Abstract
Background
In the conditioned fear paradigm, repeated pairing of an aversive unconditioned stimulus (US) (e.g. electric shock) with a neutral conditioned stimulus (CS) (e.g. bright light) results in a conditioned fear response to the light alone. Animal studies have shown that the amygdala plays a critical role in acquisition of conditioned fear responses, while the medial prefrontal cortex (including anterior cingulate), through inhibition of amygdala responsiveness, has been hypothesized to play a role in extinction of fear responses. No studies have examined neural correlates of fear conditioning and extinction in patients with post-traumatic stress disorder (PTSD).
Method
Women with early childhood sexual-abuse-related PTSD (n=8) and women without abuse or PTSD (n=11) underwent measurement of psychophysiological (skin conductance) responding as well as positron emission tomographic (PET) measurement of cerebral blood flow during habituation, acquisition and extinction conditions. During habituation subjects were repeatedly exposed to a blue square on a screen. During acquisition, exposure to the blue square (CS) was paired with an electric shock to the forearm (US). With extinction, subjects were again exposed to the blue squares without shock. On a different day subjects went through the same procedure with electric shocks administered randomly in the absence of the blue square.
Results
Skin conductance responding to the CS was consistent with the development of conditioned responses with this paradigm. PTSD patients had increased left amygdala activation with fear acquisition, and decreased anterior cingulate function during extinction, relative to controls.
Conclusions
These findings implicate amygdala and anterior cingulate in the acquisition and extinction of fear responses, respectively, in PTSD.
INTRODUCTION
Childhood sexual-abuse-related post-traumatic stress disorder (PTSD) is a major public health problem that affects about 15% of traumatized individuals and is twice as common in women as in men (Kessler et al. 1995; MacMillan et al. 1997; McCauley et al. 1997), and often leads to chronic morbidity (Saigh & Bremner, 1999). In spite of the magnitude of childhood abuse and PTSD in our society, little is known about the effects of early abuse on the brain.
Animal studies show that early stress has long-term effects on brain regions including hippocampus, amygdala, cingulate, and prefrontal cortex. Brain areas that have functional significance for PTSD and the response to threat (Bremner, 1998; Pitman, 2001) share in common the fact that they mediate different aspects of memory and visuospatial processing. Medial prefrontal cortex (Vogt et al. 1992; Devinsky et al. 1995), which is particularly sensitive to stress (Roth et al. 1988), consists of several related areas, including orbitofrontal cortex, anterior cingulate (Area 25 – subcallosal gyrus, and Area 32), and anterior prefrontal cortex (Area 9). Animals with lesions of the medial prefrontal cortex are unable to extinguish fear responses after trials of fear conditioning (Morgan et al. 1993; Morgan & LeDoux, 1995). Human subjects with lesions of the prefrontal cortex show dysfunction of normal emotions and an inability to relate in social situations that require correct interpretation of the emotional expressions of others (Damasio et al. 1994). The amygdala plays a central role in conditioned fear responses (Davis, 1992; LeDoux, 1996). The hippocampus is involved in fear responses to the context of a stressful situation (Kim & Fanselow, 1992; Phillips & LeDoux, 1992) in addition to its role in declarative memory. Posterior cingulate, parietal and motor cortex, and cerebellum are functionally related to antero-lateral prefrontal cortex (superior and middle frontal gyri) (Selemon & Goldman-Rakic, 1988), mediating visuospatial processing that is critical to survival in life-threatening situations.
One of the most important brain areas in this interconnected network mediating the stress response is the amygdala (Davis, 1992; LeDoux, 1996). In the conditioned fear response, pairing of an unconditioned stimulus (US) (e.g. electric shock) with a conditioned stimulus (CS) (e.g. bright light) leads to a fear reaction to the CS (bright light) alone. The neuroanatomy and neurophysiology of conditioned fear responses in animals have been well characterized (Hitchcock & Davis, 1986; Rosen & Davis, 1988; Hitchcock et al. 1989; Miserendino et al. 1990; Hitchcock & Davis, 1991). Lesions of the central nucleus of the amygdala have been shown to completely block fear-potentiated startle (Hitchcock & Davis, 1986; Hitchcock et al. 1989) while electrical stimulation of the central nucleus increases acoustic startle (Rosen & Davis, 1988). Pathways from the amygdala to the lateral hypothalamus effect peripheral sympathetic responses to stress (Iwata et al. 1986). Electrical stimulation of the amygdala results in peripheral signs of autonomic hyperactivity, increased catecholamine turnover, and fear-related behaviors (Chapman et al. 1954; Gunne & Reis, 1963; Hilton & Zbrozyna, 1963), while lesions of the amygdala in humans result in an impairment in fear conditioning (LaBar et al. 1995).
Studies have begun to use neuroimaging to map the neural correlates of fear and emotion in normal human subjects. Studies using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) measurement of correlates of brain blood flow found increased amygdala activity with emotional paradigms including conditioning, exposure to angry faces, and negative pictures (Cahill et al. 1996; Buchel et al. 1998; LaBar et al. 1998; Morris et al. 1998; Canli et al. 1999; Hamann et al. 1999; Canli et al. 2000). One study using pairing of an unconditioned stimulus (electric shock) with a conditioned stimulus (colored shape on a screen) found an increase in amygdala region activity as measured with fMRI during acquisition of fear responding to the conditioned stimulus (LaBar et al. 1998). Another study found increased medial prefrontal and orbitofrontal activation with extinction to classical conditioned fear in healthy subjects (Hugdahl et al. 1995).
There is some evidence for abnormalities in amygdala function in PTSD. Based on animal studies, increased startle responses are postulated to represent an increase in amygdala function. Some studies (but not others) have found abnormalities of the startle response in patients with PTSD which have been hypothesized to be mediated by the amygdala (Ornitz & Pynoos, 1989; Ross et al. 1989; Butler et al. 1990; Paige et al. 1990; Shalev et al. 1992; Morgan et al. 1995). Psychophysiology studies involving measurements of heart rate and skin conductance as markers of sympathetic function have found increased sympathetic correlates of exposure to traumatic reminders in PTSD, an effect hypothesized to be related to increased amygdala function (Blanchard et al. 1986; Pitman et al. 1987, 1990; McFall et al. 1990; Orr et al. 1993, 1998; Keane et al. 1998; Orr & Roth, 2000; Shalev et al. 2000). Orr and colleagues (2000) measured responses to fear conditioning using a pairing of conditioned stimulus (colored circles) with unconditioned stimulus (aversive electric shock) and found an increase in heart rate, skin conductance, and EMG responses both during acquisition and extinction of conditioned fear in PTSD patients relative to controls. Peri and colleagues (2000) paired colored slides with bursts of white noise and found that PTSD patients had increased heart rate response to fear acquisition (slides plus noise) and increased heart rate and skin conductance responses to extinction (slides alone) compared with traumatized non-PTSD and non-traumatized controls. Grillon & Morgan (1999) compared startle responses in Gulf War veterans with and without PTSD during exposure to two colored lights either paired or not paired to aversive electric shock. Non-PTSD subjects developed conditioned responses to the colored light previously paired with the aversive stimulus while PTSD subjects exhibited conditioned responses to both lights. Startle increased from sessions 1 to 2 in PTSD and decreased in non-PTSD, suggesting an over-generalization of fear responses in PTSD. In summary, some studies, but not others, are consistent with increased startle and enhanced conditioning in PTSD.
Neuroimaging studies have begun to map a neural circuitry of PTSD (Bremner, 1998; Pitman, 2001). Symptom provocation studies in PTSD patients found decreased prefrontal (Bremner et al. 1997, 1999a, b; Shin et al. 1997, 1999; Liberzon et al. 1999), parietal, hippocampal (Bremner et al. 1997 , 1999a), and temporal cortical function (Bremner et al. 1997, 1999a, b), and increased function in posterior cingulate, motor cortex (Bremner et al. 1999a, b), and amygdala (Rauch et al. 2000). Studies to date have not consistently implicated the amygdala in the neural circuitry of traumatic memories as measured with functional imaging. One explanation is that triggering of traumatic memories through techniques such as traumatic scripts represents a different condition than the acquisition of conditioned fear responses as measured in the laboratory. A prior study using exposure to masked fearful faces showed increased amygdala activation in PTSD (Rauch et al. 2000). No published studies to date have used fear conditioning in conjunction with functional neuroimaging in PTSD. Therefore the purpose of this study was to measure neural correlates of fear acquisition and extinction in abuse-related PTSD. We hypothesized increased amygdala function with fear acquisition, and decreased function or failure of activation in medial prefrontal cortex during fear extinction, in women with abuse-related PTSD compared with controls.
METHOD
Study subjects
Nineteen physically healthy women participated in the study. Subjects included women with a history of severe childhood sexual abuse and the diagnosis of current PTSD (n=8) and women without childhood abuse or PTSD (n=11). All subjects were recruited through newspaper advertisement. This study was approved by the Yale University Institutional Review Board for research in human subjects. Diagnosis of PTSD was established with the Structured Clinical Interview for DSM-IV (SCID; First et al. 1995). All subjects gave written informed consent for participation, were free of major medical illness on the basis of history and physical examination, laboratory testing, and electrocardiogram, were not actively abusing substances or alcohol (past 6 months) and were free of all medications for at least 4 weeks prior to the study. Subjects were not taken off medication for the purposes of participating in the study. Subjects with a serious medical or neurological illness, organic mental disorders or co-morbid psychotic disorders, retained metal, a history of head trauma, loss of consciousness, cerebral infectious disease, or dyslexia were excluded. Absence of psychiatric disorder in the non-PTSD subjects was confirmed with the SCID for Non-Patients (SCID-NP). Absence of childhood trauma in the controls was confirmed with the Early Trauma Inventory (ETI) (described below). There was no difference in age between the women with (mean=38, S.D.=10) and without (mean=36, S.D.=11) PTSD (F=0·33; df=1, 17; p=0·52).
Psychometric assessments
History of childhood abuse was assessed with the Early Trauma Inventory-Self Report Version (ETI-SR). The ETI is a 56-item clinician-administered interview that assesses physical, emotional, and sexual abuse, as well as general traumatic events. The ETI has been demonstrated to be reliable and valid in the assessment of childhood trauma (Bremner et al. 2000). PTSD subjects had an average score of 71 (34 S.D.) (consistent with a high level of trauma exposure) while non-PTSD subjects had a score of 22 (18 S.D.) on the ETI-SR.
Women with PTSD had a pattern of co-morbidity similar to prior studies of PTSD from our group and others. Six out of eight PTSD subjects (75%) fulfilled criteria for a past history of major depression and two of eight (25%) for current major depression based on the SCID interview. Two PTSD subjects (25%) had a history of current and lifetime dysthymia. In all cases the onset of the affective disorder was after the onset of PTSD. One patient (13%) fulfilled criteria for current and lifetime history of panic disorder with agoraphobia. Two PTSD subjects (25%) had current and lifetime generalized anxiety disorder, one patient (13%) had current and lifetime social phobia and one patient (13%) had current and lifetime simple phobia. One PTSD subject (13%) fulfilled criteria for a past history of alcohol dependence, one (13%) for a past history of opiate dependence, one (13%) for a past history of marijuana dependence and two (25%) for a past history of cocaine dependence. No PTSD subjects had a current history of alcohol or substance abuse or dependence.
Subjects were rated at baseline and during each scanning condition for PTSD symptoms using the PTSD Symptom Scale, a measure of PTSD symptomatology (Southwick et al. 1993); subjective distress as measured with the Subjective Units of Distress Scale (SUDS) scale, a validated measure of subjective distress on a scale of 0–100 (Wolpe & Lazarus, 1966); dissociative symptoms using the Clinician Administered Dissociative States Scale (CADSS), a validated and reliable measure of dissociative state symptomatology (Bremner et al. 1998); anxiety with the Panic Attack Symptom Scale (PASS), a measure of panic and anxiety symptom level (Southwick et al. 1993); and analogue ratings of fear as previously described (Bremner et al. 1997). All PTSD subjects were rated for baseline PTSD symptoms with the Clinician Administered PTSD Scale (CAPS; Blake et al. 1995). Mean score on the CAPS was 68 (25 S.D.).
PET imaging methods
All subjects underwent PET measurement of cerebral blood flow and psychophysiology measurement of heart rate and skin conductance during habituation, acquisition and extinction conditions, on a single day, with scanning during a control condition on another day separated by 1 week from the active condition. Subjects were randomly assigned to undergo either the active condition or the control condition first (i.e. active-control or control-active). Subjects were told at the beginning of the study that they would be exposed to electric shocks and viewing images on a screen during collection of PET and psychophysiology data. At the beginning of the study electrodes were placed on the left wrist for application of electric shock. During habituation subjects were exposed to a blue square on a screen [conditioned stimulus (CS)], 4 s in duration, followed by 6 s of a blank screen. CS exposure was repeated eight times at regular intervals over 80 s in two separate blocks separated by 8 min. One PET image of brain blood flow was obtained starting from the beginning of each of the blocks. During active fear acquisition exposure to the blue square (CS) was paired with an electric shock to the forearm [unconditioned stimulus (US)]. Electric shock (3·0 mA, 5 ms in duration) was delivered 0·5 s after the onset of the presentation of the CS by a constant current stimulator (Grass Instruments, CCU1A) attached to two pure tin disk electrodes placed on the inside of the left wrist. Subjects were asked if the stimulus was annoying, and if not the stimulus was increased until they subjectively described it as annoying. Subjects had eight paired CS-US presentations at 10-s intervals for each of two blocks. With extinction subjects were again exposed to the blue squares (CS) without shock (‘active’ extinction). On a second day subjects went through the same procedure with electric shocks delivered randomly when the blue square was not present (unpaired CS-UCS) (an equal number as on day 1) during scans 3 and 4, which served as a control for active fear acquisition. The conditioned stimulus was presented for 4 s, followed by a 6-s rest period. This was repeated continuously for an 80-s period. This was repeated for two blocks of eight CS presentations each. Following this subjects underwent extinction, where they were exposed to two blocks of presentations of CS without electric shock; again the CS was presented for 4 s with a 6-s interval between presentations, for a total of eight CS per block. Extinction blocks following active fear acquisition (paired CS-US) are referred to as active extinction. Extinction blocks following control fear acquisition (unpaired CS-US) are referred to as control extinction.
During the study period assessments were also made of psychophysiological parameters including heart rate and skin conductance. Heart rate was measured continuously every 5 s using a Polar Vantage heart rate recording device (Woodbury, NY, USA). The Polar Vantage heart rate recording apparatus was moistened with water to facilitate recording and strapped around the subject’s chest for direct measurement of heart rate. Heart rate data were transmitted from the recording device to a Polar Vantage recording device worn as a wrist watch on the subject’s wrist. Following the study session the data were downloaded to a personal computer for analysis. Heart rate over 5-s intervals was compared between a baseline period 10 min before the onset of the session (after the shock electrodes had been attached) and over 5-s intervals during the period of extinction. Skin conductance (SC) was also measured continuously during the experiment using Biograph Pro-comp equipment (Thought Technologies, Montreal, Canada). Data were collected in digital format. SC was analyzed during the acquisition and extinction phases as the delta of peak SC during exposure to CS to 1 s before exposure to CS. There were two 80-s extinction sessions with CS presented eight times in each session.
PET imaging was performed on a Posicam PET camera (Positron Corp) [in-plane resolution after filtering, 6 mm full-width half maximum (FWHM)] using methods previously described (Bremner et al. 1999a). The subject was placed in the scanner with their head held in a holder to minimize motion and positioned with the cantho-meatal line parallel to an external laser light. An intravenous line was inserted for administration of [15O]H2O. Following positioning within the camera gantry, a transmission scan of the head was obtained using an external 67Ga/68Ge rod source, in order to correct emission data for attenuation due to overlying bone and soft tissue. Subjects received a 30 mCi intravenous bolus of H2[15O] for each of the six scans which was administered at the beginning of the condition. Each scan acquisition lasted 1 min. The onset of the PET scan acquisition was timed to correspond to the point of maximum rate of increase in uptake of tracer into the brain. Subjects underwent two scans during habituation, two scans during fear acquisition, and two scans during fear extinction one day, and a similar protocol with sensitization substituted for acquisition on another day. Images were reconstructed and analyzed as previously described (Bremner et al. 1999a). Images were realigned to the first image in the scanning session using statistical parametric mapping (Friston, 1994). The mean concentration of radioactivity in each scan was obtained as an area-weighted sum of the concentration of each slice and adjusted to the nominal value of 50 ml/min per 100 g. The data were then rescaled and transformed into a common anatomical space (expressed in three dimensional x-, y- and z-coordinates) for statistical analysis (Talairach & Tournoux, 1988). After transformation, images were smoothed to 16 mm FWHM before statistical analysis.
Data analysis
Behavioral data were analyzed by repeated measures analysis of variance (ANOVA), with time as the repeated measure (behavioral condition over time) and factors of diagnosis and study day. Psychophysiological data within each of the two extinction sessions were analyzed by repeated measures ANOVA, with time as the repeated measure (psychophysiological measurement over time) and factors of diagnosis and study day. Regional cerebral blood flow was compared in subjects with and without PTSD between active and control conditions (e.g. between fear acquisition and the control condition with unpaired shock, or between extinction and habituation). Analyses were also performed to examine the interaction of group by condition (e.g. greater increases with fear acquisition versus control condition in PTSD versus control subjects, or greater decreases with extinction versus habituation on the fear acquisition day in PTSD subjects versus control subjects). Data were analyzed using SPM96 with global blood flow considered as a confounding covariate with image datasets in which the values assigned to individual voxels correspond to t statistic. Statistical images were displayed with values of z-score units >2·58 (p<0·005) and clusters of greater than 45 contingent significant voxels. This level of significance has been shown to maximally decrease the occurrence of type I and II errors in imaging studies (Reiman et al. 1997). Areas of activation were identified using standard stereotaxic coordinates based on the atlas of Talairach and Tournoux (Talairach & Tournoux, 1988). The correlation between globally normalized blood flow in the area of greatest activation in the hypothesized region of interest (amygdala) and symptoms of PTSD, dissociation, anxiety, and fear measured as described above were examined using Pearson correlations with correction for multiple comparisons (p<0·0125). Similar analyses were performed for medial prefrontal cortex. Correlations were therefore performed between only two regions in the brain and three behavioral measures.
RESULTS
Behavioral responses to fear conditioning and extinction
PTSD subjects had increased symptoms of anxiety, fear, dissociation, distress (SUDS) and PTSD at all time-points during both study days relative to non-PTSD. Repeated measures ANOVA with time as the repeated factor showed that panic anxiety symptoms measured with the PASS increased over time (F=3·24; df=6; p=0·055) and were higher at all time-points in PTSD on both study days (F=7·05; df=2; p=0·003); there was a greater increase in panic anxiety in PTSD over time within each study day compared with controls (time-by-diagnosis interaction: F=3·8; df=12; p=0·01) (Fig. 1). Dissociative symptoms were higher at all time-points on both study days in PTSD as measured by a main effect for diagnosis (F=4·81; df=2; p=0·02) but no main effects for time or study day. Similarly there was a main effect for PTSD symptoms as measured by the PTSD Symptom Scale related to PTSD subjects having higher PTSD symptoms than controls at all time-points (F=7·05; df=2; p=0·003) but no main effects for time or study day. There were significantly higher levels of fear in PTSD versus non-PTSD subjects as measured by the fear analogue scale at all time-points (main effect for diagnosis: F=23·3; df=2; p<0·001) with no main effects for time or study day. There were significantly higher levels of subjective distress in PTSD versus non-PTSD subjects as measured by the SUDS at all time-points during the study (main effect for diagnosis: F=38·6; df=2; p<0·001). SUDS ratings increased in both groups with fear conditioning (significant main effect for time; F=2·74; df=6; p=0·04), e.g. SUDS ratings in the non-PTSD subjects went from 0 (0 S.D.) at baseline to 2 (7 S.D.) for active fear acquisition and 4 (10 S.D.) for active extinction, while in PTSD subjects SUDS ratings went from 10 (2 S.D.) at baseline to 30 (20 S.D.) with active fear acquisition and 30 (20 S.D.) with fear extinction. There was no main effect for study day. PTSD patients (but not controls) had increased anxiety (as measured with the PASS) and PTSD symptoms (as measured with the PTSD Symptom Scale) at the beginning of the study and throughout the procedure on study day 1 compared to study day 2.
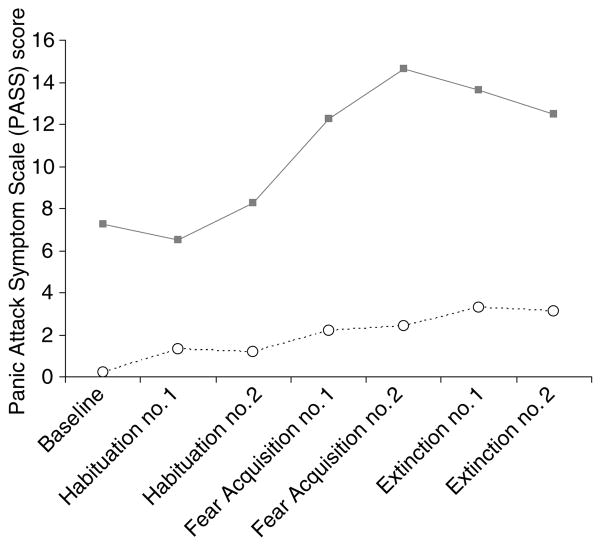
Fig. 1.
Anxiety symptoms during fear acquisition and extinction as measured with the Panic Attack Symptom Scale (PASS) on the day when electric shock was paired with exposure to the blue square, or conditioned stimulus. Post-traumatic stress disorder (PTSD) patients had elevated anxiety at baseline, and higher levels of anxiety during both acquisition and extinction of fear relative to controls as measured with the PASS. ···· ○····, Control (n=11); —■—, PTSD (n=8).
Skin conductance, blood pressure and heart responses to fear conditioning and extinction
Acquisition of fear was associated with increased skin conductance (SC) responses to CS exposure during the active versus the control conditions in all subjects for fear acquisition block 1 (F=7·17; df=3, 25; p=0·012) with no main effects for diagnosis or time (Fig. 2). There were no significant differences for block 2. When analyzed separately, differences were seen in block 1 for PTSD (F=5·42; df=1, 11; p=0·04) but not healthy subjects. Post hoc analyses showed increased SC for PTSD during the first CS-US presentation. Extinction of fear was associated with increased skin conductance (SC) responses to CS exposure during the active versus the control conditions in all subjects for extinction session 1 (F=8·43; df=3, 24; p= 0·008) with no main effects for diagnosis or time. There was a less significant difference between the active and control conditions for extinction session 2 (F=3·04; df=3, 25; p=0·09) with no main effects for time or diagnosis. When PTSD and non-PTSD subjects were examined separately, SC levels were significantly elevated in non-PTSD subjects undergoing extinction following the active compared with the control condition during session 1 (F=14·90; df=1, 14; p=0·0017), with no effect of time (F=1·24; df=1, 14; p=0·30). Post hoc analyses showed significant differences at time-points 1 and 5 for session 1 (p<0·05). There was no difference in SC in non-PTSD subjects between active and control conditions for block 2. In PTSD subjects there was not a significant difference in SC between active and control extinction conditions for PTSD subjects for session 1 (F=3·32; df=1, 11; p=0·09) or session 2 (F=1·90; df=1, 11; p=0·20) and no main effect for time (Fig. 2). There was no significant increase in heart rate (HR) in the active compared with the control condition when all subjects were combined for extinction session 1 (F=1·82; df=3, 21; p= 0·19) or extinction session 2 (F=1·67; df=3, 21; p=0·21) and no main effects for diagnosis or time for either session. In non-PTSD subjects during extinction session 1, baseline corrected mean HR during the active condition was 5·5 beats per minute (bpm) (13·3 S.D.) versus −3·2 bpm (4·3 S.D.) for the control condition (F=3·96; df=1, 10; p=0·07). In the healthy subjects for extinction session 2 there was no difference between baseline corrected mean HR during the active condition [5·5 bpm (13·3 S.D.)] compared with the control condition [−3·3 bpm (4·3 S.D.)] (F=2·72; df=1, 11; p=0·13). In PTSD subjects for extinction session 1 there was no difference between baseline corrected mean HR during the active condition [−1·5 bpm (1·9 S.D.)] compared with the control condition [−0·2 bpm (5·9 S.D.)]. Similarly for PTSD subjects for session 2 there was no difference in corrected mean HR during active [−0·5 bpm (2·7 S.D.)] and control [0·0 bpm (7·0 S.D.)] conditions.
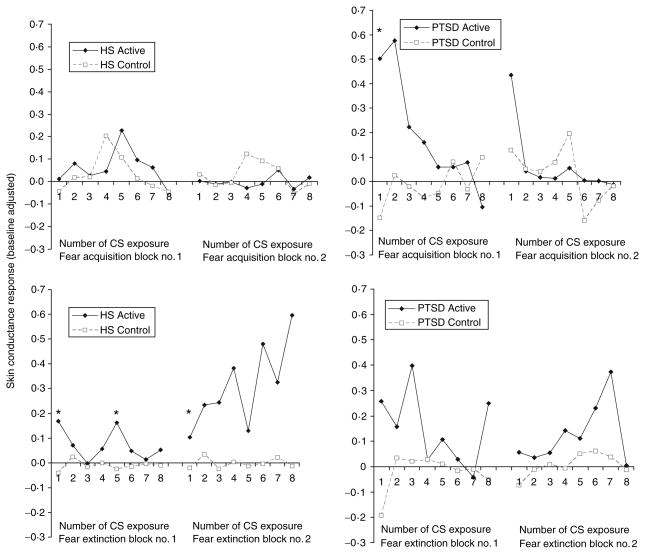
Fig. 2.
Baseline-corrected skin conductance (SC) during fear acquisition and extinction in healthy subjects and subjects with post-traumatic stress disorder (PTSD). For the active fear acquisition condition subjects were repeatedly exposed (every 10 s) to a paired conditioned stimulus (CS)–unconditioned stimulus (US) consisting of a blue square (CS) appearing on a screen for 4 s with an electric shock (US) starting half a second after the blue square first appeared on the screen. There were two blocks of eight CS–US pairings, each block associated with one PET scan acquisition of brain blood flow. The numbers on the x-axis (1–8) refer to the number of the CS presentation (eight in all for each block). The control for the fear acquisition consisted of an identical procedure except that eight shocks were delivered randomly, i.e. not paired with the CS presentation. The fear acquisition (either active or control blocks) were followed by two blocks of extinction, which consisted of presentation of blue squares for 4 s with a 6-s inter-presentation interval, repeated eight times. The active and control extinction conditions differed only in whether or not they followed the active or control fear acquisition conditions. SC levels are corrected for pre-CS baseline. Asterisks denote times where there were significant elevations in SC in the active compared with the control condition. The number of CS is listed on the x-axis. SC levels were higher for the first block of active fear acquisition versus control in all subjects combined; when the groups were examined separately, increases were only seen in PTSD. Post hoc analyses showed increased SC for the first CS–US pairing in the first block in the PTSD group (* p<0·05). Skin conductance levels were significantly elevated for the first block (but not the second block) in active versus control extinction for PTSD and non-PTSD combined. Differences were significant for non-PTSD only when groups were examined separately. Analysis of individual time-points showed elevations in non-PTSD for the first and fifth presentations of CS in block 1 and the first presentation of CS in block 2.
PTSD subjects had an increase in baseline systolic blood pressure (SBP) compared with non-PTSD subjects [118 (8 S.D.) versus 112 (12 S.D.)] (F=5·38; df=2; p=0·01). The conditioning procedure resulted in an increase in SBP in the group as a whole (main effect for time) (F=2·79; df=6; p=0·013) with no main effects for diagnosis or study day; e.g. non-PTSD went from 112 (12 S.D.) at baseline to 118 (18 S.D.) with fear acquisition block 1 and 119 (12 S.D.) with extinction block 1, while SBP in PTSD went from 118 (8 S.D.) at baseline to 120 (12 S.D.) fear acquisition block 2 to 120 (8 S.D.) extinction block 1. There was no difference for diastolic blood pressure. There were no differences in SC response during fear acquisition or extinction on the active fear acquisition day between non-PTSD or PTSD subjects who underwent active-control versus control-active study orders (i.e. no order effects).
Cerebral blood flow response to fear conditioning and extinction
PTSD subjects showed activation of the bilateral amygdala during fear acquisition compared with the control condition (Table 1). Non-PTSD subjects showed an area of activation in the region of the left amygdala (Table 2). When PTSD subjects and control subjects were directly compared, PTSD subjects showed greater activation of the left amygdala during the fear conditioning condition (pairing of US and CS) relative to the random shock control than healthy women (Fig. 3, Table 3). Other areas that showed increased activation with fear acquisition in PTSD included bilateral superior temporal gyrus [Brodmann’s Area (BA) 22], cerebellum, bilateral inferior frontal gyrus (BA 44, 45) and posterior cingulate (BA 24). Fear acquisition was associated with decreased function in medial prefrontal cortex, visual association cortex, and medial temporal cortex, inferior parietal lobule function, and other areas. Extinction of fear responses was associated with decreased function in the orbitofrontal and medial prefrontal cortex (including subcallosal gyrus, BA 25, and anterior cingulate, BA 32), visual association cortex, and other areas, in the PTSD subjects, but not in the controls (Fig. 4, Table 4).
Table 1.
Areas of increased and decreased blood flow with fear acquisition (paired US–CS) versus random shock control in women with childhood-abuse-related post-traumatic stress disorder (PTSD)
| Increased blood flow
|
Decreased blood flow
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Z-score* | Talairach coordinates
|
Brain region† | Z-score* | Talairach coordinates
|
Brain region | ||||
| x | y | z | x | y | z | ||||
| 5·24 | −20 | 6 | −12 | L amygdala | 4·82 | −46 | −18 | 58 | L postcentral gyrus (1, 2, 3) |
| 5·07 | −18 | −8 | 6 | Putamen | 4·46 | −22 | −30 | 52 | |
| 4·43 | −38 | −24 | −8 | L hippocampal region | 4·36 | 36 | −60 | 32 | R middle temporal gyrus (39) |
| 5·11 | 48 | −10 | −2 | L superior temporal gyrus (22) | 3·01 | 40 | 0 | 20 | R precentral gyrus (4, 6) |
| 3·75 | 48 | −42 | 18 | R superior temporal gyrus (22) | 2·62 | 28 | 10 | 8 | |
| 3·45 | 16 | −38 | −50 | Cerebellum | |||||
| 3·81 | 40 | 36 | 0 | R inferior frontal gyrus (44, 45) | |||||
| 3·31 | 36 | 8 | 38 | ||||||
| 3·77 | −48 | −14 | 28 | L postcentral gyrus (1, 2, 3) | |||||
| 3·63 | −8 | 4 | 54 | Posterior cingulate (24) | |||||
Z-score >2·58, p<0·005; Z-scores in bold indicate area of greatest activation in a cluster.
Numbers in parentheses represent Brodmann area number.
L, left; R, right.
Table 2.
Areas of increased and decreased blood flow with fear acquisition (paired US–CS) versus random shock control in women without abuse or post-traumatic stress disorder (PTSD)
| Increased blood flow
|
Decreased blood flow
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Z-score* | Talairach coordinates
|
Brain region† | Z-score* | Talairach coordinates
|
Brain region† | ||||
| x | y | z | x | y | z | ||||
| 5·42 | 12 | −68 | −40 | Cerebellum | 5·17 | −10 | −26 | 48 | Precuneus (7) |
| 5·23 | −2 | −82 | −10 | 4·98 | −16 | −62 | 30 | ||
| 5·30 | −2 | −82 | −10 | Visual association cortex (17, 18) | 4·40 | 36 | −38 | −14 | R fusiform gyrus (37) |
| 3·96 | 18 | 40 | 22 | R middle frontal gyrus (10) | 4·69 | 38 | −22 | 22 | R postcentral gyrus (43) |
| 3·35 | −38 | 6 | 20 | L inferior frontal gyrus (45) | 4·28 | 38 | 18 | 22 | R inferior frontal gyrus (45) |
| 3·27 | 50 | −38 | 2 | R middle temporal gyrus (21) | 3·49 | 44 | −40 | 30 | R inferior parietal lobule (40) |
| 2·90 | 52 | −28 | −12 | R middle temporal gyrus (20) | 3·91 | −52 | −14 | 52 | L postcentral gyrus (1, 2) |
| 3·23 | 46 | −20 | 0 | R superior temporal gyrus (21) | 3·71 | −60 | 8 | 10 | L precentral gyrus (6, 44) |
| 2·78 | −38 | −12 | −26 | L amygdala region | 3·60 | −66 | −18 | 22 | L postcentral gyrus (40) |
| 2·74 | −34 | 0 | −38 | L inferior temporal gyrus (20) | |||||
Z-score >2·58, p<0·005; Z-scores in bold indicate area of greatest activation in a cluster.
Numbers in parentheses represent Brodmann area number.
L, left; R, right.
Fig. 3.
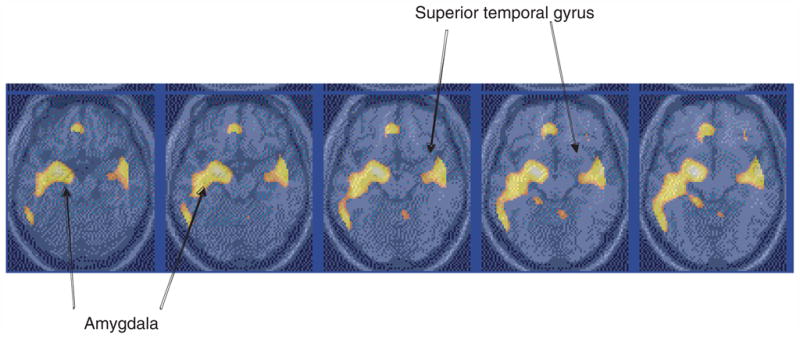
Statistical parametric map overlaid on an MRI template of areas of significantly greater increased blood flow with fear acquisition versus control in post-traumatic stress disorder (PTSD). There was increased blood flow in the amygdala in PTSD. Comparison with non-PTSD showed greater activation in PTSD. Yellow areas correspond to areas of significant activation (Z-score >3·09; p<0·001).
Table 3.
Areas of greater increases and decreases in blood flow with fear acquisition (paired US–CS) versus random shock control in women with abuse and post-traumatic stress disorder (PTSD) compared with women without abuse or PTSD
| Increased blood flow
|
Decreased blood flow
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Z-score* | Talairach coordinates
|
Brain region† | Z-score* | Talairach coordinates
|
Brain region | ||||
| x | y | z | x | y | z | ||||
| 4·85 | −8 | 6 | 52 | L posterior cingulate (6, 24, 32, 31) | 4·79 | 14 | −68 | −40 | Cerebellum |
| 4·66 | −18 | −62 | − | ||||||
| 4·02 | −6 | −28 | 46 | 4·13 | −4 | −84 | −8 | Visual cortex (18) | |
| 4·34 | 38 | 22 | 22 | R inferior frontal gyrus (46, 45, 47) | 3·91 | −26 | −26 | 54 | L precentral gyrus (4) |
| 3·58 | −44 | −44 | −50 | Cerebellum | |||||
| 3·65 | 40 | 32 | 4 | 3·07 | −54 | −52 | −34 | ||
| 3·38 | 38 | 36 | −6 | 3·01 | −30 | −36 | −50 | ||
| 4·21 | 44 | −42 | 28 | R inferior parietal lobule (40) | 3·50 | 28 | −18 | 58 | R precentral gyrus (4) |
| 4·01 | 36 | −24 | 20 | 3·40 | 14 | −40 | 44 | R precuneus (31, 7) | |
| 3·13 | 56 | −16 | 14 | R postcentral gyrus (40) | 3·23 | 14 | −44 | 36 | |
| 3·86 | 6 | −52 | −12 | Cerebellum | 3·48 | 10 | 28 | 42 | R anterior cingulate (32) |
| 3·68 | −12 | −42 | −4 | Parahippocampal gyrus (19) | 3·13 | 12 | 52 | 2 | |
| 2·91 | 12 | −22 | 2 | R hippocampal/amygdala region | 3·07 | 16 | 42 | 24 | R anterior frontal (9, 10) |
| 3·45 | −36 | −76 | 10 | L visual ass. (19) | |||||
| 3·67 | 10 | 2 | 22 | R caudate | 3·31 | −38 | −56 | 42 | L inferior parietal lobule (40) |
| 3·56 | −10 | 28 | −6 | L anterior cingulate (24, 32) | 3·14 | −36 | 24 | 36 | L middle frontal gyrus (9) |
| 3·50 | 8 | 24 | 8 | ||||||
| 3·53 | −20 | −8 | −12 | L amygdala | |||||
| 3·27 | −18 | −8 | 6 | Insula | |||||
| 3·51 | −50 | −14 | 30 | L postcentral gyrus (1, 2, 3) | |||||
| 3·15 | −40 | −26 | −10 | L middle temporal gyrus (21) | |||||
| 3·03 | −58 | −32 | 8 | L superior temporal gyrus (42) | |||||
| 2·80 | −50 | −42 | 16 | L superior temporal gyrus (22) | |||||
Z-score >2·58, p<0·005; Z-scores in bold indicate area of greatest activation in a cluster.
Numbers in parentheses represent Brodmann area number.
L, left; R, right.
Fig. 4.
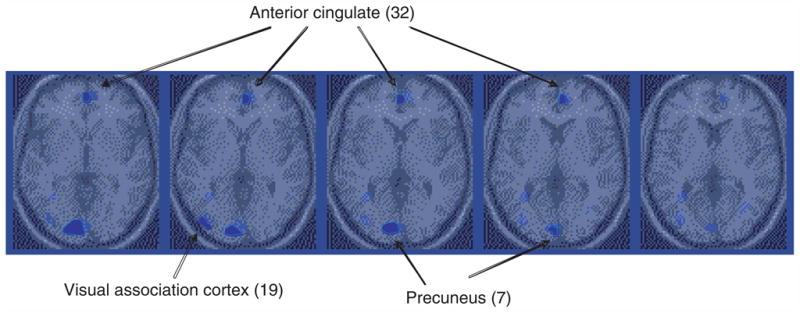
Parametric map overlaid on an MRI template of areas of areas of decreased blood flow with extinction in post-traumatic stress disorder (PTSD). Blue areas correspond to areas of significant deactivation (Z-score >3·09; p<0·001). There was decreased blood flow in the medial prefrontal cortex (anterior cingulate, BA 24, 32) in PTSD during extinction relative to control condition in PTSD, not seen in controls.
Table 4.
Areas of greater increases and decreases in blood flow with extinction to conditioned fear (US–CS pair) relative to extinction to non-paired US–CS in women with abuse and post-traumatic stress disorder (PTSD) versus controls
| Increased blood flow
|
Decreased blood flow
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Z-score* | Talairach coordinates
|
Brain region† | Z-score* | Talairach coordinates
|
Brain region | ||||
| x | y | z | x | y | z | ||||
| 3·23 | −38 | −38 | −40 | Cerebellum | 4·55 | 16 | 14 | −12 | R anterior cingulate–subcallosal gyrus (25, 32, 24) |
| 3·13 | −34 | −18 | −2 | Insula | |||||
| 3·11 | 50 | −36 | 8 | R superior temporal gyrus (22) | 3·96 | 42 | 0 | 20 | R precentral gyrus (6, 4) |
| 3·73 | 24 | −4 | 16 | ||||||
| 2·95 | 46 | −14 | 0 | 4·32 | −54 | −30 | 48 | L postcentral gyrus (1, 2, 3) | |
| 2·84 | −8 | −6 | −10 | L hippocampus | 3·52 | −58 | −2 | 30 | L precentral gyrus (6) |
| 3·48 | −56 | −8 | 38 | ||||||
| 4·30 | −18 | −34 | 18 | Fornix | |||||
| 3·96 | 28 | −54 | −42 | Cerebellum | |||||
| 2·91 | 0 | −48 | −48 | Cerebellum | |||||
| 3·91 | 20 | −76 | 12 | R visual association cortex (19) | |||||
| 3·42 | −6 | 38 | −28 | Orbitofrontal cortex (11) | |||||
| 3·40 | 4 | 14 | 60 | Anterior cingulate (32) | |||||
| 3·25 | −14 | 46 | −10 | ||||||
| 3·25 | −30 | −44 | −10 | Parahippocampal gyrus (37) | |||||
Z-score >2·58, p<0·005; Z-scores in bold indicate area of greatest activation in a cluster.
Numbers in parentheses represent Brodmann area number.
L, left; R, right.
Relationship between brain and behavioral responses to fear conditioning and extinction
Amygdala blood flow with fear acquisition was negatively correlated with medial prefrontal blood flow with fear extinction (increased blood flow in amygdala correlated with decreased blood flow in medial prefrontal cortex) in all subjects (r=−0·48; df=18; p<0·05). Increased amygdala blood flow with fear acquisition was positively correlated with PTSD (r=0·45), anxiety (r=0·44) and dissociative (r=0·80) symptom levels in PTSD (but not non-PTSD) subjects; none of these comparisons was significant after correction for multiple comparisons. There was a negative correlation between medial prefrontal blood flow during extinction and anxiety as measured with the PASS during extinction in the PTSD group only which was significant after correction for multiple comparisons (r=−0·90; df=6; p=0·006).
DISCUSSION
Acquisition of fear responses during the fear conditioning paradigm resulted in increased amygdala activation in women with abuse-related PTSD compared with women without PTSD. Other areas that showed increased activation with fear acquisition in PTSD, and were increased to a greater degree than in control subjects, included left superior temporal gyrus, right inferior frontal gyrus, cerebellum and posterior cingulate. Extinction of fear responses was associated with decreased function in the orbitofrontal and medial prefrontal cortex (including subcallosal gyrus, BA 25, and anterior cingulate, BA 32), and visual association cortex, in the PTSD subjects, but not in the controls. Decreased medial prefrontal function was associated with increased anxiety during extinction in the PTSD subjects only.
These findings are consistent with increased amygdala function during fear acquisition in PTSD. Interpreted in the context of other functional imaging studies in PTSD, one can tentatively conclude that in order for increased amygdala function to be observed it is necessary to utilize the appropriate task. Imaging studies inducing memories of traumatic reminders and/or PTSD symptoms with scripts, combat-related slides and/or sounds, or pharmacological challenge have not consistently resulted in increased amygdala activation in PTSD. These studies all involved enteroceptive or internally generated emotional states which may or may not actually correspond to classical states of fear. It should be noted that there are a number of emotional states that characterize PTSD in addition to exaggerated fear responses to threat. These include symptoms of dissociation, feeling worse with traumatic reminders, amnesia, and flashbacks, which involve visual imagery of the traumatic event which plays back in front of the patient’s eyes like a movie. Put more simply, fear conditioning, although helpful especially as an animal model, does not account for all of the aspects of the presentation of PTSD. The two studies which involved exteroceptive or externally generated stress, exposure to masked fearful faces (Rauch et al. 2000) and the current study involving fear conditioning (electric shock paired with exposure to blue squares) did demonstrate increased amygdala activation. These findings are consistent with the hypothesis of Reiman and colleagues (1997) that exteroceptive threatening stimuli involve amygdala function more than enteroceptive states. Although the current study requires replication, these studies do suggest that the exaggerated fear response in PTSD can at least be partially accounted for by increased amygdala function.
Brain areas besides the amygdala that activated during fear acquisition are similar to those that have been shown to activate in prior studies of PTSD symptom induction using a variety of tasks. Prior studies of PTSD used a variety of tasks, including script-driven imagery or exposure to traumatic slides and sounds, or retrieval of emotionally valenced words (Bremner et al. 2003b), to activate specific memories associated with the traumatic event. The findings of the current study are congruent with prior studies of PTSD in showing decreased function in medial prefrontal cortex, visual association cortex, and medial temporal cortex, alterations in inferior parietal lobule function, and increased function in posterior cingulate and inferior frontal gyrus. The findings suggest that an interrelated network of brain regions, all of which happen to be involved in memory function, are dysfunctional in PTSD.
Extinction of fear resulted in a decrease in blood flow in the medial prefrontal cortex/anterior cingulate in PTSD. An inability to extinguish fear responses can be considered to be one of the most elemental aspects of the presentation of PTSD patients. Decreased function in the medial prefrontal cortex was not seen in the healthy controls; in fact, controls had increased function in this area with extinction. As reviewed above, medial prefrontal cortex (including anterior cingulate) (Vogt et al. 1992; Devinsky et al. 1995) consists of several related areas, including orbitofrontal cortex, anterior cingulate (Area 25 – subcallosal gyrus, and Area 32), and anterior prefrontal cortex (Area 9). Inhibitory inputs from the medial prefrontal cortex to the amygdala are hypothesized to represent the neural circuit that mediates extinction (Morgan & LeDoux, 1995). Human subjects with lesions of the prefrontal cortex show dysfunction of normal emotions and an inability to relate in social situations that require correct interpretation of the emotional expressions of others (Damasio et al. 1994). These findings, interpreted together with the findings in the current study, suggest that dysfunction of the medial prefrontal cortex/anterior cingulate in PTSD may underlie both altered social behavior and a failure of extinction of fear responses.
The psychophysiological findings in the current study are of interest relative to the three other published studies of psychophysiological responding to classical fear conditioning in PTSD. We found increased skin conductance responses to the conditioned stimulus during the extinction phase in the group as a whole; when PTSD and non-PTSD groups were examined separately, skin conductance responses that were specific to the conditioned stimulus showed a pattern of greater magnitude in the non-PTSD versus PTSD group. This finding is consistent with a prior study of Grillon & Morgan (1999) who compared startle responses at baseline and during aversive conditioning which involved a pairing of colored lights with and without an aversive electric shock (CS+ and CS−) on two separate days. During session 1, non-PTSD patients showed increased startle to CS+ compared with CS− during the conditioning and extinction phase, while PTSD subjects showed increased responses to both CS+ and CS− during extinction. The authors concluded that PTSD patients have an over-generalization of fear responses and/or deficits in learning to discriminate safety signals from threat cues. Orr and co-workers (2000) used a classical fear conditioning paradigm exposing subjects to a colored circle with (CS+) and without (CS−) electric shock. PTSD patients had larger skin conductance and heart rate responses to CS+ versus CS− during acquisition compared with controls, and higher skin conductance responses to CS during extinction, which was seen for both CS+ and CS−. However, PTSD patients showed a significant differential in SC response between CS+ and CS− during extinction, while the controls did not. Peri and co-workers (2000) exposed subjects to colors paired with an aversive burst of white noise (CS+) and colored slides without white noise (CS−). All subjects showed increased skin conductance responses during acquisition and extinction of fear. PTSD patients had greater skin conductance responses to CS− during acquisition and greater responses to both CS+ and CS− during extinction than controls. PTSD patients had increased heart rate response to CS+ during acquisition and extinction compared with controls. These data suggest that PTSD patients may be unable to differentiate threat and safety cues. Future studies are required in this area.
There are several limitations to the current study. PTSD subjects did not clearly show greater conditioning than non-PTSD subjects based on the psychophysiology data. However, not all studies have consistently shown increased conditioning in PTSD. The pattern of SC response to CS in the extinction phase did not show a pattern of decreased response over time with re-exposure to CS, at least based on mean SC responses to CS. The lack of statistically significant increases in SC in response to CS in block 2 (but not block 1) suggests, however, that there were less consistent increases in SC in response to CS over time during the extinction phase. The intervals between shocks paired with the blue square were fixed, therefore subjects could have responded to the time interval rather than the blue square. The limited time-frame of one minute for PET image acquisition, however, limits the ability to have a variable interval for shock and conditioned stimulus exposure. Functional MRI would avoid this problem; fMRI also avoids problems related to rapid habituation of fear response. However, acquisition of psychophysiology information to verify conditioning is more difficult with fMRI because of interference from the magnet with the recording. Also, there are artifacts related to the brain–air interface which complicate the measurement of amygdala activity with fMRI, although improvements in technology are addressing these methodological issues. Subjects underwent both scanning with a paired shock and blue square and unpaired shock and blue square. Therefore when subjects underwent the second scan day with unpaired blue square and shock they would associate the blue square with shock. The fear acquisition condition compared paired shock and blue square with unpaired shock and blue square, while the extinction paradigm compared exposure to blue square after the paired shock–blue square condition with the acquisition condition (initial exposure to blue square). This prevents direct comparisons of the fear acquisition and extinction paradigms. Our study design also confounded ability to measure conditioned responses during the fear acquisition phase with response to the UCS, since the UCS onset was soon after the CS onset. Brain imaging studies involving comparisons of multiple small areas of the brain (voxels, or picture elements from the brain image) are potentially subject to error related to multiple comparisons. Application of Bonferroni corrections (dividing 0·05 by the number of comparisons, in this case the number of voxels) results in a situation where it is not possible to measure differences owing to the fact that these analyses involve hundreds or even thousands of voxels. This study adopted the approach of Reiman and colleagues (1997) of using a statistical threshold that they have shown minimizes the possibility of both Type I and Type II errors. In addition, we selected a minimum cluster of voxels (size of brain activation) to exclude potentially spurious results. The current study did not assess sexually abused women without PTSD, so we cannot comment on whether or not the changes are specific to PTSD. They could have represented a non-specific response to childhood abuse. The activation paradigm did not involve a trauma-specific task, so it appears reasonable to use non-traumatized controls as a first step; however, future studies should compare PTSD women with abused non-PTSD women to assess whether or not the findings are specific to PTSD. The correlational analyses involved multiple comparisons with a small population sample; these results are presented as results of exploratory analyses and require replication. There were high rates of co-morbidity of PTSD with other psychiatric diagnoses, similar to other studies in PTSD. For example, 25% had past cocaine abuse, and cocaine dependence has been associated with alterations in amygdala function. Our PTSD patients, however, had been abstinent from substances for one or more years.
In summary, the current results are consistent with increased amygdala function with fear acquisition and decreased medial prefrontal function with extinction in PTSD. These results, however, require replication.
Acknowledgments
We thank Helen Sayward MS and Sinead Quinn MS for image processing and data analysis, and Tammi Rowe-Padhy MS, Sara Dubuque MS, Nadeem Afzal MD and Sajid Siddiq MD for assistance in data collection. This study was supported by NIMH 1R01MH56120-01A1 and a Veterans Administration Career Development Award to Dr Bremner, the Emory Conte Center for the Neurosciences of Mental Health (1P50 MH58922), and the VA National Center for Post-Traumatic Stress Disorder.
Footnotes
DECLARATION OF INTEREST
References
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS. The development of a clinician-administered PTSD scale. Journal of Trauma and Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Kolb LC, Gerardi RJ, Ryan P, Pallmeyer TP. Cardiac response to relevant stimuli as an adjunctive tool for diagnosing post-traumatic stress disorder in Vietnam veterans. Behaviour Therapy. 1986;17:592–606. [Google Scholar]
- Bremner JD. Neuroimaging in posttraumatic stress disorder. Psych Annal. 1998;28:445–450. [Google Scholar]
- Bremner JD, Innis RB, Ng CK, et al. PET measurement of cerebral metabolic correlates of yohimbine administration in posttraumatic stress disorder. Archives of General Psychiatry. 1997;54:246–256. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam F, et al. Measurement of dissociative states with the Clinician Administered Dissociative States Scale (CADSS) Journal of Trauma and Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999a;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib L, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological Psychiatry. 1999b;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depression and Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, et al. Neural correlates of the classical neutral and emotional Stroop in women with abuse-related posttraumatic stress disorder. Biological Psychiatry. 2003a doi: 10.1016/j.biopsych.2003.10.001. (in press) [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, et al. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder (PTSD) related to early childhood sexual abuse. Biological Psychiatry. 2003b;53:289–299. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Butler RW, Braff DL, Rausch JL, Jenkins MA, Sprock J, Geyer MA. Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. Am J Psychiatry. 1990;147:1308–1312. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- Cahill J, Haier RJ, Fallon RJ, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Nature and Neuroscience. 1996;2:289–293. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JDE, Cahill J. Event-related activation in the amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Glover G, Gabrieli JDE. FMRI identifies a network of structures correlated with retention of positive and negative emotional memory. Psychobiology. 1999;27:441–452. [Google Scholar]
- Chapman WP, Schroeder AF, Guyer G, et al. Physiological evidence concerning the importance of the amygdaloid nuclear region in the integration of circulating functions and emotion in man. Science. 1954;129:949–950. doi: 10.1126/science.120.3127.949. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM IV – Patient Edition (SCID-P) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- Friston K. Statistical parametric mapping. In: Thatcher R, Hallett M, Zeffro T, John E, Huerta M, editors. Functional Neuroimaging: Technical Foundations. Academic Press; San Diego, CA: 1994. pp. 79–93. [Google Scholar]
- Grillon C, Morgan CA. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Gunne LM, Reis DJ. Changes in brain catecholamines associated with electrical stimulation of amygdaloid nucleus. Life Sciences. 1963;11:804–809. doi: 10.1016/0024-3205(63)90090-0. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature and Neuroscience. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hilton SM, Zbrozyna AW. Amygdaloid region for defense reactions and its efferent pathway to the brain stem. Journal of Physiology. 1963;165:160–173. doi: 10.1113/jphysiol.1963.sp007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behavioural Neuroscience. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behavioural Neuroscience. 1991;105:826–842. doi: 10.1037//0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Sananes CB, Davis M. Sensitization of the startle reflex by footshock: blockade by lesions of the central nucleus of the amygdala or its efferent pathway to the brainstem. Behavioural Neuroscience. 1989;103:509–518. doi: 10.1037//0735-7044.103.3.509. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Berardi A, Thompson WL, et al. Brain mechanisms in human classical conditioning: a PET blood flow study. Neuroreport. 1995;6:1723–1728. doi: 10.1097/00001756-199509000-00005. [DOI] [PubMed] [Google Scholar]
- Iwata J, LeDoux JE, Meeley MP, Arneric S, Reis DJ. Intrinsic neurons in the amygdaloid field projected to by the medial geniculate body mediate emotional responses conditioned to acoustic stimuli. Brain Research. 1986;383:195–214. doi: 10.1016/0006-8993(86)90020-x. [DOI] [PubMed] [Google Scholar]
- Keane TM, Kolb LC, Kaloupek D, et al. Utility of psychophysiological measurement in the diagnosis of post-traumatic stress disorder: results from a Department of Veterans Affairs Cooperative Study. Journal of Consulting and Clinical Psychology. 1998;66:914–923. doi: 10.1037//0022-006x.66.6.914. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. Journal of Neuroscience. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. Simon & Schuster; New York: 1996. [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, et al. Brain activation in PTSD in response to trauma-related stimuli. Biological Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Fleming JE, Trocme N, et al. Prevalence of child physical and sexual abuse in the community: results from the Ontario Health Supplement. Journal of the American Medical Association. 1997;278:131–135. [PubMed] [Google Scholar]
- McCauley J, Kern DE, Kolodner K, et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. Journal of the American Medical Association. 1997;277:1362–1368. [PubMed] [Google Scholar]
- McFall ME, Murburg MM, Ko GN, Veith RC. Autonomic responses to stress in Vietnam combat veterans with posttraumatic stress disorder. Biological Psychiatry. 1990;27:1165–1175. doi: 10.1016/0006-3223(90)90053-5. [DOI] [PubMed] [Google Scholar]
- Miserendino MJD, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Morgan CA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioural Neuroscience. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Grillon C, Southwick SM, Davis M, Charney DS. Fear-potentiated startle in posttraumatic stress disorder. Biological Psychiatry. 1995;38:378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neuroscience Letters. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Pynoos RS. Startle modulation in children with posttraumatic stress disorder. American Journal of Psychiatry. 1989;146:866–870. doi: 10.1176/ajp.146.7.866. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Metzger LJ, Ahern CE, Berry NJ, Pitman RK. Psychophysiological assessment of women with posttraumatic stress disorder resulting from childhood sexual abuse. Journal of Consulting and Clinical Psychology. 1998;66:906–913. doi: 10.1037//0022-006x.66.6.906. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109:290–298. [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, Herz LR. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. Journal of Abnormal Psychology. 1993;102:152–159. doi: 10.1037//0021-843x.102.1.152. [DOI] [PubMed] [Google Scholar]
- Orr SP, Roth WT. Psychophysiological assessment: clinical applications for PTSD. Journal of Affective Disorders. 2000;61:225–240. doi: 10.1016/s0165-0327(00)00340-2. [DOI] [PubMed] [Google Scholar]
- Paige SR, Reid GM, Allen MG, Newton JEO. Psychophysiological correlates of posttraumatic stress disorder in Vietnam veterans. Biological Psychiatry. 1990;27:419–420. doi: 10.1016/0006-3223(90)90552-d. [DOI] [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in post-traumatic stress disorder. Biological Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioural Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. Journal of Clinical Psychiatry. 2001;62:47–54. [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgus DF, Altman B, de Jong JB, Herz LR. Psychophysiologic responses to combat imagery of Vietnam veterans with posttraumatic stress disorder versus other anxiety disorders. Journal of Abnormal Psychology. 1990;99:49–54. doi: 10.1037//0021-843x.99.1.49. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgus DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Archives of General Psychiatry. 1987;44:970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000:47. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, et al. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Davis M. Enhancement of acoustic startle by electrical stimulation of the amygdala. Behavioural Neuroscience. 1988;102:195–202. doi: 10.1037//0735-7044.102.2.195. [DOI] [PubMed] [Google Scholar]
- Ross RJ, Ball WA, Cohen ME, Silver SM, Morrison AR, Dinges DF. Habituation of the startle reflex in post-traumatic stress disorder. J Neuropsychiatry. 1989;1:305–307. doi: 10.1176/jnp.1.3.305. [DOI] [PubMed] [Google Scholar]
- Roth RH, Tam SY, Ida Y, Yang JX, Deutch AY. Stress and the mesocorticolimbic dopamine systems. Annals of the New York Academy of Science. 1988:149–157. doi: 10.1111/j.1749-6632.1988.tb42102.x. [DOI] [PubMed] [Google Scholar]
- Saigh PA, Bremner JD. Posttraumatic Stress Disorder: A Comprehensive Text. Allyn & Bacon; Needham Heights, MA: 1999. [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. Journal of Neuroscience. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev AY, Orr SP, Peri T, Schreiber S, Pitman RK. Physiologic responses to loud tones in Israeli patients with posttraumatic stress disorder. Archives of General Psychiatry. 1992;49:870–875. doi: 10.1001/archpsyc.1992.01820110034005. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Peri T, Brandes D, Freedman S, Orr SP, Pitman RK. Auditory startle response in trauma survivors with posttraumatic stress disorder: a prospective study. Am J Psychiatry. 2000;157:255–261. doi: 10.1176/appi.ajp.157.2.255. [DOI] [PubMed] [Google Scholar]
- Shin LM, Kosslyn SM, McNally RJ, et al. Visual imagery and perception in posttraumatic stress disorder: a positron emission tomographic investigation. Archives of General Psychiatry. 1997;54:233–237. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- Shin LH, McNally RJ, Kosslyn SM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Americal Journal of Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Morgan CA, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Archives of General Psychiatry. 1993 doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux J. Co-Planar Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wolpe J, Lazarus AA. Behavioral Therapy Techniques. Pergamon Press; New York: 1966. [Google Scholar]