Abstract
Context
We previously used positron emission tomography (PET) measurement of brain metabolism with 18fluorodeoxyglucose to show that patients receiving selective serotonin reuptake inhibitors (SSRIs) who have a tryptophan depletion–induced return of depressive symptoms have an acute decrease in metabolism in orbitofrontal cortex, dorsolateral prefrontal cortex, and thalamus. Many patients with depression in remission while taking norepinephrine reuptake inhibitors (NRIs) (but not SSRIs) experience a return of depressive symptoms with depletion of norepinephrine and dopamine using α-methylparatyrosine (AMPT).
Objective
To assess brain metabolic correlates of AMPT administration in patients with depression in remission while receiving NRIs.
Design, Setting, and Participants
Randomized, controlled, double-blind trial in which 18 patients recruited in 1997–2000 from the general community who had depression in remission while taking NRIs had PET imaging in a psychiatric research unit following AMPT and placebo administration.
Interventions
After initial medication with desipramine and follow-up until response, patients underwent active AMPT (five 1-g doses administered orally over 28 hours) and placebo (diphenhydramine hydrochloride, five 50- mg doses administered similarly) catecholamine depletion challenges in randomized order of assignment, after which PET imaging was performed on day 3 of each condition. Both study conditions were performed 1 week apart.
Main Outcome Measures
Regional brain metabolism rates in patients with and without AMPT-induced return of depressive symptoms.
Results
AMPT-induced return of depressive symptoms was experienced by 11 of the 18 patients and led to decreased brain metabolism in a number of cortical areas, with the greatest magnitude of effects in orbitofrontal (P=.002) and dorsolateral prefrontal (P=.03) cortex and thalamus (P=.006). Increased resting metabolism in prefrontal and limbic areas predicted vulnerability to return of depressive symptoms.
Conclusions
Different neurochemical systems that mediate depression may have effects on a common brain circuitry. Baseline metabolism in successfully treated depressed patients may predict vulnerability to future episodes of depression.
Major depression is an important public health problem that affects about 16% of people in the United States at some time in their lives.1 A major effort of the past few decades has been elucidating biological mechanisms that underlie symptoms of depression. Understanding the biology of depression may help in the treatment of this disabling disorder. Two classic biological models for depression have been the serotonin and norepinephrine hypotheses of depression.
Multiple lines of evidence support the serotonergic hypothesis of depression.2,3 Serotonergic neurons have their cell bodies in the brain stem (dorsal raphe). These cell bodies give rise to long axons that project throughout the brain. Treatment with selective serotonin reuptake inhibitors (SSRIs) is efficacious for treatment of depression. Studies have found a decrease in the serotonin metabolite 5-hydroxyindole-acetic acid in the cerebrospinal fluid4,5 and in postmortem brain,6 decreased serotonin concentrations in platelets,7 increased 5-hydroxytryptamine receptor binding in frontal cortex in some studies8–11 but not in others,12 and reduction of prolactin response to the serotonin agents fenfluramine13 and clomipramine14 and the serotonin precursor L-tryptophan15 in patients with depression. Dietary depletion of tryptophan, an amino acid that is the precursor of serotonin, is associated with decreased levels of plasma tryptophan16 as well as a decrease in brain serotonin levels.17 Tryptophan depletion also results in a transient return of depressive symptoms in more than half of patients who have been successfully treated with SSRIs,18–20 but depletion does not worsen mood in healthy persons16 or in patients with untreated depression.21 Patients with treated depression who have stopped SSRIs have a return of symptoms of depression with tryptophan depletion.22 These studies suggest that alterations in serotonergic function mediate, at least in part, symptoms of depression.
Alterations in noradrenergic function have also been hypothesized to underlie symptoms of depression.3,23 The majority of the noradrenergic neurons in the brain have their cell bodies in the pons (brain stem), with long axons that project throughout the brain, releasing transmitter in multiple cortical and subcortical sites, including pre-frontal, parietal, and sensory cortex and hippocampus. The noradrenergic hypothesis of depression was originally based on the finding that the antihypertensive medication reserpine, which acts by interfering with uptake and storage of norepinephrine and dopamine in intracellular storage vesicles, is associated with symptoms of depression in many patients.24 Treatment with selective norepinephrine reuptake inhibitors (NRIs) is also efficacious for treatment of depression in the majority of cases. Norepinephrine reuptake inhibitors decrease sensitivity of the α2 autoreceptor and increase sensitivity of the α1 receptor, leading to increased noradrenergic transmission. Norepinephrine reuptake inhibitors also lead to a reduction in β receptors after 2 weeks, which is the time frame of action of the antidepressive effects.25 These medications lead to a reduction in whole-body norepinephrine turnover in patients treated for depression.26 Other evidence for alterations in noradrenergic function in depressed patients includes a well-replicated finding of blunted growth hormone response to the α2 agonist clonidine (the growth hormone response to clonidine is believed to be related to postsynaptic stimulation of the α2 receptor).27–29 Postmortem studies of the brains of depressed patients found decreased density of the noradrenergic transporter30 and upregulation of tyrosine hydroxylase.31 Studies of norepinephrine metabolites in urine and cerebrospinal fluid in patients with major depression have been inconclusive.23
α-Methylparatyrosine (AMPT) is a competitive inhibitor of the rate-limiting enzyme of catecholamine synthesis, tyrosine hydroxylase.32 AMPT results in a decrease in urine,33, 34 plasma,35–38 and cerebrospinal39 levels of catecholamine metabolites; it has no effect on mood in healthy persons36,40 or in drug-free depressed patients,35 but more than half of patients with treated depression who are taking NRIs have a transient return of depressive symptoms following AMPT administration.37,38 In one study, 71% of drug-free patients who remitted from depression had a return of depressive symptoms.41 These studies are consistent with alterations in noradrenergic function underlying symptoms of depression.
Both noradrenergic and serotonergic systems therefore appear to modulate symptoms of depression. Tryptophan depletion–induced return of depressive symptoms has been observed in patients who responded to SSRIs (but not patients treated with NRIs),18 while patients with depression who were successfully treated with NRIs (but not patients taking SSRIs) were susceptible to AMPT-induced return of depressive symptoms.38 These studies suggest that different neuro-chemical systems may result in the same phenotypical outcome of depression. It is not clear if there are 2 separate etiologies for depression (eg, serotonergic or noradrenergic dysfunction) or whether these diverse neurochemical systems act on common brain regions and/or have common postsynaptic effects that are involved in the mediation of symptoms of depression.42
Brain imaging studies have begun to map out a common circuit of brain regions that are thought to mediate symptoms of depression. Deficits in hippocampal structure and function have been hypothesized to underlie both the deficits in emotional dysregulation as well as cognitive dysfunction associated with depression.43 Deficits in pre-frontal cortical function have also been hypothesized to underlie symptoms of depression.44,45 Areas of prefrontal cortex implicated in depression include dorsolateral prefrontal cortex (involved in working memory) and medial prefrontal cortex (mediating emotion), which consists of several related areas, including orbitofrontal cortex, anterior cingulate (area 25, sub-callosal gyrus; area 24, subgenual gyrus; and area 32, the Stroop area), and anterior prefrontal cortex (area 9). Alterations in the amygdala have also been hypothesized in depression.
Imaging studies in depression showed alterations in hippocampus, amygdala, and prefrontal cortex.43,46,47 Magnetic resonance imaging (MRI) studies in patients with depression showed smaller hippocampal volumes and other alterations in hippocampal structure48–56 (although see Pantel et al57 and Ashtari et al58) and smaller subgenual (anterior cingulate) cortical59 and orbitofrontal cortical60,61 volumes. Changes in both amygdala structure48,62 and function63,64 were found in depression. Multiple positron emission tomography (PET) and single-photon emission computed tomography studies found decreased left65–68 and bilateral69–71 dorsolateral prefrontal cortex function at baseline in patients with untreated major depression. Some PET studies also found decreased metabolism at baseline in medial prefrontal cortex/anterior cingulate.47,59,70,72 Other PET studies in patients with depression and a range of comorbid conditions found decreased metabolism and/or blood flow in the prefrontal cortex, including orbitofrontal cortex and anterior cingulate.73–76 Patients with depression showed a blunted metabolic response to challenge with the serotonergic agent fenfluramine in left prefrontal and temporal-parietal cortex relative to controls.77 Successful response to treatment was associated with changes in the prefrontal cortex.78,79
To assess neural correlates of serotonergic contributions to depression, we used PET to measure brain metabolism in conjunction with administration of tryptophan depletion and placebo in patients with depression in remission while taking SSRIs. Depressed patients in remission with a tryptophan depletion–induced return of depressive symptoms were compared with depressed patients without a tryptophan depletion–induced return of depressive symptoms. Patients with a tryptophan depletion–induced return of depressive symptoms (but not nondepressed patients) had decreased metabolism in dorsolateral prefrontal cortex, orbitofrontal cortex, and thalamus on the tryptophan depletion day in comparison with the placebo day. Relapse-prone patients had increased resting (ie, on the placebo day) limbic and prefrontal cortical metabolism.44 What was unanswered by this study was whether the findings were related to serotonergic alterations in these brain regions (eg, alterations in postsynaptic serotonin receptors or in serotonin neurotransmitter release) or whether there was a regional brain abnormality (not linked to a specific neurochemical system) that accounted for the findings.
One way to address this question is to probe alternative neurochemical systems that have been implicated in depression, such as the noradrenergic system. The purpose of the current study was to assess neural correlates of AMPT-induced return of depressive symptoms in patients with depression in remission while taking NRIs. Based on the fact that the orbitofrontal and dorso-lateral prefrontal cortex receive important innervation from the noradrenergic system (and are involved in a circuit with the thalamus) and that decreases were observed in these areas with tryptophan depletion–induced depressive relapse, we hypothesized that AMPT-induced relapse would be associated with a decrease in metabolism in orbitofrontal cortex, dorsolateral prefrontal cortex, and thalamus.
METHODS
Participants
Twenty-three men and women with a history of major depression in remission who were taking NRIs participated in the study. None had participated in a previously published study of depression. All were free of major medical illness on the basis of history and physical examination, laboratory testing, and electrocardiogram, were not actively abusing substances or alcohol (in the past 6 months), and were free of all medications at initial recruitment. Participants with symptoms of depression were recruited by newspaper advertisement. Participants were started on desipramine (if there was no contraindication) and followed up by a psychiatrist in an outpatient research clinic in an academic setting until they had a response to medication. After they had a response to medication as defined by the Hamilton Depression Rating Scale (HDRS) criteria outlined herein, they were entered into the PET portion of the study. If they did not have a response, if possible, they were switched to another NRI (nortriptyline). Participants with a serious medical or neurological illness, organic mental disorder, comorbid psychotic disorder or posttraumatic stress disorder based on the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (SCID),80 premenstrual dysphoric disorder, history of alcohol or substance abuse or dependence, retained metal, or history of head trauma, loss of consciousness, cerebral infectious disease, or dyslexia were excluded.
This study was approved by the Yale University Human Investigation Committee. All participants gave written informed consent and were paid for their participation. Informed consent included a description of the possibility that AMPT would result in a return of depressive symptoms, as well as the possible adverse effects of AMPT. This study included provision for supportive treatment of depression in an inpatient psychiatric research unit; however, all participants had a return of normal mood within 6 hours of the last AMPT dose, consistent with prior studies of AMPT, which have found no episodes among individuals who had depressive symptoms to last more than 8 hours after the last AMPT dose.35–38,41,81
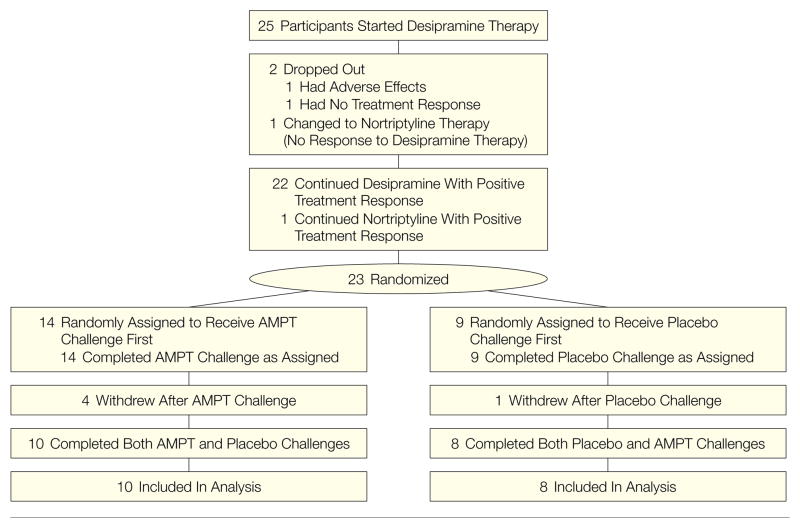
Twenty-five participants were started on desipramine, 1 was switched to nor-triptyline because of nonresponse, 2 dropped out because of adverse effects or nonresponse, 23 started the PET imaging protocol, and 5 did not complete the second test day (Figure 1). Four of these noncompleting participants received AMPT on the first day and 1 received placebo on the first day. One participant who received AMPT was removed from the protocol because of adverse effects of AMPT; the other 3 participants who received AMPT on day 1 refused or did not follow up with completion of day 2. These participants were clinically noted to have had a return of depressive symptoms with AMPT. The participant who received placebo on day 1 did not follow up with completion of day 2.
Figure 1. Patient Flow Through the Study.
Of 25 patients who started desipramine, 2 dropped out of the study because of adverse effects or nonresponse, 1 did not respond to desipramine and was switched to nortriptyline, 23 started the positron emission tomography (PET) part of the study, and 18 completed both test days. AMPT indicates α-methylparatyrosine.
Eighteen participants completed both test conditions (9 women and 9 men). Of these, 11 had an AMPT-induced return of depressive symptoms and 7 did not. Mean (SD) age was 43 (13) years. No women were postmenopausal. All participants were right-handed. Participants had been treated with desipramine or nortriptyline in an open-label fashion until they achieved remission of depression with dosage adjustments made by a study psychiatrist based on clinical response. Desipramine dosages ranged from 75 to 300 mg/d (mean dosage, 150 mg/d), with desipramine blood levels in the 150 to 300 ng/mL range. Nortriptyline dosage was 150 mg/d, with blood levels in the 50 to 150 ng/mL range. Mean (SD) number of depressive episodes was 17 (28) (range, 1–99). Full remission of depression was achieved after a mean (SD) of 7 (8) weeks (range, 2–36 weeks). Participants continued taking desipramine after remission until they completed PET scanning and then were referred for follow-up treatment. At the time of scanning, participants had been treated for a mean (SD) of 13 (10) weeks (range, 5–46 weeks).
Diagnosis of major depression was established using the SCID.80 All of the depressed patients had current and lifetime unipolar major depression. For 3 patients (17%), this was the first episode of depression; the other patients had recurrent depression. Two of the 18 depressed patients (11%) fulfilled criteria for a lifetime history of dysthymia based on the SCID. One patient (6%) had a lifetime (not current) history of agoraphobia. One patient had a lifetime (not current) history of social phobia. One patient had a lifetime (not current) history of simple phobia. Two patients (11%) had a lifetime history of panic disorder without agoraphobia; in one of these patients, the diagnosis was current. One patient had lifetime (not current) cocaine dependence, 2 patients (11%) had lifetime (not current) marijuana dependence, and 4 patients (22%) had lifetime (not current) alcohol dependence.
AMPT and Placebo Administration
Participants underwent active AMPT (five 1-g doses administered orally over 28 hours) and placebo (diphenhydramine hydrochloride, five 50-mg doses administered similarly) catecholamine depletion challenges in a randomized double-blind design, with randomized order of assignment.35,37,38,41 Both study conditions were performed 1 week apart. Fourteen of 23 participants underwent the AMPT test first. Of the participants who completed both tests, 9 of 18 underwent the AMPT test first. Diphen-hydramine was used as an active placebo to approximate the level of sedation induced by AMPT.35,37,38 The course of AMPT administration and PET imaging was based on our prior studies showing the maximal behavioral effects of AMPT after 3 days of administration.35,37,38 Medication was prepared by an onsite research pharmacist who assigned participants to an AMPT-placebo or placebo-AMPT test order using a random number generator. Medication was dispensed in numbered bottles and administered by a psychiatrist and research nurse in a research study unit. All raters, participants, psychiatrists, nurses, imaging personnel, and data analysts were blinded to assignment until the data analysis was completed. Participants were recruited between 1997 and 2000.
Each study condition involved outpatient visits on 4 days. Behavioral ratings and blood samples for monoamine metabolites were obtained daily (8:00 AM to 9:00 AM and 3:00 PM to 4:00 PM) during days 2 and 3 and once in the mornings of days 1 and 4. Medication capsules containing AMPT (1 g) or diphenhydramine hydrochloride (50 mg) were given during day 2 (9:00 AM, noon, and 7:00 PM) and day 3 (9:00 AM and noon). Investigators were blinded to study condition. Vital signs were obtained 3 times daily. Daily urinalysis was performed to allow for early detection of potential urinary crystal formation. To minimize the risk of urinary crystal formation, participants drank 2 L of water per day. Positron emission tomographic scanning was performed at 11:00 AM on day 3.
Symptoms of depression were measured using the HDRS.82 AMPT-induced changes in mood were measured as the change in score on the HDRS between AMPT baseline (ie, AMPT day 1) and the time of the PET scan (AMPT day 3), subtracting the HDRS change from day 1 to day 3 of the placebo session. We have used this method of analyzing response in prior studies of tryptophan depletion44 and AMPT in depression.35,41 Return of depressive symptoms was categorized as an increase with AMPT of 9 points and a 50% increase from baseline on the HDRS defined in this fashion.
PET and MRI Scanning Methods
Two PET scans were performed 1 week apart in conjunction with the AMPT and placebo conditions. The PET scans took place on day 3 of the study condition at 11:00 AM, 3 hours after the last dose of AMPT or placebo. Participants were scanned with a Posicam 6.5 PET camera (Positron Corp, Houston, Tex). The Posicam 6.5 is a 21-slice camera with 5.125-mm interslice thickness. Inherent resolution in plane is 5.8 mm and 11.9 mm in the Z axis; system sensitivity is 165 kilocounts/s per μCi/cm3.83 An intravenous line was inserted in the hand and warmed with a heating pad for measurement of arterialized venous blood samples. This method has been shown to yield equivalent values of metabolism to arterial line placement.84 Participants were then placed in the scanner with the head held in a head holder to minimize patient motion. The head was positioned with the canthomeatal line parallel to the external laser light. Following positioning within the camera gantry, a transmission scan of the head was obtained using an external gallium citrate Ga 67/germanium Ge 68 rod source. These data were used to correct emission data from attenuation due to overlying bone and soft tissue. Participants then received an intravenous injection of 5 mCi (185 MBq) of 18fluorodeoxyglucose in a single bolus followed by scanning of the brain from 30 to 50 minutes after injection with eyes open in a dimly lit room. Twenty-three arterialized venous blood samples were obtained at multiple points after injection for measurement of radioactivity in plasma, used for construction of a plasma time-activity curve. Three blood samples were also obtained for measurement of plasma glucose concentrations. There were no differences between plasma glucose concentrations during the AMPT (85 [SD, 13] mg/100 mL) and placebo (85 [SD, 13] mg/100 mL) tests. Brain and tissue measurements were used for calculation of cerebral glucose metabolic rate (in milligrams per minute per 100 mL) using the formula of Sokoloff et al.85 Glucose uptake rates were also calculated by dividing brain activity by the product of body weight and injected dose. Images were attenuation-corrected based on the transmission scan and reconstructed on a Sparc Workstation (Sun Microsystems, Sunnyvale, Calif). A 20-cm cylindrical fluid-filled phantom with a known amount of radioactivity was scanned on the same day to obtain calibration factors for each of the 21 slices of the camera for conversion of radioactivity into units of millicuries per milliliter.
Magnetic resonance imaging scans were obtained in all participants for coregistration with PET and determination of regions of interest from MRI scans resliced to correspond to PET slices. Magnetic resonance imaging scans in the same participants were obtained on a 1.5-T General Electric Signa scanner (Milwaukee, Wis). Scan acquisition included axial slices, 3-mm slice thickness, T1-weighted images (repetition time=25 ms; echo time=5 ms; number of excitations = 2; matrix, 256×256; field of view, 24 cm).
Image Processing and Analysis Methods
Positron emission tomography and MRI scans were transferred to a Sun Workstation (Sun Microsystems, Mountain View, Calif) for analysis. A surface matching algorithm and the ANALYZE (Mayo Clinic, Rochester, Minn) software package, version 5.0, was used for coregistration of images.86 Brain surfaces from PET and MRI were matched using this program. The MRI was resliced to correspond to the 21 PET slices. Using this technique, we have shown a registration error of 2.86 mm.87 Regions of interest were drawn on resliced MRI scans using specific criteria based on anatomical landmarks with a method that we have shown to be highly reliable. These regions of interest use anatomical landmarks from the MRI. We have previously published the details and the reliability of these methods.88 Multiple brain regions were selected for analysis. These regions correspond to the same regions measured in our prior study of neural correlates of tryptophan depletion–induced return of depressive symptoms,44 since a primary aim of the current study was to replicate the brain findings of that study using provocation of a different neurochemical system. The only additional brain regions added were a region of dorsolateral prefrontal cortex more superior to the area measured in that prior study (to correspond to the area where alterations in neuronal morphology were found in postmortem brain89) and the subgenual prefrontal cortex (for similar reasons).90 Global brain metabolism was calculated as the mean of brain tissue activity in all slices.
Data Analysis
Specific regions of interest were selected based on the results of our prior PET study of neural correlates of tryptophan depletion–induced depression44 and on prior studies of neural correlates of depression. Regions were separated into those that were and were not hypothesized to change with AMPT-induced return of depressive symptoms. Regions hypothesized to change included dorsolateral prefrontal cortex (middle frontal gyrus), orbitofrontal cortex, and thalamus. Other regions from the prior study that showed reduction with depressive symptoms after correction for multiple comparisons included parietal and temporal cortex, postcentral gyrus, superior frontal gyrus, and anterior cingulate.
Data were analyzed for regional brain metabolic rates following administration of placebo or AMPT using repeated-measures analysis of variance with drug (AMPT vs placebo) as the repeated factor, depression status (those with vs without a return of depressive symptoms) (Figure 2) and hemisphere (left vs right) as factors in the analysis, and baseline brain metabolism (during the placebo condition) as a covariate. Data analysis examined the interaction between depression status and drug. Because of the highly variable measurement of glucose metabolic rate, glucose uptake rates were used as the primary outcome. A secondary analysis examined the ratio of regional to whole-brain metabolism. Bonferroni corrections were performed to correct for multiple comparisons for nonhypothesized regions (P=.05/10=.005). This study was not analyzed as an intention-to-treat analysis.
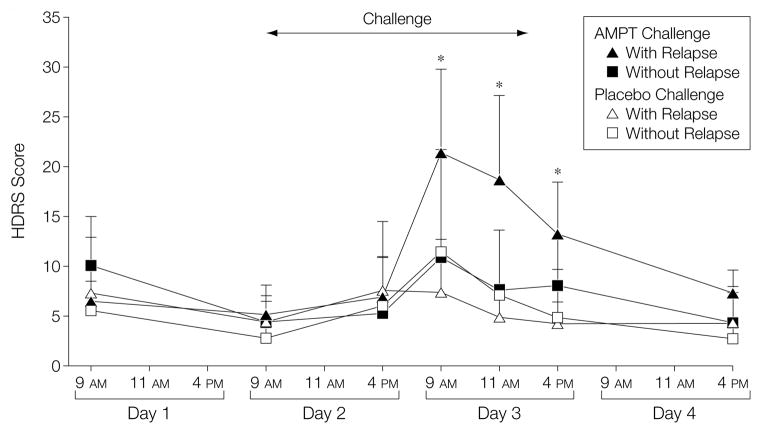
Figure 2. Effects of AMPT and Placebo (Diphenhydramine) on Symptoms of Depression as Measured With the HDRS.
Patients are divided into those with (n=11) and without (n=7) an α-methylparatyrosine (AMPT)–induced return of depressive symptoms. AMPT (but not placebo) resulted in an increase in depressive symptoms at all points on day 3 of the study (the day of the positron emission tomography scan) in patients defined as having a return of depressive symptoms (P<.001 as indicated by asterisks). HDRS indicates Hamilton Depression Rating Scale. Error bars indicate SDs.
Brain behavioral correlations were performed by comparing the relationship between the placebo-corrected change in HDRS with AMPT (as described herein) and the changes in regional brain metabolism with AMPT and placebo.
Data were analyzed using SAS statistical software, version 8 (SAS Institute Inc, Cary, NC).
RESULTS
Effects of AMPT on Regional Cerebral Metabolism
Metabolic (glucose uptake) rate data are presented in the Table. Patients with AMPT-induced return of depressive symptoms showed decreased brain metabolism after controlling for baseline metabolism (measured during the placebo condition) in a number of cortical regions with AMPT in comparison with placebo, with the greatest magnitude of effect in dorsolateral prefrontal cortex, orbitofrontal cortex (Figure 3A), and thalamus. Patients without AMPT-induced return of depressive symptoms tended to show the opposite response (eg, in orbitofrontal cortex; Figure 3B). The value for the interaction between depression status and drug (AMPT vs placebo) for these regions is presented in the Table. There were no significant interactions between relapse status and drug for the ratio of regional to global brain metabolic rates.
Table.
Metabolic Rates in Patients With and Without an AMPT-Induced Return of Depressive Symptoms*
| With Relapse (n = 11)
|
Without Relapse (n = 7)
|
Depletion-Depression Interaction
|
||||||
|---|---|---|---|---|---|---|---|---|
| Brain Regions | Placebo, Mean (SD) | AMPT, Mean (SD) | % Change | Placebo, Mean (SD) | AMPT, Mean (SD) | % Change | F Value | P Value |
| Brain Regions Hypothesized to Change With AMPT | ||||||||
| Orbitofrontal cortex | 10.78 (6.04) | 8.27 (3.30) | −23 | 6.28 (3.64) | 9.53 (4.71) | 52 | 10.27 | .002 |
|
| ||||||||
| Middle frontal gyrus | 12.65 (6.87) | 9.87 (3.82) | −22 | 7.68 (4.42) | 11.32 (4.79) | 47 | 4.68 | .04 |
|
| ||||||||
| Superior dorsolateral prefrontal cortex | 13.4 (6.65) | 9.36 (4.17) | −30 | 8.04 (4.93) | 11.00 (5.91) | 37 | 5.32 | .03 |
|
| ||||||||
| Thalamus | 9.48 (3.77) | 7.41 (2.58) | −22 | 5.59 (3.58) | 8.15 (3.64) | 46 | 8.76 | .006 |
|
| ||||||||
| Brain Regions Not Hypothesized to Change or With Less Evidence for Change With AMPT | ||||||||
| Temporal cortex | 12.13 (5.94) | 9.01 (3.44) | −26 | 7.17 (4.37) | 10.10 (4.44) | 41 | 11.76 | .001 |
|
| ||||||||
| Superior frontal gyrus | 12.39 (6.56) | 9.11 (3.52) | −26 | 7.04 (4.11) | 10.87 (5.34) | 54 | 6.08 | .02 |
|
| ||||||||
| Inferior frontal gyrus | 12.59 (6.47) | 9.52 (3.51) | −24 | 7.66 (4.73) | 11.15 (5.62) | 46 | 4.07 | .05 |
|
| ||||||||
| Parietal cortex | 12.53 (4.66) | 9.16 (3.58) | −27 | 7.57 (4.46) | 10.65 (6.26) | 41 | 5.55 | .03 |
|
| ||||||||
| Caudate | 10.26 (6.07) | 8.63 (3.85) | −16 | 6.81 (5.02) | 10.60 (5.56) | 56 | 1.35 | .25 |
|
| ||||||||
| Putamen | 11.66 (5.03) | 9.95 (3.63) | −15 | 7.07 (5.10) | 11.38 (5.07) | 61 | 4.53 | .04 |
|
| ||||||||
| Anterior cingulate | 12.04 (6.00) | 9.10 (3.68) | −24 | 6.76 (4.17) | 9.84 (4.91) | 46 | 6.50 | .02 |
|
| ||||||||
| Postcentral gyrus | 12.03 (5.16) | 9.13 (3.83) | −24 | 7.59 (4.73) | 10.42 (5.77) | 37 | 5.21 | .03 |
|
| ||||||||
| Hippocampus | 7.39 (3.12) | 5.73 (2.25) | −22 | 4.55 (3.06) | 6.73 (2.69) | 48 | 7.63 | .009 |
|
| ||||||||
| Subcallosal region | 9.47 (5.27) | 7.86 (3.26) | −17 | 6.16 (4.03) | 9.18 (4.08) | 49 | 1.49 | .24 |
|
| ||||||||
| Parahippocampus | 7.23 (2.72) | 5.83 (2.13) | −19 | 4.34 (2.41) | 6.57 (3.11) | 51 | 7.13 | .01 |
|
| ||||||||
| Amygdala | 7.34 (3.42) | 5.83 (2.30) | −21 | 4.64 (3.06) | 6.61 (3.05) | 42 | 3.42 | .07 |
|
| ||||||||
| Midbrain | 7.08 (2.50) | 6.01 (1.79) | −15 | 4.42 (2.48) | 6.58 (3.07) | 49 | 5.32 | .03 |
|
| ||||||||
| Global | 7.64 (3.25) | 6.00 (2.85) | −21 | 4.42 (2.54) | 6.46 (2.85) | 46 | 2.27 | .15 |
Abbreviation: AMPT, α-methylparatyrosine.
Metabolic rates shown in this table are glucose uptake rates, calculated as regional activity in microcuries per cubic centimeter/[injected dose in microcuries × body weight in pounds].
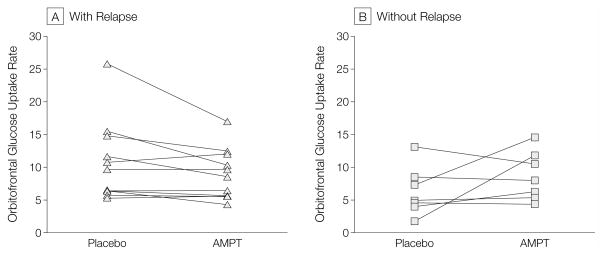
Figure 3. Orbitofrontal Glucose Uptake Rates in Patients With (n=11) and Without (n=7) AMPT-Induced Return of Depressive Symptoms.
Glucose uptake rates were calculated as regional activity in microcuries per cubic centimeter/[injected dose in microcuries × body weight in pounds]. Patients with a return of depressive symptoms showed decreased orbitofrontal metabolism with α-methylparatyrosine (AMPT). Patients without an AMPT-induced return of depressive symptoms showed a pattern of increase in orbitofrontal cortical metabolism with AMPT.
Patients who had an AMPT-induced return of depressive symptoms had increased resting metabolic rates in several prefrontal and limbic brain regions. These included orbitofrontal cortex, middle frontal gyrus, hippocampus, parahippocampus, and amygdala. There were also elevations in temporal and parietal cortex not observed in our prior study (P<.005).44
Relationship Between Depression and Brain Metabolism
The relationship between symptoms of depression during PET scanning and brain metabolism was examined by calculating the baseline-corrected change in HDRS score for the AMPT vs placebo tests and correlating that with the change in regional brain metabolism for AMPT and placebo tests. Correlations were examined for brain regions with the most significant change (dorsolateral prefrontal cortex, orbitofrontal cortex, and thalamus). There were modest negative correlations between increased symptoms of depression and decreased brain metabolism with AMPT for middle frontal gyrus (r = −0.46; df=17; P=.05), orbitofrontal cortex (r=−0.40; df=17; P=.10), thalamus (r=−0.44; df=17; P=.07), temporal cortex (r=−0.47; df=17; P=.045), and hippocampus (r=−0.53; df=17; P=.03). There were positive correlations between change in brain metabolism with AMPT in middle frontal gyrus, orbito-frontal cortex, and thalamus (range of r values, 0.95–0.97).
Relationship Between Clinical Factors and Biological Outcomes
There were no significant relationships between brain metabolism and age or sex. Number of lifetime depressive episodes was significantly correlated with decreased orbitofrontal metabolism with AMPT (r = −0.71; df = 11; P<.05); this effect was primarily related to a strong correlation in patients who had a return of depressive symptoms with AMPT (r=−0.74) and was not significant in patients who did not experience a return of depressive symptoms (r=−0.28). There were no differences in brain metabolic responses to AMPT by order of assignment to AMPT or placebo.
COMMENT
AMPT-induced return of depressive symptoms was associated with decreased metabolism in multiple cortical regions, with the greatest effects in orbitofrontal cortex, dorsolateral pre-frontal cortex, and thalamus. These frontal regions receive important nor-adrenergic innervation and are involved in a functional circuit with the thalamus. These findings are consistent with our prior study of tryptophan depletion–induced return of depressive symptoms, which found decreases in these areas with return of depressive symptoms. In the current study, we also found greater decreases in temporal and parietal cortex than we found in that prior study. Greater decreases in metabolism in these regions could not be explained by greater magnitude of catecholamine depletion with AMPT in the relapsing patients. Decreased metabolism in these regions was not significant when the ratio of regional to whole-brain metabolism was examined, demonstrating the strong global changes with AMPT-induced return of depressive symptoms. Increased resting metabolism during the placebo test was found in patients vulnerable to AMPT-induced return of depressive symptoms in prefrontal and limbic regions (orbitofrontal cortex, middle frontal gyrus, hippocampus, parahippocampus, and amygdala). This was also reported in our prior tryptophan depletion study. In the current study, number of lifetime episodes of depression was correlated with decreased AMPT-induced metabolism in orbitofrontal cortex.
Interpreted in conjunction with our prior PET study and prior studies of AMPT and tryptophan depletion–induced return of depressive symptoms, the results suggest that norepinephrine and serotonin modulate common brain regions to mediate symptoms of depression. Both of the serotonin and norepinephrine neuro-transmitter systems have their cell bodies in the brainstem, with long neurons that project throughout the brain, globally modulating neuronal function. The research suggests that disruption of either neurochemical system can lead to depression, probably by affecting the common brain regions that they innervate. As reviewed in the introduction to this article, disruption of these systems causes mood changes in patients with a history of depression who experience remission while taking antidepressants, or in patients with a history of depression who are in remission and recently stopped medication.22 However, patients with current depression and healthy persons without a history of depression are unaffected by disruption of these neuro-transmitter systems. This is primarily related to a ceiling effect, whereby patients with depression have a limit in the degree to which they can develop additional symptoms of depression.
We found that increased resting baseline metabolism in prefrontal and limbic regions was associated with vulnerability to return of depressive symptoms. This also was reported in our prior study of tryptophan depletion. Prior studies have shown that vulnerability to tryptophan depletion–induced return of depressive symptoms predicts long-term vulnerability to relapse when medications are withdrawn.91 It appears that alterations in brain function continue to persist, even after patients have been successfully treated for depression. Consistent with this idea is the finding that some patients with a history of depression are vulnerable to tryptophan depletion–induced return of depressive symptoms, even when they are not taking medications, although healthy participants without a history of depression are not vulnerable to the development of depressive symptoms. The number of episodes of depression has been correlated with hippocampal atrophy,48,56 and it is known that the risk of depressive recurrence increases with the number of episodes of depression experienced during an individual’s lifetime. This suggests that changes in the brain underlie the development of vulnerability to depressive recurrence. Consistent with that idea is the finding in the current study that number of episodes of depression correlate with decreased orbitofrontal metabolism with AMPT. It will be important to more carefully describe the brain-related variables that underlie this vulnerability to depressive recurrence. Brain imaging may also be developed to predict who will be vulnerable to depressive recurrence if antidepressant medications are discontinued.
Prior studies in depression have found structural alterations in the brain regions implicated in this study. For example, studies have found reductions in volume of orbitofrontal cortex in patients with depression,60,61 while other studies found hippocampal volume reductions.48,56 However since this study has a within-subject design, changes in metabolism between placebo and AMPT tests are not likely to be affected by structural alterations.
There are number of limitations of this study that are worth mentioning. The results relied on the use of glucose uptake rates, since the plasma data were too unreliable for use in quantitation of glucose metabolic rates in the brain. Several patients dropped out of the study after the first test day. This was largely related to the fact that patients had a return of depressive symptoms with AMPT on that first day and did not wish to continue. Sample size is limited and the current study needs to be replicated with a larger sample. AMPT has sedating effects that may confound the depressogenic effects and may interfere with the blinded administration of the medication. We attempted to control for this by using the sedating medication diphen-hydramine as the placebo, which, in our prior studies, resulted in an equally sedating effect. Diphenhydramine may also have effects on brain metabolism that are currently unknown. However, it is anticipated that sedating medications would result in decreased global brain metabolism. It is possible that the changes in brain function are not related to symptoms of depression. This study is cross-sectional, and other factors may contribute to the symptoms of depression observed in this study. The current study did not include a never-depressed group of healthy persons for comparison. However, prior studies described in the introduction to this article have not found effects of AMPT on mood in healthy, never-depressed individuals. We did not control for stage of menstrual cycle. It would have been difficult, however, to perform all scans at the same stage of the menstrual cycle because the PET study days were 1 week apart and there was a random assignment to AMPT or placebo on the first scan day. We did not assess smoking status, which could affect plasma glucose levels, among other things.
The mechanisms by which AMPT affects regional brain metabolism are not entirely clear. Administration of AMPT with depletion of norepinephrine and dopamine resulted in decreased metabolism (from an elevated baseline) in patients who developed depressive symptoms, and increased metabolism (from a relatively lower baseline) in patients who did not develop depressive symptoms. The findings suggest a dose-response effect or a variation in receptor responsiveness that differs depending on vulnerability to return of depressive symptoms in individual participants. At the level of the postsynaptic receptor, norepinephrine has a stimulatory effect with binding to α1 receptors, an inhibitory effect with α2 receptors, and excitatory and inhibitory effects with β receptors. Animal studies have shown that stimulation of the norepinephrine system (with medications such as the α-adrenergic receptor blocker phentolamine and phenylethylamine) resulted in a dose-dependent effect, with increased cerebral blood flow at lower doses and decreased blood flow at high doses.92,93 Administration of the α2 antagonist yohimbine, which stimulates release of norepinephrine in the brain, resulted in decreased brain metabolism in frontal, parietal, sensory, and olfactory cortex.94,95 Electrical stimulation of the locus coeruleus (brainstem site of most of the noradrenergic cell bodies) resulted in a decrease in metabolism or blood flow in several cortical and subcortical regions,96–99 while lesions of the locus resulted in increased metabolism.100–102 Therefore, the brain response to norepinephrine depletion with AMPT in patients without a return of depressive symptoms can be considered to be the “normal” response. This response was similar to what was found in animal studies; ie, increased metabolism with removal of norepinephrine. The question remains: Why do patients who have a return of their depressive symptoms have decreased function? It appears that the decreased function in the prefrontal and orbito-frontal cortex represents a neural correlate of the symptoms of depression. The fact that depressive symptoms can recur with serotonergic or noradrenergic depletion suggests that this may not be secondary to a change in the post-synaptic receptor in vulnerable patients but rather a common alteration in an intracellular process in specific brain areas, including orbitofrontal cortex.
Acknowledgments
Funding/Support: This study was supported by a VA Merit Review Grant to Dr Charney, the National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD) Young Investigator Award and National Institute of Mental Health grant 1R01MH56120 to Dr Bremner, and a Veterans Administration Career Development Award grant to Dr Bremner.
We thank Helen Sayward, MS, for image processing and data analysis; Robert Stur-wold, PharmD, for performing randomization and medication preparation; and Cathy Colonese, RN, and Jacque Piscitelli, MS, for assistance in data collection.
Footnotes
Author Contributions: As principle investigator, Dr Bremner had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bremner, Charney.
Acquisition of data: Bremner, Vythilingam, Ng, Vermetten, Nazeer, Oren, Berman.
Analysis and interpretation of data: Bremner, Ng.
Drafting of the manuscript: Bremner, Berman, Charney.
Critical revision of the manuscript for important intellectual content: Vythilingam, Ng, Vermetten, Nazeer, Oren, Charney.
Statistical expertise: Bremner.
Obtained funding: Bremner, Charney.
Administrative, technical, or material support: Bremner, Ng, Nazeer, Oren, Berman, Charney.
Study supervision: Bremner, Vythilingam, Oren, Charney.
References
- 1.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Risch SC, Nemeroff CB. Neurochemical alterations of serotonergic neuronal systems in depression. J Clin Psychiatry. 1992;53:3–7. [PubMed] [Google Scholar]
- 3.Delgado PL. Depression: the case for a mono-amine deficiency. J Clin Psychiatry. 2000;61(suppl 6):S7–S11. [PubMed] [Google Scholar]
- 4.Asberg M, Thoren P, Traskman L, Bertilsson L, Ring-berger V. Serotonin depression: a biological subgroup within the affective disorders? Science. 1976;191:478–480. doi: 10.1126/science.1246632. [DOI] [PubMed] [Google Scholar]
- 5.Jones JS, Stanley B, Mann JJ, et al. CSF 5-HIAA and HVA concentrations in elderly depressed patients who attempted suicide. Am J Psychiatry. 1990;147:1225–1227. doi: 10.1176/ajp.147.9.1225. [DOI] [PubMed] [Google Scholar]
- 6.Mann JJ, Arango V, Marzuk PM. Evidence for the 5-HT hypothesis of suicide: a review of post-mortem studies. Br J Psychiatry Suppl. 1989 December;:7–14. [PubMed] [Google Scholar]
- 7.Mann JJ, McBride A, Anderson GM, Mieczkowski TA. Platelet and whole blood serotonin content in depressed inpatients: correlations with acute and lifetime psychopathology. Biol Psychiatry. 1992;32:243–257. doi: 10.1016/0006-3223(92)90106-a. [DOI] [PubMed] [Google Scholar]
- 8.Mann JJ, Stanley M, McBride A, McEwen BS. Increased serotonin-2 and beta-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry. 1986;43:954–959. doi: 10.1001/archpsyc.1986.01800100048007. [DOI] [PubMed] [Google Scholar]
- 9.Stanley M, Mann JJ. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet. 1983;1:214–216. doi: 10.1016/s0140-6736(83)92590-4. [DOI] [PubMed] [Google Scholar]
- 10.Arango V, Ernsberger P, Marzuk PM, et al. Autoradiographic demonstration of increased serotonin 5-HT-2 and alpha-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry. 1990;47:1038–1047. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- 11.Ferrior IN, McKeith IG, Cross AJ, Perry EK, Candy JM, Pemy RH. Postmortem neurochemical studies in depression. Ann N Y Acad Sci. 1986;487:128–142. doi: 10.1111/j.1749-6632.1986.tb27893.x. [DOI] [PubMed] [Google Scholar]
- 12.Lowther S, De Paermentier F, Crompton MR, Katona CL, Horton RW. Brain 5-HT-2 receptors in suicide victims: violence of death, depression and effects of antidepressant medication. Brain Res. 1994;642:281–289. doi: 10.1016/0006-8993(94)90932-6. [DOI] [PubMed] [Google Scholar]
- 13.Muhlbauer HD, Muller-Oerlinghausen B. Fenfluramine stimulation of serum cortisol in patients with major affective disorders and healthy controls: further evidence for a central serotonergic action of lithium in man. J Neural Transm. 1985;61:81–94. doi: 10.1007/BF01253053. [DOI] [PubMed] [Google Scholar]
- 14.Golden RN, Hsiao J, Lane E, et al. Abnormal neuroendocrine responsivity to acute i.v. clomipramine challenge in depressed patients. Psychiatry Res. 1990;3:39–47. doi: 10.1016/0165-1781(90)90107-g. [DOI] [PubMed] [Google Scholar]
- 15.Heninger GR, Charney DS, Sternberg DE. Serotonergic function in depression: prolactin response to intravenous tryptophan in depressed patients and healthy subjects. Arch Gen Psychiatry. 1984;41:398–402. doi: 10.1001/archpsyc.1984.01790150088012. [DOI] [PubMed] [Google Scholar]
- 16.Delgado PL, Charney DS, Price LH, Landis H, Heninger GR. Neuroendocrine and behavioral effects of dietary tryptophan restriction in healthy subjects. Life Sci. 1989;45:2323–2332. doi: 10.1016/0024-3205(89)90114-8. [DOI] [PubMed] [Google Scholar]
- 17.Young SN, Ervin FR, Pihl RO, Finn P. Biochemical aspects of tryptophan depletion in primates. Psychopharmacology. 1989;98:508–511. doi: 10.1007/BF00441950. [DOI] [PubMed] [Google Scholar]
- 18.Delgado PL, Miller HL, Salomon RM, et al. Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biol Psychiatry. 1999;46:212–220. doi: 10.1016/s0006-3223(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 19.Delgado PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR. Serotonin function and the mechanism of antidepressant action: reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47:411–418. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- 20.Spillmann MK, Van der Does AJ, Rankin MA, et al. Tryptophan depletion in SSRI-recovered depressed outpatients. Psychopharmacology. 2001;155:123–127. doi: 10.1007/s002130000669. [DOI] [PubMed] [Google Scholar]
- 21.Delgado PL, Price LH, Miller AH, et al. Serotonin and the neurobiology of depression: effects of tryptophan depletion in drug-free depressed patients. Arch Gen Psychiatry. 1994;51:865–874. doi: 10.1001/archpsyc.1994.03950110025005. [DOI] [PubMed] [Google Scholar]
- 22.Moreno FA, Gelenberg AJ, Heninger GR, et al. Tryptophan depletion and depressive vulnerability. Biol Psychiatry. 1999;46:498–505. doi: 10.1016/s0006-3223(99)00095-5. [DOI] [PubMed] [Google Scholar]
- 23.Delgado PL, Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry. 2000;61(suppl 1):S5–S12. [PubMed] [Google Scholar]
- 24.Goodwin F, Bunney W. Depressions following reserpine: a reevaluation. Semin Psychiatry. 1971;3:435–448. [PubMed] [Google Scholar]
- 25.Charney DS, Menkes DB, Heninger GR. Receptor sensitivity and the mechanism of action of anti-depressants. Arch Gen Psychiatry. 1981;38:1160–1180. doi: 10.1001/archpsyc.1981.01780350094011. [DOI] [PubMed] [Google Scholar]
- 26.Golden RN, Markey SP, Risby ED, Rudorfer MV, Cowdry RW, Potter WZ. Antidepressants reduce whole-body norepinephrine turnover while enhancing 6-hydroxymelatonin output. Arch Gen Psychiatry. 1988;45:150–154. doi: 10.1001/archpsyc.1988.01800260060008. [DOI] [PubMed] [Google Scholar]
- 27.Charney DS, Heninger GR, Sternberg F, Hafsted KM, Giddings S, Landis DH. Adrenergic receptor sensitivity in depression. Arch Gen Psychiatry. 1982;39:290–294. doi: 10.1001/archpsyc.1982.04290030030005. [DOI] [PubMed] [Google Scholar]
- 28.Matussek N, Ackenheil M, Hippius H, et al. Effect of clonidine on growth hormone release in psychiatric patients and controls. Psychiatry Res. 1980;2:25–36. doi: 10.1016/0165-1781(80)90004-9. [DOI] [PubMed] [Google Scholar]
- 29.Siever LJ, Uhde TW, Silberman EK, et al. Evaluation of alpha-adrenergic responsiveness to clonidine challenge and noradrenergic metabolism in the affective disorders and their treatment. Psychopharmacol Bull. 1982;18:118–119. [PubMed] [Google Scholar]
- 30.Klimek V, Stockmeier C, Overholser JC, et al. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ordway G, Smith I, Haycock J. Elevated tyrosine hydroxylase in the locus coeruleus of suicide victims. J Neurochem. 1994;62:680–685. doi: 10.1046/j.1471-4159.1994.62020680.x. [DOI] [PubMed] [Google Scholar]
- 32.Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase: the initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- 33.Engelman K, Horwitz D, Jequier E, Sjoerdsma A. Biochemical and pharmacological effects of alpha-methyltyrosine in man. J Clin Invest. 1968;47:577–594. doi: 10.1172/JCI105754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendels J, Frazer A. Brain biogenic amine depletion and mood. Arch Gen Psychiatry. 1974;30:447–451. doi: 10.1001/archpsyc.1974.01760100019004. [DOI] [PubMed] [Google Scholar]
- 35.Miller HL, Delgado PL, Salomon RM, Heninger GR, Charney DS. Effects of alpha-methyl-para-tyrosine (AMPT) in drug-free depressed patients. Neuropsychopharmacology. 1996;14:151–157. doi: 10.1016/0893-133X(95)00072-L. [DOI] [PubMed] [Google Scholar]
- 36.Salomon RM, Miller HL, Krystal JH, Heninger GR, Charney DS. Lack of behavioral effects of mono-amine depletion in healthy subjects. Biol Psychiatry. 1997;41:58–64. doi: 10.1016/0006-3223(95)00670-2. [DOI] [PubMed] [Google Scholar]
- 37.Delgado PL, Miller HL, Salomon RM, et al. Mono-amines and the mechanism of antidepressant action: effects of catecholamine depletion on mood of patients treated with antidepressants. Psychopharmacol Bull. 1993;29:389–396. [PubMed] [Google Scholar]
- 38.Miller HL, Delgado PL, Salomon RM, et al. Clinical and biochemical effects of catecholamine depletion on antidepressant-induced remission of depression. Arch Gen Psychiatry. 1996;53:117–128. doi: 10.1001/archpsyc.1996.01830020031005. [DOI] [PubMed] [Google Scholar]
- 39.Brodie H, Murphy DL, Goodwin F, Bunney W. Catecholamines and mania: the effect of alpha-methyl-para-tyrosine on manic behavior and catecholamine metabolism. Clin Pharmacol Ther. 1971;12:218–224. doi: 10.1002/cpt1971122part1218. [DOI] [PubMed] [Google Scholar]
- 40.McCann UD, Thorne D, Hall M, et al. The effects of l-dihydroxyphenylalanine on alertness and mood in alpha-methyl-para-tyrosine-treated healthy humans: further evidence for the role of catecholamines in arousal and anxiety. Neuropsychopharmacology. 1995;13:41–52. doi: 10.1016/0893-133X(94)00134-L. [DOI] [PubMed] [Google Scholar]
- 41.Berman RM, Narasimhan M, Miller HL, et al. Transient depressive relapse induced by catecholamine depletion: potential phenotypic vulnerability marker? Arch Gen Psychiatry. 1999;56:395–403. doi: 10.1001/archpsyc.56.5.395. [DOI] [PubMed] [Google Scholar]
- 42.Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996;29:2–11. doi: 10.1055/s-2007-979535. [DOI] [PubMed] [Google Scholar]
- 43.Bremner JD. Structural changes in the brain in depression and relationship to symptom recurrence. CNS Spectrums. 2002;7:129–139. doi: 10.1017/s1092852900017442. [DOI] [PubMed] [Google Scholar]
- 44.Bremner JD, Innis RB, Salomon RM, et al. PET measurement of cerebral metabolic correlates of depressive relapse. Arch Gen Psychiatry. 1997;54:364–374. doi: 10.1001/archpsyc.1997.01830160092012. [DOI] [PubMed] [Google Scholar]
- 45.George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. Depression. 1994;2:59–72. [Google Scholar]
- 46.Steffens DC, Krishnan KR. Structural neuroimaging and mood disorders: recent findings, implications for classification, and future directions. Biol Psychiatry. 1998;43:705–712. doi: 10.1016/s0006-3223(98)00084-5. [DOI] [PubMed] [Google Scholar]
- 47.Mayberg HS, Brannan SK, Mahurin RK, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 48.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller H, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–117. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 49.Sha PJ, Ebmeier KP, Glabus MF, Goodwin GM. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Br J Psychiatry. 1998;172:527–532. doi: 10.1192/bjp.172.6.527. [DOI] [PubMed] [Google Scholar]
- 50.Krishnan KR, Doraiswamy PM, Figiel GS, et al. Hippocampal abnormalities in depression. J Neuropsychiatry Clin Neurosci. 1991;3:387–391. doi: 10.1176/jnp.3.4.387. [DOI] [PubMed] [Google Scholar]
- 51.Sheline YI, Sanhavi M, Mintun MA, Gado MU. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vakili K, Pillay SS, Lafer B, et al. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- 53.Steffens DC, Byrum CE, McQuoid DR, et al. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- 54.Mervaala E, Fohr J, Konogen M, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30:117–125. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- 55.Sheline YI. 3D MRI studies of neuroanatomical changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- 56.Sheline YI, Wang P, Gado M, Csernansky J, Vannier M. Hippocampal atrophy in major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pantel J, Schroder J, Essig M, et al. Quantitative magnetic resonance imaging in geriatric depression and primary degenerative dementia. J Affect Disord. 1997;42:69–83. doi: 10.1016/s0165-0327(96)00105-x. [DOI] [PubMed] [Google Scholar]
- 58.Ashtari M, Greenwald BS, Kramer-Ginsberg E, et al. Hippocampal/amygdala volumes in geriatric depression. Psychol Med. 1999;29:629–638. doi: 10.1017/s0033291799008405. [DOI] [PubMed] [Google Scholar]
- 59.Drevets WC, Price JL, Simpson JR, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 60.Lai TJ, Payne ME, Byrum CE, Steffens D, Krishnan KR. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48:971–975. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- 61.Bremner JD, Vythilingam M, Vermetten E, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 62.Tebartz van Elst L, Woermann FG, Lemieux L, Trimble MR. Amygdala enlargement in dysthymia: a volumetric study of patients with temporal lobe epilepsy. Biol Psychiatry. 1999;46:1614–1623. doi: 10.1016/s0006-3223(99)00212-7. [DOI] [PubMed] [Google Scholar]
- 63.Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 64.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 65.Ebert D, Feistel H, Barocka A. Effects of sleep deprivation on the limbic system and the frontal lobes in affective disorders: a study with Tc-99m-HMPAO SPECT. Psychiatry Res. 1991;40:247–251. doi: 10.1016/0925-4927(91)90016-j. [DOI] [PubMed] [Google Scholar]
- 66.Bench CJ, Friston KJ, Brown RG, Scott LC, Frack-owiak RS, Dolan RJ. The anatomy of melancholia: focal abnormalities of cerebral blood flow in major depression. Psychol Med. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- 67.Baxter LR, Schwartz JM, Phelps ME, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–249. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 68.Martinot JL, Hardy P, Feline A, et al. Left pre-frontal glucose hypometabolism in the depressed state: a confirmation. Am J Psychiatry. 1990;147:1313–1317. doi: 10.1176/ajp.147.10.1313. [DOI] [PubMed] [Google Scholar]
- 69.Mayberg HS, Lewis PJ, Regenold W, Wagner HN. Paralimbic hypoperfusion in unipolar depression. J Nucl Med. 1994;35:929–934. [PubMed] [Google Scholar]
- 70.Mayberg HS. Frontal lobe dysfunction in secondary depression. J Neuropsychiatry Clin Neurosci. 1994;6:428–442. doi: 10.1176/jnp.6.4.428. [DOI] [PubMed] [Google Scholar]
- 71.Biver F, Goldman S, Delvenne V, et al. Frontal and parietal metabolic disturbances in unipolar depression. Biol Psychiatry. 1994;36:381–388. doi: 10.1016/0006-3223(94)91213-0. [DOI] [PubMed] [Google Scholar]
- 72.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 73.Mayberg HS, Starkstein SE, Peyser CE, Brandt J, Dannals RF, Folstein SE. Paralimbic frontal lobe hypometabolism in depression associated with Huntington’s 99mTc-exametazine with single photon emission tomography in major depression before and after treatment. J Affect Disord. 1993;29:245–255. [Google Scholar]
- 74.Ring RA, Bench CJ, Trimble MR, Brooks DJ, Frack-owiak RS, Dolan RJ. Depression in Parkinson’s disease: a positron emission study. Br J Psychiatry. 1994;165:333–339. doi: 10.1192/bjp.165.3.333. [DOI] [PubMed] [Google Scholar]
- 75.Mayberg HS, Starkstein SE, Sadzot B, et al. Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson’s disease. Ann Neurol. 1990;28:57–64. doi: 10.1002/ana.410280111. [DOI] [PubMed] [Google Scholar]
- 76.George MS, Ketter TA, Parekh PI, et al. Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop) J Neuropsychiatry Clin Neurosci. 1997;9:55–63. doi: 10.1176/jnp.9.1.55. [DOI] [PubMed] [Google Scholar]
- 77.Mann JJ, Malone KM, Diehl DJ, Perel J, Cooper TB, Mintun MA. Demonstration of in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. Am J Psychiatry. 1996;153:174–182. doi: 10.1176/ajp.153.2.174. [DOI] [PubMed] [Google Scholar]
- 78.Brody AL, Saxena S, Stoessel P, et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry. 2001;58:631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- 79.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 80.First MB, Spitzer RL, Williams JB, Gibbon M. Structured Clinical Interview for DSMIV-Patient Edition (SCID-P) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 81.McCann UD, Thorne D, Hall M, et al. The effects of l-dihydroxyphenylalanine on alertness and mood in alpha-methyl-para-tyrosine-treated healthy humans: further evidence for the role of catecholamines in arousal and anxiety. Neuropsychopharmacology. 1995;13:41–52. doi: 10.1016/0893-133X(94)00134-L. [DOI] [PubMed] [Google Scholar]
- 82.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;12:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mulaney NA, Gould LK, Hartz RK, et al. Design and performance of Posicam 6.5 BGO Positron camera. J Nucl Med. 1990;31:610–616. [PubMed] [Google Scholar]
- 84.Brownell GL, Kearfott KJ, Kairento AL, et al. Quantitation of regional cerebral glucose metabolism. J Comput Assist Tomogr. 1983;7:919–924. doi: 10.1097/00004728-198310000-00038. [DOI] [PubMed] [Google Scholar]
- 85.Sokoloff L, Reivich M, Kennedy C, et al. The [14]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 86.Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989;13:433–454. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- 87.Zubal IG, Zhang L, Tagare B, Duncan JS. 3-D registration of SPECT and MRI brain images. J Nucl Med. 1993;34:187. [Google Scholar]
- 88.Bremner JD, Bronen RA, de Erasquin G, et al. Development and reliability of a method for using magnetic resonance imaging for the definition of regions of interest for positron emission tomography. Clin Positron Imaging. 1998;1:145–159. doi: 10.1016/s1095-0397(98)00015-6. [DOI] [PubMed] [Google Scholar]
- 89.Rajkowska NA, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neuronal and glial pre-frontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 90.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moreno FA, Heninger GR, McGahuey CA, Delgado PL. Tryptophan depletion and risk of depression relapse: a prospective study of tryptophan depletion as a potential predictor of depressive episodes. Biol Psychiatry. 2000;48:327–329. doi: 10.1016/s0006-3223(00)00893-3. [DOI] [PubMed] [Google Scholar]
- 92.Raichle ME, Hartman BK, Eichling JO, Sharpe LG. Central noradrenergic regulation of cerebral blood flow and vascular permeability. Proc Natl Acad Sci U S A. 1975;72:3726–3730. doi: 10.1073/pnas.72.9.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCulloch J, Harper AM. Factors influencing the response of the cerebral circulation to phenylethylamine. Neurology. 1979;29:201–207. doi: 10.1212/wnl.29.2.201. [DOI] [PubMed] [Google Scholar]
- 94.Savaki HE, Kadekaro M, McCulloch J, Sokoloff L. The central noradrenergic system in the rat: metabolic mapping with alpha-adrenergic blocking agents. Brain Res. 1982;234:65–79. doi: 10.1016/0006-8993(82)90473-5. [DOI] [PubMed] [Google Scholar]
- 95.Inoue M, McHugh M, Pappius HM. The effect of alpha-adrenergic receptor blockers prazosin and yohimbine on cerebral metabolism and biogenic amine content of traumatized brain. J Cereb Blood Flow Metab. 1991;11:242–252. doi: 10.1038/jcbfm.1991.56. [DOI] [PubMed] [Google Scholar]
- 96.Abraham WC, Delanoy RL, Dunn AJ, Zornetzer SF. Locus coeruleus stimulation decreases deoxyglucose uptake in ipsilateral mouse cerebral cortex. Brain Res. 1979;172:387–392. doi: 10.1016/0006-8993(79)90552-3. [DOI] [PubMed] [Google Scholar]
- 97.De la Torre JC, Surgeon JW, Walker RH. Effects of locus coeruleus stimulation on cerebral blood flow in selected brain regions. Acta Neurol Scand. 1977;64(suppl):104–105. [PubMed] [Google Scholar]
- 98.Katayama Y, Ueno Y, Tsukiyama T, Tsubokawa T. Long lasting suppression of firing of cortical neurons and decrease in cortical blood flow following train pulse stimulation of the locus coeruleus in the cat. Brain Res. 1981;216:173–179. doi: 10.1016/0006-8993(81)91285-3. [DOI] [PubMed] [Google Scholar]
- 99.Goadsby PJ, Duckworth JW. Low frequency stimulation of the locus coeruleus reduces regional blood flow in the spinalized cat. Brain Res. 1989;476:71–77. doi: 10.1016/0006-8993(89)91537-0. [DOI] [PubMed] [Google Scholar]
- 100.Schwartz WJ. 6-Hydroxydopamine lesions of rat locus coeruleus alter brain glucose consumption, as measured by the 2-deoxy-D-[C-14]glucose technique. Neurosci Lett. 1978;7:141–150. doi: 10.1016/0304-3940(78)90158-1. [DOI] [PubMed] [Google Scholar]
- 101.Bates D, Weinshilboum RM, Campbell RJ, Sundt TM. The effect of lesions in the locus coeruleus on the physiological responses of the cerebral blood vessels in cats. Brain Res. 1977;136:431–443. doi: 10.1016/0006-8993(77)90068-3. [DOI] [PubMed] [Google Scholar]
- 102.Savaki HE, Graham DI, Grome JJ, McCulloch J. Functional consequences of unilateral lesion of the locus coeruleus: a quantitative [14C]2-deoxyglucose investigation. Brain Res. 1984;292:239–249. doi: 10.1016/0006-8993(84)90760-1. [DOI] [PubMed] [Google Scholar]