MA, Bevan MJ. Increased peptide promiscuity provides a rationale for the lack of N regions in the neonatal T cell repertoire. Immunity. 1995;3:793–800. doi: 10.1016/1074-7613(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 32.Conde C, Weller S, Gilfillan S, Marcellin L, Martin T, Pasquali JL. Terminal deoxynucleotidyl transferase deficiency reduces the incidence of autoimmune nephritis in (New Zealand Black x New Zealand White)F1 mice. J Immunol. 1998;161:7023–7030. [PubMed] [Google Scholar]
- 33.Robey IF, Peterson M, Horwitz MS, Kono DH, Stratmann T, Theofilopoulos AN, Sarvetnick N, Teyton L, Feeney AJ. Terminal deoxynucleotidyltransferase deficiency decreases autoimmune disease in diabetes-prone nonobese diabetic mice and lupus-prone MRL-Fas(lpr) mice. J Immunol. 2004;172:4624–4629. doi: 10.4049/jimmunol.172.7.4624. [DOI] [PubMed] [Google Scholar]
- 34.Gilfillan S, Bachmann M, Trembleau S, Adorini L, Kalinke U, Zinkernagel R, Benoist C, Mathis D. Efficient immune responses in mice lacking N-region diversity. Eur J Immunol. 1995;25:3115–3122. doi: 10.1002/eji.1830251119. [DOI] [PubMed] [Google Scholar]
- 35.Gilfillan S, Waltzinger C, Benoist C, Mathis D. More efficient positive selection of thymocytes in mice lacking terminal deoxynucleotidyl transferase. Int Immunol. 1994;6:1681–1686. doi: 10.1093/intimm/6.11.1681. [DOI] [PubMed] [Google Scholar]
- 36.Cooper CJ, Turk G, Sun M, Farr AG, Fink PJ. Cutting Edge: TCR revision occurs in germinal centers. J Immunol. 2004;173:6532–6536. doi: 10.4049/jimmunol.173.11.6532. [DOI] [PubMed] [Google Scholar]