Abstract
Background
Animal studies have shown that stress is associated with damage to the hippocampus, inhibition of neurogenesis, and deficits in hippocampal-based memory dysfunction. Studies in patients with posttraumatic stress disorder (PTSD) found deficits in hippocampal-based declarative verbal memory and smaller hippocampal volume, as measured with magnetic resonance imaging (MRI). Recent preclinical evidence has shown that selective serotonin reuptake inhibitors promote neurogenesis and reverse the effects of stress on hippocampal atrophy. This study assessed the effects of long-term treatment with paroxetine on hippocampal volume and declarative memory performance in PTSD.
Methods
Declarative memory was assessed with the Wechsler Memory Scale–Revised and Selective Reminding Test before and after 9–12 months of treatment with paroxetine in PTSD. Hippocampal volume was measured with MRI. Of the 28 patients who started the protocol, 23 completed the full course of treatment and neuropsychological testing. Twenty patients were able to complete MRI imaging.
Results
Patients with PTSD showed a significant improvement in PTSD symptoms with treatment. Treatment resulted in significant improvements in verbal declarative memory and a 4.6% increase in mean hippocampal volume.
Conclusions
These findings suggest that long-term treatment with paroxetine is associated with improvement of verbal declarative memory deficits and an increase in hippocampal volume in PTSD.
Keywords: Posttraumatic stress disorder, memory, hippocampus, stress, paroxetine, selective serotonin reuptake inhibitors
Introduction
A wide range of evidence from animal studies has demonstrated memory deficits and damage to the hippocampus, a brain area that plays a critical role in learning and memory, after exposure to stress (Lupien and Lepage 2001; Sapolsky 1996; Vermetten and Bremner 2002). Several mechanisms have been proposed for this finding, including high levels of glucocorticoids released during stress (McEwen et al 1992; Sapolsky et al 1990), increased production of corticotrophin-releasing factor (Brunson et al 2001), increased release of excitatory amino acids (Moghaddam 2002; Moghaddam et al 1994), inhibition of neurogenesis (Gould et al 1997), and/or stress-related inhibition of brain-derived neurotrophic factor (BDNF) (Nibuya et al 1995; Smith et al 1995). Treatment with selective serotonin reuptake inhibitors (SSRIs) has demonstrated reversal of hippocampal atrophy and promotion of neurogenesis (Duman et al 2001; Malberg et al 2000).
Adults with posttraumatic stress disorder (PTSD) related to combat exposure or childhood physical or sexual abuse have been shown to have reduced hippocampal size on magnetic resonance imaging (MRI) when compared with healthy or trauma control subjects (Bremner et al 1995a, 1997; Gurvits et al 1996; Stein et al 1997; Villarreal et al 2002.) Trauma at different stages of life (early childhood abuse as well as trauma in later life [e.g., due to combat]) has been thought to influence hippocampal volume. Several clinical studies have also reported alterations in learning and memory in patients with PTSD, which are consistent with both deficits in encoding on explicit memory tasks and deficits in retrieval, as well as enhanced encoding or retrieval for specific trauma-related material (Andrews et al 2000; Buckley et al 2000; Gilbertson et al 2001; Pitman 1989; Roca and Freeman 2001; Vasterling et al 1998; Wolfe and Schlesinger 1997). The majority of these studies found deficits in verbal memory, with a relative absence of deficits in tasks of attention or visuospatial memory. The alterations varied from self-reported difficulties in memory (Thygesen et al 1970) to impairments of verbal declarative memory (Bremner et al 1993a, 1995b; Gilbertson et al 2001; Jenkins et al 1998; Moradi et al 1999; Sutker et al 1991; Uddo et al 1993; Yehuda et al 1995). These studies, which involved heterogenous groups of trauma populations and comorbidity status, all reported specific deficits in explicit memory function in PTSD (with no change in intelligence quotient). Some studies report that the memory impairments could not be accounted for by attentional disturbances or intellectual functioning (Gilbertson et al 2001; Vasterling et al 2002); others do report evidence of attentional and/or executive dysfunction in PTSD (Semple et al 1996). Some studies did not report specificity of verbal declarative memory deficits in PTSD (Crowell et al 2002; Dalton and Pederson 1989; Zalewski et al 1994).
Studies in patients with epilepsy have shown a correlation between deficits in verbal declarative memory and decreased density of neurons in the hippocampus (Sass et al 1990). Patients with Cushing’s disease (in which there are abnormally high levels of cortisol in the plasma) had deficits in verbal declarative memory and smaller hippocampal volume; smaller hippocampal volume was correlated with elevations in plasma cortisol levels. Treatment of hypercortisolemia was associated with a significant increase in hippocampal volume and improvement in verbal declarative memory function, suggesting that hippocampal damage is reversible (Bourdeau et al 2002; Starkman et al 1999, 2003).
Neurons within the hippocampus were found to be unique within the brain in showing the capacity to regenerate themselves (Gould et al 1998). Studies in animals have demonstrated that several agents (e.g., phenytoin, tianeptine, dehydroepiandrosterone, and the SSRI fluoxetine) might block or modulate the effects of stress on the hippocampus (Czeh et al 2001; Malberg et al 2000; Manev et al 2001; Watanabe et al 1992). Selective serotonin reuptake inhibitors also promote neurogenesis in the hippocampus through a regulation of BDNF and cyclic adenosine monophosphate (cAMP), with long-term effects on brain function (Duman et al 2001). In these studies it was chronic, but not acute, administration of these drugs that induced the changes in morphology (Nibuya et al 1996).
Several prior studies have demonstrated the efficacy of paroxetine on clinical measures of PTSD (Marshall et al 1998, 2001; Tucker et al 2001), but no studies have yet looked at the effects of paroxetine—or any medication, for that matter—on verbal declarative memory or other cognitive functions in PTSD. An important question related to the effects of stress on the hippocampus was whether these memory deficits and the hippocampal atrophy in traumatized patients with PTSD are reversible. The purpose of this study was, therefore, to assess the effects of treatment with the SSRI paroxetine on these parameters. We hypothesized that patients who responded to treatment with paroxetine would also demonstrate an improvement in declarative memory performance and show increased hippocampal volume.
Methods and Materials
Subjects
Patients with symptoms of PTSD were recruited by advertisements in newspapers and flyers. Some patients in this study were part of a national double-blind trial of paroxetine compared with placebo in PTSD. After 12 weeks of participation in the study, subjects were offered participation in an extension phase of 9 months open-label treatment of 10–50 mg paroxetine per day. After initial phone screening, a total of 46 patients were invited to participate in the study. Eighteen patients did not complete the baseline assessments or were found ineligible (because of positive drugs screen, score of less than 50 on the Clinician-Administered PTSD Scale [CAPS], or incomplete baseline assessment). Twenty-eight patients were found to be eligible and started the medication phase. Eleven patients of this group participated in the double-blind trial and received placebo or 20–50 mg of paroxetine for 12 weeks before open-label treatment. Seventeen patients started directly with the open-label treatment. The patients who had participated in the double-blind study were washed out according to the study protocol, including patients who had received active drug and patients who might have received placebo. The blind, however, was not broken for this purpose. Of the total patient sample that started the long-term medication phase, five patients did not finish this long-term treatment phase, owing to noncompliance; 23 patients completed the study. Demographic and clinical characteristics of these 23 patients are shown in Table 1.
Table 1.
Patient Demographics and Clinical Characteristics
| Men (n = 9)
|
Women (n = 14)
|
Total (n = 23)
|
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 47.2 | 6.0 | 44.0 | 9.7 | 45.3 | 8.4 |
| Years of Education | 15.3 | 2.8 | 15.8 | 3.1 | 15.6 | 2.9 |
| Race | ||||||
| Caucasian | 8 | 13 | 21 | |||
| African American | 1 | 0 | 1 | |||
| Hispanic | 0 | 1 | 1 | |||
| Asian | 0 | 0 | 0 | |||
| Other | 0 | 0 | 0 | |||
| Age of Onset | ||||||
| <18 | 3 | 9 | 12 | |||
| ≥18 | 6 | 5 | 11 | |||
| Trauma | ||||||
| Childhood sexual abuse | 2 | 8 | 10 | |||
| Childhood physical abuse | 2 | 1 | 3 | |||
| Assault | 1 | 3 | 4 | |||
| Combat | 2 | 0 | 2 | |||
| Witness Death | 1 | 1 | 2 | |||
| Motor vehicle accident | 1 | 0 | 1 | |||
| Torture | 0 | 1 | 1 | |||
| PTSD Diagnosis | ||||||
| Only diagnosis | 6.0 | 7.0 | 13 | |||
| Comorbidity | ||||||
| Comorbidity | 3.0 | 7.0 | 10 | |||
| MDD-current | 2.0 | 3.0 | 5 | |||
| MDD-lifetime | 3.0 | 8.0 | 11 | |||
| Dysthymia | 0.0 | 1.0 | 1 | |||
| PD w/o agoraphobia | 1.0 | 1.0 | 2 | |||
| GAD | 2.0 | 3.0 | 5 | |||
| Social phobia | 1.0 | 2.0 | 3 | |||
| ETI | ||||||
| Trauma | 18.0 | 8.6 | 23.2 | 15.2 | 21.4 | 13.4 |
| Physical | 14.4 | 7.2 | 11.3 | 10.1 | 12.3 | 9.2 |
| Emotional | 23.8 | 10.2 | 18.8 | 14.7 | 20.5 | 13.7 |
| Sexual | 10.8 | 7.8 | 11.7 | 12.2 | 11.0 | 10.7 |
| Sum score | 67.4 | 23.9 | 64.5 | 40.9 | 65.4 | 35.5 |
| WAIS-R | ||||||
| Verbal | 111.7 | 21.7 | 112.5 | 21.8 | 122.2 | 21.2 |
| Performance | 109.3 | 14.4 | 104.8 | 17.0 | 106.5 | 15.9 |
| Full scale | 116.0 | 12.7 | 106.9 | 14.4 | 110.2 | 14.2 |
| CAPS | ||||||
| Intrusion | 24.1 | 6.8 | 24.4 | 10.2 | 24 | 8.5 |
| Avoidance | 33.1 | 9.4 | 33.2 | 13.1 | 33 | 11.3 |
| Arousal | 24.7 | 7.6 | 23.6 | 9.8 | 24 | 8.7 |
| Total | 82.0 | 21.9 | 81.3 | 30.9 | 82 | 26.4 |
PTSD, Posttraumatic Stress Disorder; MDD, major depressive disorder; PD, panic disorder; GAD, generalized anxiety disorder; ETI, Early Trauma Inventory; WAIS-R, Wechsler Adult Intelligence Scale-Revised; CAPS, Clinician-Administered PTSD Scale.
All baseline assessments, including neuropsychological testing and MRI acquisition, were performed before subjects started in the double-blind trial or open-label medication phase. A placebo control was not included in this study because it was not known whether relatively short-term treatment (e.g., 12 weeks) would result in hippocampal changes, and it was considered unethical to provide long-term placebo treatment. All participating patients were outpatients. They were assessed for the presence of a diagnosis of PTSD based on the DSM-IV criteria for PTSD as determined with the Structural Clinical Interview for DSM-IV (Spitzer 1994), consensus diagnosis by three research psychiatrists (EV, MV, and JDB), and a score of greater than 50 on the CAPS, current symptoms version (Blake et al 1995). In patients with comorbid disorders, PTSD symptom onset had to predate the onset of comorbid psychopathology. Patients were excluded who had a serious medical or neurologic illness on the basis of history and physical examination, laboratory testing, and electrocardiogram; a history of psychotherapeutic treatment within 3 months of the start of the study; or a positive toxicology screening. Female subjects were required to have a negative pregnancy test before the study. Patients with a history of alcohol or drug abuse 6 months before the study, as well as current alcohol abuse or drug use, were excluded from participation. All patients were medication free for at least 4 weeks before participation in the study. During the study, no concomitant antidepressant or neuroleptic medication was allowed. After complete explanation of the study procedures and before entering the study, written informed consent was obtained. This study was approved by the investigational review board of Yale University and was prepared in accordance with the ethical standards laid down in the Declaration of Helsinki.
History of childhood trauma was assessed with the Early Trauma Inventory (ETI). This is a 56-item instrument for measurement of childhood traumatic experiences that assesses physical, emotional, and sexual abuse, as well as general traumatic events. The ETI has been demonstrated to be reliable and valid in the assessment of childhood trauma (Bremner et al 2000).
The mean age of the patients was 45.3 years (SD = 8.4). The patient group consisted of 14 women (average age 44.0 years, SD = 9.7) and 9 men (average age 47.2 years, SD = 6.0). Their mean education level was 15.6 years (SD = 2.9). Of the participating patients, 21 were white (91.3%), one was black (4.3%), and one was Hispanic (4.3%). Trauma exposure occurred in 12 patients in childhood and in 11 patients in adult life. Some patients who demonstrated symptoms related to an adult trauma later reported also having had childhood traumatic experiences with which they also would have met criterion A1 of the DSM-IV diagnosis (“experienced, witnessed or have been confronted with an event that involved actual or threatened death or serious injury, or a threat to the physical integrity of self or others”). Two patients were Vietnam combat veterans, the others were civilian trauma patients.
Of the 23 study completers, 11 patients (48%) had a lifetime diagnosis of major depressive disorder, five (21%) had a current depressive disorder, and one (4%) was also diagnosed with dysthymia. Two patients (8%) also had a comorbid panic disorder without agoraphobia, five (21%) met criteria for generalized anxiety disorder, and three (13%) met criteria for social phobia. Thirteen patients (56%) had PTSD as a sole diagnosis without comorbidity (see Table 1). There was no significant difference in the male versus the female patient group on the scores on ETI, Wechsler Adult Intelligence Scale–Revised (WAIS-R), or CAPS.
Neuropsychological Assessments
Before patients started the medication phase, three standardized neuropsychological tests were administered.
To assess the intellectual level for each subject, patients were administered four tests of the WAIS-R (Wechsler 1981): the arithmetic, vocabulary, picture arrangement, and block design test.
Two subtests of the Wechsler Memory Scale–Revised (WMS-R; Wechsler 1987) were administered. The tests included logical memory (free recall of two story narratives, which represents verbal memory) and figural memory (which represents visual memory and involved reproduction of designs after a 6-sec presentation). For both subtests, immediate and delayed (after a 30-minute interval) reproduction were tested, and a percentage of retention was computed (calculated as delayed/ immediate × 100).
The verbal and visual components of the Selective Reminding Test (SRT; Hannay and Levin 1985) were administered. The verbal task is a measure of verbal learning in which words are presented for immediate recall. On subsequent trials only the words not recalled on the prior trial are presented. The task is complete after two consecutive perfect recall trials or 12 presentations. The visual component is modeled on the verbal test; simple figurative designs are presented one at a time for 3 sec each, followed by an opportunity to draw all from memory. Each design that is not accurately reproduced on a given trial is shown again until perfect recall is attained or 12 trials are reached. Five indexes of learning and memory are obtained from each of the selective reminding tests: total recall, long-term retrieval, long-term storage, list learning (continuous long-term retrieval), and delayed recall.
Magnetic Resonance Imaging
Magnetic resonance images were acquired on a 1.5-T GE Electric Signa Camera (General Electric Medical Systems, Milwaukee, Wisconsin) with a protocol previously described (Bremner et al 1995a). Only sagittal images were acquired. Acquisition parameters were as follows: T1-weighted, three-dimensional volume spoiled gradient recall sequence, repetition time = 24 msec, echo time = 5 msec, 45° flip angle, 256 × 196 matrix, field of view = 30 cm, number of excitations = 2, slice thickness = 1.2 isotropic voxel, no skip (total scan time 20 min). Images were transferred through a computer network to a Sun Sparc80 Workstation (Sun Microsystems, Santa Clara, California), on which volumetric measurements were performed. No software or hardware upgrades were made during the course of the study.
Treatment Phase
Medication was prescribed in the first visit after the pretreatment assessments. The patients who started with the double-blind phase for a period of 12 weeks were tapered off and then started with open-label paroxetine at a dose of 10 mg daily and were titrated up to 20 mg in 4 days. All other patients started directly on open-label paroxetine in this way. If patients reported adverse effects or were concerned about any symptoms during treatment, they could call a 24-hour answering service that was linked to an on-call physician. If patients did not respond to the medication, the dosage was increased in 10 mg increments up to a maximum of 50 mg/day. This was similar for the double-blind and for the open-label phase. Follow-up visits were scheduled every other week from weeks 1 to 12 and every 4 weeks from weeks 12 to 36. No systematic psychotherapeutic procedure was administered in the study phase. Medication was dispensed every visit to enhance compliance and reduce chance of misuse. Average dose of paroxetine in participating patients (n = 28) was 20 mg (range 10–30 mg). Five patients did not complete the study owing to medication noncompliance, for a total completion sample of n = 23. Main reason for drop-out was protocol noncompliance: two patients reported to have used drugs (cannabis and cocaine) while using medication, one patient failed to attend follow-up visits as of week 24 of the study, one was relocated because of the start of a new job right after completion of the baseline assessments and therefore could not continue, and one patient was hospitalized and could for this reason not attend to follow-up visits. One patient participated for 14 weeks then, despite good clinical efficacy, stopped taking the medication because of complaints of loss of libido. He used herbal medication for 6 weeks, during which his libido returned as well as his PTSD symptoms. He requested a restart in the study, used paroxetine for 9 months and finished the protocol. All other patients were maximally compliant with the study protocol.
After 36–48 weeks, at the time of study completion, the CAPS as well as WMS-R and SRT were readministered. Magnetic resonance image acquisition was also repeated. After study completion, patients were referred for ongoing treatment if necessary.
Statistical Analyses
Clinical effect was analyzed with a paired-samples t test. Before analysis of neurocognitive data, we examined demographic variables, trauma variables, treatment, and clinical outcomes in the patients who completed the treatment phase. Paired-samples t tests were used to compare pretreatment and posttreatment assessments on each of the subcomponents of the WMS-R and SRT. Two-tailed tests of significance were used throughout. Based on our prior studies, we specifically hypothesized improvement in WMS-R percent retention and SRT long-term retrieval for verbal tests only. Visual measures were performed to demonstrate specificity. Significance was defined as p < .05. Morphometric data were subjected to repeated-measures analyses of variance (ANOVA) with side (left/right) as the repeated factor. This method was used to analyze hippocampal volume before and after treatment. The potentially confounding factor of whole brain volume was added in the analysis. Here, significance was also defined as p < .05.
Volumetric Analyses
Volumetric measurement of the hippocampus was performed in one run by a single trained rater (EV) who was blind to treatment phase. All MRI scans were stripped of the header. The commercial software package Analyze (Biomedical Imaging Resource, Mayo Foundation, Rochester, Minnesota; Robb et al 1989) was used to reslice MRI coronal scans to correct for head rotation and to create slices in a parallel–oblique coronal plane perpendicular to the long axis of each hippocampus (right and left) separately. First, correction for head rotation was achieved with use of anatomic landmarks, including the internal auditory canal and the seventh and eighth cranial nerve. Then, two midhippocampal points separated by 15 mm were selected to construct a line that defines the long axis of the hippocampus. A third midhippocampal point in the opposite hippocampus was then selected to define a plane parallel to the long axes of both hippocampi. A series of oblique images was constructed perpendicular to this plane to create images orthogonal to the long axis of the hippocampus. Measurement of whole hippocampal volume was performed by drawing hippocampal volume with a mouse-driven cursor on 1.0-mm-thick slices from posterior to anterior in every consecutive slice, starting at the slice where the pulvinar of the thalamus interrupts the fornix superiorly (used as the posterior landmark of the hippocampus). The superior border of the hippocampus was determined, including gray matter, alveus, and fimbriae. The inferior border was assessed, including the subiculum. A straight line from the inferior subcortical white matter extending medially was used to disconnect the parahippocampal gyrus from the subiculum. Working from posterior to anterior, in several slices around the area displaying the basilar artery, both hippocampus and amygdala were visible. The uncal recess of the temporal horn of the lateral ventricle was used as the most reliable way to separate the hippocampal head from the amygdala. If the uncal recess was not prominent, we traced along the alveus or connected the inferior horn of the lateral ventricle to the sulcus at the inferior margin of the semilunar gyrus. Previous studies have demonstrated high interrater reliability for hippocampal measurements (Bremner et al 1997; Schmahl et al 2003; Vythilingam et al 2002). Accuracy of method of hippocampal volume measurement for this data set was checked with reliability analysis. Intraclass correlation coefficient α for hippocampal volume was .925. Whole brain volumes were assessed by tracing the outline of the brain (excluding cerebellum) in all axial slices in which it was visualized. Variance in the hippocampal measurements and in the whole brain volumes pre- and posttreatment was comparable.
Results
Paroxetine treatment resulted in a mean 54% reduction (85.3% [SD 22.45%] to 38.6% [SD 28.2%]) in PTSD symptoms as measured with mean changes from baseline on the CAPS total score [t(1,22) = 7.10, p = .000] among study completers. Improvement was equally strong on all symptom cluster scores (re-experiencing, avoidance/ numbing, hyperarousal) (Figure 1).
Figure 1.
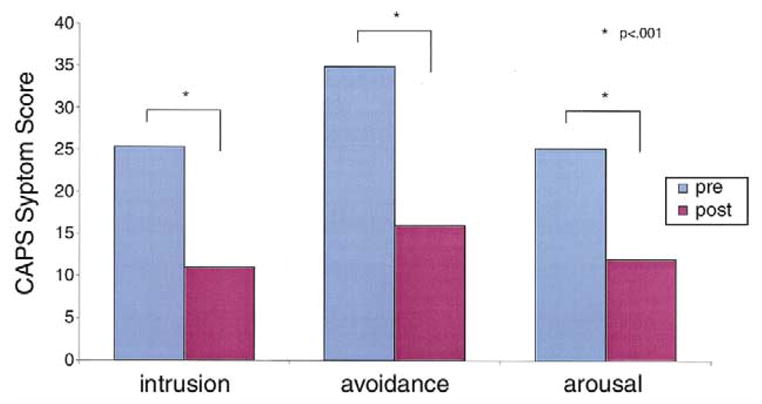
Changes in the Clinician-Administered Posttraumatic Stress Disorder (PTSD) Scale (CAPS) after long-term treatment.
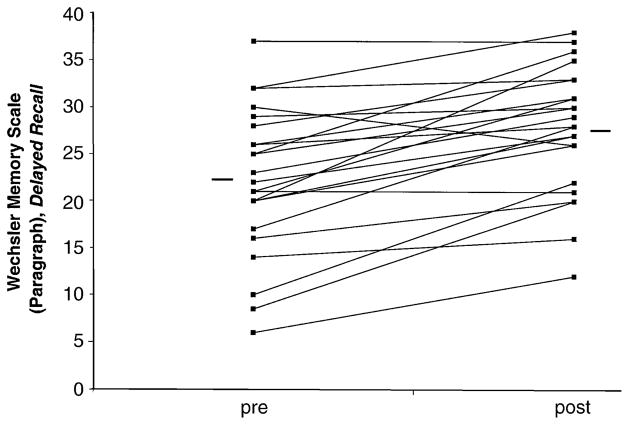
Treatment also resulted in significant improvements in verbal declarative memory, as measured with the WMS-R paragraph recall, for delayed recall [22.1–27.7, t(1,22) = −5.755, p = .000] and percent retention [80.2–91.1, t(1,22) = −3.41, p = .003] but not immediate recall [27.3–30.3, t(1,22) = −1.842, p = .079). Visuospatial memory on the WMS-R significantly improved for immediate [32.5–35.8, t(1,21) = −2.857, p = .009] but not for delayed recall [23.6–30.6, t(1,21) = −2.723, p = .13] nor for percent retention [72.6–84.4; t(1,20) = −1.96, p= .064]. Improvements were significant on all subscales of the verbal component of the SRT, including long-term retrieval and delayed recall, which was not significant in the visual component of the scale [11.6–11.7; t(1,22) =−.646, p = .525] (see Table 2).
Table 2.
Memory Performance before and after Long-Term Treatment with Paroxetine in Posttraumatic Stress Disorder
| Baseline
|
Posttreatment
|
t | p | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Wechsler Memory Scale–Revisedf | |||||||
| Logical memory | |||||||
| Immediate recall | 27.3 | 7.6 | 30.3 | 6.6 | −1.842 | .079 | ns |
| Delayed recall | 22.1 | 7.8 | 27.7 | 6.7 | −5.755 | .000 | a |
| Percent retention | 80.2 | 18.4 | 91.1 | 11.8 | −3.41 | .003 | a |
| Figural memory | |||||||
| Immediate recall | 32.5 | 4.5 | 35.8 | 3.3 | −2.857 | .009 | a |
| Delayed recall | 23.6 | 11.1 | 30.6 | 7.2 | −2.723 | .130 | ns |
| Percent retention | 72.6 | 29.3 | 84.4 | 16.9 | −1.96 | .064 | ns |
| Selective Reminding Test | |||||||
| Verbal | |||||||
| Total recall | 116.1 | 12.2 | 129.3 | 9.4 | −5.809 | .000 | a |
| Long-term storage | 114.4 | 13.6 | 128.8 | 9.4 | −5.993 | .000 | a |
| Long-term retrieval | 108 | 16.1 | 125.1 | 12.9 | −6.177 | .000 | a |
| Continuous long-term retrieval | 89.8 | 27.4 | 114.7 | 26.4 | −4.685 | .000 | a |
| Delayed recall | 10.4 | 1.2 | 11.2 | .7 | −2.398 | .025 | b |
| Visual | |||||||
| Total recall | 132.7 | 11.1 | 139.2 | 2.7 | −3 | .007 | a |
| Long-term storage | 132.6 | 10.6 | 138.6 | 3.6 | −2.597 | .016 | a |
| Long-term retrieval | 131.3 | 10.8 | 138 | 3.5 | −3.039 | .006 | a |
| Continuous long-term retrieval | 126 | 19.8 | 137.2 | 4.2 | −2.892 | .008 | a |
| Delayed recall | 11.6 | .9 | 11.8 | .4 | −.646 | .525 | ns |
p <.01
p <.05
The individual pretreatment and posttreatment effects per patient are displayed in Figure 2.
Figure 2.
Individual difference in memory performance on Wechsler Memory Scale (paragraph), delayed recall, before and after treatment. Each line connects the individual score pre- and posttreatment. The horizontal lines indicate the mean group score.
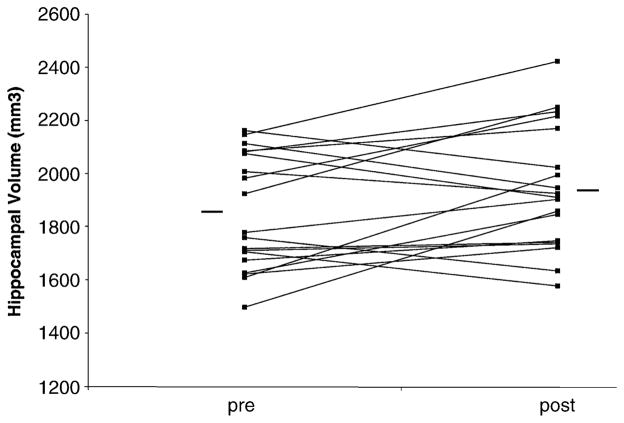
Repeated-measures ANOVA with side as the repeated measure showed a main effect for treatment related to a 4.6% increase in mean hippocampal volume (1857.3 mm3 [SD 225.6] to 1942.9 mm3 [SD 243.2]) with treatment [F(1,36) = 8.775, p = .005]. Increased hippocampal volume was seen for both left (5.6%) (1807.6 mm3 [SD 255.5, p = .025] to 1909.3 mm3 [SD 236.9]) and right (3.7%) (1906.9 mm3 [SD 195.8] to 1976.7 mm3 [SD 249.6, p = .046]) hippocampus. There was no main effect for side (left/right) [F(1,36) = .413, p = .525]. There was no change in whole brain volume with treatment (360,871 mm3 to 359,932 mm3) (p = .17). Increase in hippocampal volume was significant after adding whole brain volume before and after treatment to the model. Individual pretreatment and posttreatment effects per patient are displayed in Figure 3.
Figure 3.
Individual differences in hippocampal volume before and after treatment. Each line connects the individual score of pre- and posttreatment. The horizontal lines indicate the mean group volume.
There was no correlation between change in CAPS or memory and change in hippocampal volume with treatment. There were no significant gender-specific differences in the patients on the visual memory components of WMS-R or SRT. Analyzing the data per gender did not change the specificity of the improvement in delayed verbal memory as opposed to visual memory. No significant relationships were found between age, ETI index score, trauma onset, and WAIS-R with memory improvement. A positive relationship was found between ETI index score and improvement with treatment on the CAPS avoidance subscale [F(1,17) = 4.78, p < .05]. A positive correlation was found between absence of lifetime depression and improvement on CAPS [F(1,18) = 2,72, p = .007]. There was no correlation with increase in hippocampal volume in this group.
Discussion
Our findings of clinical efficacy of paroxetine are in line with earlier reports of open-label (Marshall et al 1998; Smajkic et al 2001) and placebo-controlled studies (Marshall et al 2001; Tucker et al 2001) that showed a similar drop in CAPS scores in PTSD. Treatment led to a significant improvement in verbal declarative memory function. The difference in immediate and delayed memory performance, which is best reflected by the percent retention score, improved significantly for verbal memory. This was not seen for figural memory. Patients with PTSD showed a 4.6% increase in hippocampal volume with 9–12 months of treatment. These findings suggest that clinical successful treatment with paroxetine results in reversal of stress-induced hippocampal atrophy in patients with a history of traumatic stress and the diagnosis of PTSD. Changes in hippocampal volume were not associated with improvement in PTSD symptoms measured with the CAPS. This is the first published study in humans in which an increase in hippocampal volume after treatment was found and the first study of the effects of treatment on cognitive function in PTSD.
Our findings are consistent with preclinical studies showing that SSRIs in the hippocampus promote neurogenesis and reverse hippocampal damage (Duman et al 2001). Animal studies have demonstrated several agents with potentially beneficial effects on stress-induced hippocampal damage. It has been found that phenytoin blocks the effects of stress on the hippocampus, probably through modulation of excitatory amino acid–induced neurotoxicity (Watanabe et al 1992). Other agents, including tianeptine, dehydroepiandrosterone, and fluoxetine have similar effects (Czeh et al 2001; Kimonides et al 1998; Malberg et al 2000). These medications might share a common mechanism of action through upregulation of cAMP response element binding protein (CREB) that might lead to regulation of expression of specific target genes involved in structural modeling of the hippocampus. Such treatment effects on BDNF and tyrosine receptor kinase B (trkB) messenger ribonucleic acid (mRNA) can have long-term effects on brain structure and function. Preclinical studies showed that chronic, but not acute, administration of several different classes of antidepressants, including serotonin- and norepinephrine-selective reuptake inhibitors, increase the expression of CREB mRNA (Frechilla et al 1998; Nibuya et al 1996). Interestingly, in contrast, chronic administration of several nonantidepressant psychotropic drugs did not influence expression of CREB mRNA, which demonstrates the pharmacologic specificity of this effect (Nibuya et al 1996).
Although several structural MRI studies reported smaller hippocampal volume in patients with PTSD, there is controversy regarding the nature and source of these hippocampal volume changes in PTSD. A recently published study supported smaller hippocampal volume as a vulnerability factor to PTSD (Gilbertson et al 2002). This study questions the nature of the hippocampal volume reductions in PTSD as being secondary to the neurotoxic effects of trauma. To attribute smaller hippocampal volume in PTSD as being entirely related to hereditary factors must be taken with caution. Multiple lines of evidence demonstrate that exposure to repeated stressors increases the probability of pathology with re-exposure to stress (Bremner et al 1993b). Also, if only genetic factors are related to smaller hippocampal volume in PTSD, then it would be unlikely that SSRI treatment would lead to an increase in volume.
We did not find a significant relationship between improvement on memory performance and hippocampal volume. The small magnitude of change in hippocampal volume might limit our ability to find correlations between this and other parameters. The hypotheses that increased hippocampal volume is secondary to a promotion of neurogenesis by SSRIs should be interpreted with caution. There are other possible explanations for our findings, such as changes in water content in the hippocampus that could lead to an increase in hippocampal volume. Other limitations should be considered when interpreting our findings. We did not include other brain regions, except for whole brain, to assess specificity of changes in hippocampal volume; for example, a comparable effect for caudate nuclei volumes (5.7% increase) was found in first-episode schizophrenic patients taking typical antipsychotic medication (Chakos et al 1994; Lieberman et al 2001). We did not include a wait-list or control group that would have enabled us to assess the specific effects of treatment and take into consideration time effects that are independent of group and treatment. We also did not include a control group because we speculated that long-term treatment might be required for effects on memory and hippocampal volume and did not feel that it was ethical to treat patients with placebo for 9–12 months. Future studies should include control groups and shorter periods of treatment. There might have been a practice or learning effect related to the repeat of neuropsychological testing before and after treatment (Ryan et al 1981; Theisen et al 1998). This is probably unlikely, however, because of the long period of 1 year before the second testing session and the relative lack of improvement in visual compared with verbal memory. We also did not look at depression posttreatment. It might also be that increase in hippocampal volume is related to improvement of depressive symptoms.
These findings might have clinical implications for PTSD. If treatment with medications such as paroxetine reverses stress-induced hippocampal damage, it might lead to an improvement in memory and cognition in PTSD. Our study, showing an improvement in hippocampal based declarative memory function with paroxetine in PTSD, is consistent with this. Also, reversal of hippocampal atrophy with improvement in memory function might help PTSD patients deal with stressful traumatic memories and cope better with daily life hassles and lead to a better long-term recovery.
Acknowledgments
This work was supported by National Institute of Mental Health ROI MH56120, by a grant from GlaxoSmithKline, by the National Center for Posttraumatic Stress Disorder, and a Veterans Affairs Career Development Award to JDB.
We thank Christian Schmahl, M.D., and Sajid Siddiq, M.D., for expert assistance in image processing and analysis; Heather Douglas Palumberi, M.A., Tammy Rowe, B.A., and Jacque Piscatelli, R.N., for assistance in patient recruitment and assessment; and Terry Hickey, R.T.N.M., and Hedy Sarofin, R.T.R., for assistance with MRI scanning.
References
- Andrews B, Brewin CR, Ochera J, Morton J, Bekerian DA, Davies GM, et al. The timing, triggers and qualities of recovered memories in therapy. Br J Clin Psychol. 2000;39(Pt 1):11–26. doi: 10.1348/014466500163077. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bourdeau I, Bard C, Noel B, Leclerc I, Cordeau MP, Belair M, et al. Loss of brain volume in endogenous Cushing’s syndrome and its reversibility after correction of hypercortisolism. J Clin Endocrinol Metab. 2002;87:1949–1954. doi: 10.1210/jcem.87.5.8493. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al. MRI-based measurement of hippocampal volume in patients with combat- related post-traumatic stress disorder. Am J Psychiatry. 1995a;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Capelli S, Delaney R, McCarthy G, et al. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res. 1995b;59:97–107. doi: 10.1016/0165-1781(95)02800-5. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, et al. Deficits in short-term memory in posttraumatic stress disorder. Am J Psychiatry. 1993a;150:1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse in combat-related posttraumatic stress disorder. Am J Psychiatry. 1993b;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: The Early Trauma Inventory. Depress Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci U S A. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD: A review of the empirical literature. Clin Psychol Rev. 2000;20:1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, et al. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Crowell T, Kieffer K, Siders C, Vanderploeg R. Neuro-psychological findings in combat-related posttraumatic stress disorder. Clin Neuropsychol. 2002;16:310–321. doi: 10.1076/clin.16.3.310.13851. [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton JE, Pederson SL. Effects of post-traumatic stress disorder on neuropsychological test performance. Int J Clin Neuropsychol. 1989;11:121–124. [Google Scholar]
- Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology. 2001;25:836–844. doi: 10.1016/S0893-133X(01)00358-X. [DOI] [PubMed] [Google Scholar]
- Frechilla D, Otano A, Del Rio J. Effect of chronic antidepressant treatment on transcription factor binding activity in rat hippocampus and frontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:787–802. doi: 10.1016/s0278-5846(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Gurvits TV, Lasko NB, Orr SP, Pitman RK. Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J Trauma Stress. 2001;14:413–432. doi: 10.1023/A:1011181305501. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko N, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannay HJ, Levin HS. Selective reminding test: An examination of the equivalence of four forms. J Clin Exp Neuropsychol. 1985;7:251–263. doi: 10.1080/01688638508401258. [DOI] [PubMed] [Google Scholar]
- Jenkins MA, Langlais PJ, Delis D, Cohen R. Learning and memory in rape victims with posttraumatic stress disorder. Am J Psychiatry. 1998;155:278–279. doi: 10.1176/ajp.155.2.278. [DOI] [PubMed] [Google Scholar]
- Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: Can’t live with it, can’t live without it. Behav Brain Res. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev R, Uz T, Manev H. Fluoxetine increases the content of neurotrophic protein S100beta in the rat hippocampus. Eur J Pharmacol. 2001;420:R1–R2. doi: 10.1016/s0014-2999(01)00989-x. [DOI] [PubMed] [Google Scholar]
- Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: A fixed- dose, placebo-controlled study. Am J Psychiatry. 2001;158:1982–1988. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- Marshall RD, Schneier FR, Fallon BA, Knight CB, Abbate LA, Goetz D, et al. An open trial of paroxetine in patients with noncombat-related, chronic posttraumatic stress disorder. J Clin Psychopharmacol. 1998;18:10–18. doi: 10.1097/00004714-199802000-00003. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D, Gannon MN, et al. Paradoxical effects of adrenal steroids on the brain: Protection versus degeneration. Biol Psychiatry. 1992;31:177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: Implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML, Stein-Behrens B, Sapolsky R. Glucocorticoids mediate the stress-induced extra-cellular accumulation of glutamate. Brain Res. 1994;655:251–254. doi: 10.1016/0006-8993(94)91622-5. [DOI] [PubMed] [Google Scholar]
- Moradi AR, Doost HT, Taghavi MR, Yule W, Dalgleish T. Everyday memory deficits in children and adolescents with PTSD: Performance on the Rivermead Behavioural Memory Test. J Child Psychol Psychiatry. 1999;40:357–361. [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK. Post-traumatic stress disorder, hormones, and memory. Biol Psychiatry. 1989;26:221–223. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC. Analyze: A comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989;13:433–454. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- Roca V, Freeman TW. Complaints of impaired memory in veterans with PTSD. Am J Psychiatry. 2001;158:1738–1739. doi: 10.1176/appi.ajp.158.10.1738-a. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Morris J, Yaffa S, Peterson L. Test-retest reliability of the Wechsler Memory Scale, Form I. J Clin Psychol. 1981;37:847–848. doi: 10.1002/1097-4679(198110)37:4<847::aid-jclp2270370429>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass KJ, Spencer DD, Kim JH, Westerveld M, Novelly RA, Lencz T. Verbal memory impairment correlates with hippocampal pyramidal cell density. Neurology. 1990;40:1694–1697. doi: 10.1212/wnl.40.11.1694. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Semple WE, Goyer PF, McCormick R, Compton-Toth B, Morris E, Donovan B, et al. Attention and regional cerebral blood flow in posttraumatic stress disorder patients with substance abuse histories. Psychiatry Res. 1996;67:17–28. doi: 10.1016/0925-4927(96)02735-7. [DOI] [PubMed] [Google Scholar]
- Smajkic A, Weine S, Djuric-Bijedic Z, Boskailo E, Lewis J, Pavkovic I. Sertraline, paroxetine, and venlafaxine in refugee posttraumatic stress disorder with depression symptoms. J Trauma Stress. 2001;14:445–452. doi: 10.1023/A:1011177420069. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R. Structural Clinical Interview for DSM-IV. New York: New York State Psychiatric Institute; 1994. [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Biol Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Schteingart DE. Improvement in learning associated with increase in hippocampal formation volume. Biol Psychiatry. 2003;53:233–238. doi: 10.1016/s0006-3223(02)01750-x. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Sutker PB, Winstead DK, Galina ZH, Allain AN. Cognitive deficits and psychopathology among former prisoners of war and combat veterans of the Korean conflict. Am J Psychiatry. 1991;148:67–72. doi: 10.1176/ajp.148.1.67. [DOI] [PubMed] [Google Scholar]
- Theisen ME, Rapport LJ, Axelrod BN, Brines DB. Effects of practice in repeated administrations of the Wechsler Memory Scale Revised in normal adults. Assessment. 1998;5:85–92. doi: 10.1177/107319119800500110. [DOI] [PubMed] [Google Scholar]
- Thygesen P, Hermann K, Willanger R. Concentration camp survivors in Denmark: Persecution, disease, compensation. Dan Med Bull. 1970;17:65–108. [PubMed] [Google Scholar]
- Tucker P, Zaninelli R, Yehuda R, Ruggiero L, Dillingham K, Pitts CD. Paroxetine in the treatment of chronic posttraumatic stress disorder: Results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001;62:860–868. doi: 10.4088/jcp.v62n1105. [DOI] [PubMed] [Google Scholar]
- Uddo M, Vasterling JT, Braily K, Sutker PB. Memory and attention in posttraumatic stress disorder. J Psychopathol Behav Asess. 1993;15:43–52. [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Jr, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress. I. Preclinical studies. Depress Anxiety. 2002;15:126–147. doi: 10.1002/da.10016. [DOI] [PubMed] [Google Scholar]
- Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, et al. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry. 2002;52:119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2:431–435. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. WMS-R: Wechsler Memory Scale-Revised Manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Wolfe J, Schlesinger LK. Performance of PTSD patients on standard tests of memory. Implications for trauma. Ann N Y Acad Sci. 1997;821:208–218. doi: 10.1111/j.1749-6632.1997.tb48280.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Keefe RS, Harvey PD, Levengood RA, Gerber DK, Geni J, et al. Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1995;152:137–139. doi: 10.1176/ajp.152.1.137. [DOI] [PubMed] [Google Scholar]
- Zalewski C, Thompson W, Gottesman II. Comparison of neuropsychological test performance in PTSD, generalized anxiety disorder, and control Vietnam veterans. Assessment. 1994;1:133–142. doi: 10.1177/1073191194001002003. [DOI] [PubMed] [Google Scholar]