Abstract
Objective
Studies in nonhuman primates suggest that high levels of cortisol associated with stress have neurotoxic effects on the hippocampus, a brain structure involved in memory. The authors previously showed that patients with combat-related posttraumatic stress disorder (PTSD) had deficits in short-term memory. The purpose of this study was to compare the hippocampal volume of patients with PTSD to that of subjects without psychiatric disorder.
Method
Magnetic resonance imaging was used to measure the volume of the hippocampus in 26 Vietnam combat veterans with PTSD and 22 comparison subjects selected to be similar to the patients in age, sex, race, years of education, socioeconomic status, body size, and years of alcohol abuse.
Results
The PTSD patients had a statistically significant 8% smaller right hippocampal volume relative to that of the comparison subjects, but there was no difference in the volume of other brain regions (caudate and temporal lobe). Deficits in short-term verbal memory as measured with the Wechsler Memory Scale were associated with smaller right hippocampal volume in the PTSD patients only.
Conclusions
These findings are consistent with a smaller right hippocampal volume in PTSD that is associated with functional deficits in verbal memory.
Patients with combat-related posttraumatic stress disorder (PTSD) clinically demonstrate alterations in memory, including nightmares, flashbacks, intrusive memories, and amnesia for war experiences. In addition, descriptions from all wars of this century document alterations in memory occurring in combat veterans during or after the stress of battle. These include forgetting one's name or identity and forgetting events that had just taken place during the previous battle (1, 2), as well as gaps in memory that continue to recur for many years after the war (3). Servicemen who had been prisoners of war during the Korean conflict were found to have an impairment in short-term verbal memory, as measured by the logical memory component of the Wechsler Memory Scale, in comparison with veterans of the Korean war who did not have a history of imprisonment (4). We also found deficits in short-term verbal memory, as measured by the logical memory component of the Wechsler Memory Scale, in Vietnam combat veterans with combat-related PTSD in comparison with healthy subjects who were matched for age, years of education, and alcohol abuse (5).
Several lines of evidence suggest a relation between stress and damage to the hippocampus (6). The hippocampus and the adjacent perirhinal, parahippocampal, and entorhinal cortex play an important role in short-term memory (7). Studies in humans have shown that reductions in hippocampal volume secondary to either neurosurgery (8) or the pathophysiological effects of epilepsy (9) are associated with deficits in short-term memory as measured by the Wechsler Memory Scale. Monkeys exposed to the extreme stress of improper caging have shown increased glucocorticoid release as well as damage to the CA2 and CA3 subfields of the hippocampus (10). Studies in a variety of animal species suggest that direct glucocorticoid exposure results in a loss of neurons and a decrease in dendritic branching in the hippocampus (11, 12) with associated deficits in memory function (13). The mechanism of action of glucocorticoid toxicity is probably through an increase in the vulnerability of neurons to the toxicity of excitatory amino acids (14–16). Studies using computed tomography in human subjects who are exposed to high levels of glucocorticoids secondary to glucocorticoid steroid therapy (17, 18) or who have affective disorders (also felt to be related to stress) (19) have shown changes in brain structure, including ventricular enlargement and widening of the cortical sulci. Magnetic resonance imaging (MRI) studies in patients with affective disorders have shown a smaller right hippocampal volume (20) and temporal lobe volume (21) in bipolar disorder and abnormalities of the hippocampus, including alterations in T1 (22), but no change in hippocampal volume (23) in major depression. One MRI study (24) found a relation between deficits in short-term memory and smaller hippocampal volume, as well as higher plasma cortisol levels and smaller hippocampal volume, in patients with Cushing's disease. Stress in both healthy human subjects (25) and soldiers undergoing random artillery bombardment (26) results in an increase in urinary cortisol, suggesting the possibility that exposure to the extreme stress of combat may be associated with damage to the hippocampus.
The purpose of this study was to use MRI to measure the volume of the hippocampus and comparison brain structures in patients with PTSD and in matched comparison subjects. We hypothesized that PTSD would be associated with smaller hippocampal volume in relation to that of the comparison subjects. We also hypothesized that smaller hippocampal volume would be associated with deficits in short-term verbal memory in patients with PTSD.
METHOD
Subjects
The patient group consisted of 26 Vietnam veterans with a history of combat-related PTSD who were recruited from the inpatient unit of the National Center for Posttraumatic Stress Disorder, Division of Clinical Neurosciences, West Haven Veterans Affairs Medical Center, over a 3-year period. Each veteran gave informed consent for participation, met the DSM-III-R criteria for PTSD on the basis of the Structured Clinical Interview for DSM-III-R (SCID) (27), had a score of more than 107 (consistent with the diagnosis of PTSD) on the Mississippi Scale for Combat-Related Posttraumatic Stress Disorder (28), and was judged by consensus diagnosis of three psychiatrists (including J.D.B. and S.M.S.) to meet the criteria for PTSD. Exposure to combat in Vietnam was confirmed by official military records of service in Vietnam when these were available.
Patients were excluded if they had a history of psychotropic medication use within the past 3 weeks, meningitis, traumatic brain injury, neurological disorder, HIV-positive status, current alcohol or substance abuse or lifetime schizophrenia according to the SCID, or shrapnel or other foreign bodies that would preclude MRI scanning. Patients were observed for a 2-month period as inpatients, with frequent toxicology screens for validation of drug- and alcohol-free status. The study group consisted of 25 patients who had never lost consciousness for longer than 10 minutes, and one patient who had had a loss of consciousness lasting 1 hour and was matched with a comparison subject who had a similar history. None of the patients had a history of loss of consciousness within the past year. About 80% of PTSD patients have comorbid diagnoses of lifetime alcohol and/or drug dependence, and it was felt that excluding these patients would result in a nonrepresentative group. We therefore decided to select the comparison subjects for a history of alcohol dependence as described below.
Patients were evaluated with the SCID for comorbid psychiattic diagnoses; SCID data were not available for one patient. Data on the comorbid diagnoses of the other 25 patients are shown in table 1.
TABLE 1.
Comorbid Diagnoses of 25 Male Patients With PTSDa
| Diagnosis | N | % |
|---|---|---|
| Major depression | ||
| Current | 12 | 48 |
| Lifetime | 17 | 68 |
| Dysthymia | ||
| Current | 8 | 32 |
| Lifetime | 8 | 32 |
| Bipolar disorder | ||
| Mixed, manic, or depressed | 0 | 0 |
| Not otherwise specified | ||
| Current | 1 | 4 |
| Lifetime | 2 | 8 |
| Panic disorder | ||
| With agoraphobia | ||
| Current | 8 | 32 |
| Lifetime | 8 | 32 |
| Without agoraphobia | ||
| Current | 3 | 12 |
| Lifetime | 3 | 12 |
| Agoraphobia without panic disorder | ||
| Current | 6 | 24 |
| Lifetime | 6 | 24 |
| Social phobia | ||
| Current | 4 | 16 |
| Lifetime | 5 | 20 |
| Obsessive-compulsive disorder | ||
| Current | 7 | 28 |
| Lifetime | 7 | 28 |
| Generalized anxiety disorder | ||
| Current | 1 | 4 |
| Lifetime | 1 | 4 |
| Simple phobia | ||
| Current | 1 | 4 |
| Lifetime | 1 | 4 |
| Psychotic disorder not otherwise specified | ||
| Current | 1 | 4 |
| Lifetime | 1 | 4 |
| Bulimia | ||
| Current | 1 | 4 |
| Lifetime | 1 | 4 |
| Alcohol dependence, lifetime | 19 | 76 |
| Alcohol abuse, lifetime | 2 | 8 |
| Sedative/hypnotic/anxiolytic | ||
| Dependence | 8 | 32 |
| Abuse | 0 | 0 |
| Cannabis | ||
| Dependence | 14 | 56 |
| Abuse | 2 | 8 |
| Stimulant | ||
| Dependence | 10 | 40 |
| Abuse | 1 | 4 |
| Opiate | ||
| Dependence | 6 | 24 |
| Abuse | 0 | 0 |
| Cocaine | ||
| Dependence | 10 | 40 |
| Abuse | 1 | 4 |
| Hallucinogen/phencyclidine | ||
| Dependence | 6 | 24 |
| Abuse | 0 | 0 |
| Multiple drug | ||
| Dependence | 8 | 32 |
| Abuse | 0 | 0 |
According to data from the Structured Clinical Interview for DSM-III-R.
The 22 comparison subjects were carefully selected to be similar to the patients in age, sex, race, handedness, height, weight, years of education, socioeconomic status, and years of alcohol abuse. The comparison subjects were largely recruited from a crew of construction workers but also included other nonprofessional workers, in order for them to be similar in socioeconomic status to the patients. The Addiction Severity Index interview (29) was used to assess total years of lifetime alcohol abuse, defined as drinking to the point of intoxication (three or more drinks per day) on a regular basis (3 or more days per week). We did not attempt to select comparison subjects so as to have a group with a history of substance abuse similar to that of the patients. The rationale for this was the difficulty of matching subjects on more than 10 factors and the fact that we are not aware of any evidence connecting other substances of abuse (besides alcohol) and hippocampal damage. Potential comparison subjects were excluded if they were judged to have a history of psychiatric disorder on the basis of a psychiatric interview or if they met the other exclusion criteria outlined for the patients. Informed consent was obtained from all comparison subjects.
Clinical assessments administered as part of this study included the Mississippi Scale for Combat-Related Posttraumatic Stress Disorder (28), a self-report measure of current PTSD symptom severity; the Combat Exposure Scale (30), a self-report instrument; and the Dissociative Experiences Scale (31), a self-report instrument for the assessment of general dissociative symptoms, which are increased in PTSD (32). Verbal memory was assessed with the logical component and visual memory with the figural component of the Wechsler Memory Scale, with percent retention calculated as delayed recall divided by immediate recall multiplied by 100, as described in detail in our previous publication (5). Wechsler Memory Scale data were available for 14 comparison subjects and 21 PTSD patients. Of these subjects, all of the comparison subjects and 16 of the 20 PTSD patients had been included in our previously reported study group (5).
MRI
Magnetic resonance images of 3-mm contiguous brain slices were obtained with a 1.5-T General Electric Signa device (figure 1). Images were acquired with a spoiled GRASS (gradient recall acquisition in the steady state) sequence, with TR=25 msec, TE=5 msec, number of excitations=2, matrix=256×256 pixels, and field of view=16 cm. This protocol provides high contrast between gray and white matter regions. An initial sagittal localizing sequence was obtained to determine the long axis of the hippocampus. Coronal and axial sections were then obtained perpendicular and parallel, respectively, to the long axis of the hippocampus.
FIGURE 1.
Coronal Slice of a Magnetic Resonance Image at the Level of the Hippocampus Between the Bifurcation of the Basilar Artery and the Superior Colliculi in a Healthy Subjecta
Images were transferred by means of magnetic tape to an IBM-30486-based personal computer for morphometric assessments. We used a PC-based program called MIND, which was developed by CORITechs, Inc. (New Haven, Conn.). The MIND program permits reslicing of MRI images in any plane and volumetric assessments. Regions of interest are circumscribed by using a cursor-driven system, aided by contrast- and edge-enhancing filters. Pixels within the region of interest are tabulated by the computer. Data are then entered into an Excel spreadsheet together with matrix and field of view information for the calculation of area.
Volume measurements of the hippocampus were performed independently by two investigators (J.D.B. and P.R.) who were blind to the subjects’ diagnoses. The body of the hippocampus was measured because it is the easiest hippocampal segment to define (33). First, corrections for head rotation were achieved with the use of anatomical landmarks, including the internal auditory canal and the seventh and eighth cranial nerve. Then, two midhippocampal points separated by 15 mm and a third midhippocampal point in the opposite hippocampus were selected to define a plane parallel to the long axes of both hippocampi. A series of oblique images was constructed perpendicular to this plane to create images orthogonal to the long axis of the hippocampus. (The purpose of reslicing the MRI to create images perpendicular to the long axis of the hippocampus was for reproducibility of measurement.) The outline of the hippocampus was then traced with a mouse-driven cursor on five coronal sections (15 mm) between the superior colliculus and the bifurcation of the basilar artery, with the first slice anterior to the superior colliculus (33). The first slice in which the hippocampus was traced was the slice anterior to the superior colliculus. Cross-sectional areas were measured in each of the five slices, summed, and multiplied by the slice thickness (3 mm) to obtain the volume of the hippocampal body segment. The mean of the two raters’ measurements was obtained for the final value of hippocampal volume. In two cases there was more than a 20% discrepancy between measurements by the two operators. These scans were blindly reexamined and a consensus measurement was determined.
Volumetric assessments of two other regions, the temporal lobe and caudate, were done for purposes of comparison. These regions were selected because they are gray matter regions that may be used to measure the specificity of possible decreases in hippocampal volume and because there are no theoretical reasons to suspect that these regions would be smaller in patients with PTSD. The volume of the temporal lobe was obtained by measuring the cross-sectional area of all coronal slices of the temporal lobe anterior to the superior colliculus, summing, multiplying by the slice thickness, and subtracting the volume of the hippocampus (33). The anterior border of the caudate was identified as the first section containing caudate and corresponded with the genu of the corpus callosum and the anterior horns of the lateral ventricles. The posterior border was defined as the section before the trigone of the lateral ventricle and the splenium of the corpus callosum. Volume measurements included both the head and the body of the caudate and were determined by measuring cross-sectional areas in all coronal slices in which the head and body of the caudate were identified with the use of these criteria, then summing and multiplying by the slice thickness. Volumes reported for the temporal lobe and caudate are from measurements performed by a single rater (J.D.B.).
The use of methods such as ratios of brain region to whole brain to control for differences in brain size among subjects is accompanied by problems that include an increase in variability, which may interfere with the detection of true differences between subject groups (34). We elected to address the issue of controlling for brain size by matching patients with comparison subjects of similar height and weight, since brain size is related to total body size.
Interrater reliability was determined with the intraclass correlation coefficient (ICC) (35) and one-way analysis of variance (ANOVA) for volumetric assessments of the hippocampus by two raters (J.D.B. and P.R.) using resliced MRI scans and of the temporal lobe in a subset of 15 subjects by two raters (J.D.B. and T.M.S.) using nomesliced MRI scans. Test-retest reliability was determined for assessments of hippocampal volume by a single rater (J.D.B.) using resliced MRI scans of 33 subjects. The data in table 2 demonstrate that there was excellent interrater reliability for hippocampal and temporal lobe volume measurements and excellent test-retest reliability for hippocampal measurements.
TABLE 2.
Interrater Reliability and Test-Retest Reliability for Volume Measurements of the Hippocampus and Other Brain Regionsa
| Intraclass Correlation Coefficient | Analysis of Variance |
||
|---|---|---|---|
| Measure | F | df | |
| Interrater reliability for volumeb | |||
| Hippocampus | |||
| Left | 0.69 | 5.44* | 47, 48 |
| Right | 0.80 | 8.79* | 47, 48 |
| Mean | 0.78 | 8.11* | 47, 48 |
| Temporal lobe | |||
| Left | 0.69 | 5.38* | 14, 15 |
| Right | 0.91 | 20.66* | 14, 15 |
| Mean | 0.76 | 7.18* | 14, 15 |
| Test-retest reliability, hippocampusc | |||
| Left | 0.78 | 8.25* | 32, 33 |
| Right | 0.70 | 5.70* | 32, 33 |
| Mean | 0.75 | 7.03* | 32, 33 |
Values approaching 1.00 represent a high level of agreement.
Based on 48 assessments of the hippocampus and 15 assessments of the temporal lobes.
Based on 33 assessments.
p<0.01.
Data Analysis
Repeated measures ANOVA with side (left versus right) as the repeated measure was used to compare left and right hippocampal volume (as well as left and right caudate and left and right temporal lobe volume) in the patients and the comparison subjects. Analysis of covariance (ANCOVA) was used to compare left and right hippocampal volume in the patients and comparison subjects while controlling for differences between the two groups in years of education and years of alcohol abuse. Two-tailed nonpaired t tests were used to compare hippocampal volume in the PTSD patients with and without comorbid depression and alcohol/drug abuse.
RESULTS
Hippocampal Volume
The PTSD patients and comparison subjects did not differ in factors that could affect hippocampal volume, including age, sex, race/ethnicity, years of education, height, weight, handedness, and years of alcohol abuse (table 3).
TABLE 3.
Demographic Variables for Male Patients With PTSD and for Matched Comparison Subjects
| Variable | Patients With PTSD (N=26) | Comparison Subjects (N=22) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 46.0 | 1.8 | 44.5 | 7.3 |
| Education (years) | 12.7 | 2.4 | 14.2 | 2.9 |
| Height (in) | 69.2 | 2.7 | 69.5 | 3.5 |
| Weight (lb) | 182 | 26 | 179 | 31 |
| Alcohol abuse (years)a | 10.0 | 9.2 | 7.5 | 9.7 |
| N | % | N | % | |
|---|---|---|---|---|
| Race/ethnicity | ||||
| White | 25 | 96 | 17 | 77 |
| Black | 1 | 4 | 4 | 18 |
| Hispanic | 0 | 0 | 1 | 5 |
| Handedness | ||||
| Right | 22 | 85 | 20 | 91 |
| Left | 4 | 15 | 2 | 9 |
Defined as total number of years of alcohol abuse in a lifetime as assessed by the Addiction Severity Index interview. A year of alcohol abuse is defined in the index as drinking more than 3 oz of alcohol a day for 3 or more days per week in a typical month.
Repeated measures ANOVA did not show a significant difference between patients and comparison subjects when left and right hippocampal volumes were combined in a single model (table 4). There was no significant main effect of side (left versus right hippocampal volume) (F=2.05, df=1, 46, p=0.16) or interaction between side and diagnosis (F=2.32, df=1, 46, p=0.13). When handedness (left versus right) was entered as a factor in the model, there was no interaction between handedness and side (left versus right hippocampal volume). There was an 8.0% smaller right hippocampal volume in the PTSD patients in relation to the comparison subjects, which was statistically significant by univariate analysis. A 3.8% smaller volume of the left hippocampus was not significant (table 4). In the ANCOVA with years of education and years of alcohol abuse as covariates, there continued to be a significant difference in right hippocampal volume between the PTSD patients and the comparison subjects (F=3.86, df=1, 44, p<0.05).
TABLE 4.
Volume of the Hippocampus in Male Patients With PTSD and in Matched Comparison Subjects
| Volume (mm3) |
||||||
|---|---|---|---|---|---|---|
| Patients With PTSD (N=26) |
Comparison Subjects (N=22) |
Analysis of Variance |
||||
| Hippocampal Region | Mean | SD | Mean | SD | F (df=l, 46) | p |
| Left | 1186 | 138 | 1233 | 163 | 1.20 | 0.28 |
| Right | 1184 | 142 | 1286 | 175 | 5.02 | 0.03 |
| Mean | 1185 | 123 | 1260 | 160 | 3.38 | 0.07 |
Volume of Comparison Regions
Repeated measures ANOVA for temporal lobe volume, with side (left versus right) as the repeated factor, did not show a difference between patients and comparison subjects (i.e., no main effect for diagnosis) (table 5). There was a significant main effect for side (left versus right temporal lobe volume) {F=41.19, df=1, 44, p=0.0001) but no Side by Diagnosis interaction, suggesting greater right temporal lobe volume relative to left temporal volume in both groups. Univariate analyses did not show a difference for the left or the right temporal lobe, when examined alone, between patients and comparison subjects. Repeated measures ANOVA for caudate volume, with side (left versus right) as the repeated factor, showed no main effect for diagnosis, a significant main effect for side (left versus right caudate volume) (F=20.03, df=1, 42, p=0.0001), and no Side by Diagnosis interaction, suggesting that the right caudate is greater in both PTSD patients and comparison subjects. Univariate analyses did not show a difference in the left or the right caudate, when examined alone, between patients and comparison subjects. The patients had nonsignificantly smaller volumes of the left temporal lobe (5.0%), right temporal lobe (7.6%), left caudate (7.3%), and right caudate (6.3%) (table 5). We did not find a relation between volume of the hippocampus and volumes of the caudate and temporal lobes in either the patients or the comparison subjects. There was a significant correlation within the group of comparison subjects between mean temporal lobe and caudate volumes (r=0.74, df=16, p=0.0005), which was not seen within the group of PTSO patients. This correlation was seen on both the left and the right sides in the comparison subject group.
TABLE 5.
Volume of Brain Structures Other Than the Hippocampus in Male Patients With PTSD and in Matched Comparison Subjects
| Volume (mm3) |
||||||
|---|---|---|---|---|---|---|
| Patients With PTSD (N=26) |
Comparison Subjects (N=20)a |
Analysis of Variance |
||||
| Brain Region | Mean | SD | Mean | SD | F (df=l, 44) | p |
| Temporal lobeb | ||||||
| Left | 52,044 | 7,259 | 54,807 | 9,005 | 1.32 | 0.26 |
| Right | 58,773 | 9,247 | 63,606 | 10,923 | 2.69 | 0.11 |
| Mean | 55,408 | 7,362 | 59,206 | 9,019 | 2.53 | 0.12 |
| Caudate | ||||||
| Left | 3,056 | 578 | 3,310 | 907 | 0.34 | 0.56 |
| Right | 3,254 | 669 | 3,474 | 828 | 0.20 | 0.66 |
| Mean | 3,155 | 608 | 3,392 | 860 | 0.27 | 0.60 |
Data for the caudate and temporal lobe in two comparison subjects were missing.
As described in the Method section, the volume of the hippocampus was subtracted from that of the temporal lobe.
Hippocampal Volume and Memory
PTSD patients had lower scores in relation to those of the comparison subjects on the Wechsler Memory Scale logical component (verbal memory) for the subscales of immediate recall (mean=11.1, SD=3.4, and mean=19.8, SD=6.6, respectively; t=4.6, df=33, p=0.0002), delayed recall (mean=7.2, SO=2.8, and mean=17.0, SD=6.5, respectively; t=5.4, df=33, p=0.0001), and percent retention (mean=65.0%, SD=20.0%, and mean=85.2%, SD=13.2%, respectively; t=3.4, df=33, p<0.002). On the Wechsler Memory Scale figural component (visual memory) there were no significant differences between the PTSD patients and comparison subjects in scores on immediate recall (mean=9.0, SD= 2.8, and mean=11.1, SD=2.8, respectively; t=2.1, df=33, p=0.05), delayed recall (mean=7.2, SD=4.1, and mean=9.1, SD=4.0, respectively; t=1,4, df=33, p=0.17), and percent retention (mean=72.9%, SD=30.3%, and mean=80.4%, SD=21.7%, respectively; t=0.80, df=33, p=0.43).
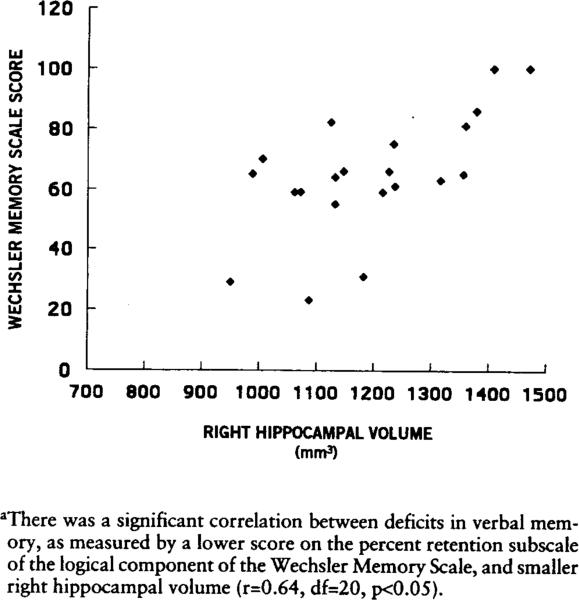
There was a positive correlation between right hippocampal volume and score on the percent retention subscale of the Wechsler Memory Scale logical component (verbal memory) for the PTSO patients (i.e., smaller right hippocampal volume was correlated with lower score on the Wechsler Memory Scale) (figure 2). There was no correlation between verbal memory scores and left hippocampal volume or bilateral caudate or temporal lobe volumes in either the patients or the comparison subjects, or between either left or right hippocampal volume and scores on any Wechsler Memory Scale figural component (visual memory) subscales for either patients or comparison subjects.
FIGURE 2.
Relation Between Verbal Memory and Right Hippocampal Volume in Patients With PTSDa
Relation Between Hippocampal Volume and Demographic and Clinical Factors
There was no significant correlation between age, years of education, years of alcohol abuse, height, or weight and left or right hippocampal volume in either the patients or the comparison subjects examined separately. In the PTSO patients there was no significant correlation between hippocampal volume and PT5D symptoms as measured with the Mississippi scale, dissociative symptoms as measured with the Dissociative Experiences Scale, or level of combat exposure.
Hippocampal Volume and Comorbidity With Depression and Alcohol/Drug Abuse
Table 6 shows the hippocampal volumes of the subjects grouped according to comorbid diagnoses. There was no difference in either left or right hippocampal volume between the PTSD patients with and the PTSD patients without a lifetime history of major depression. There was no difference between the PTSD patients with and without a history of current dysthymia in either left or right hippocampal volume. In addition to using years of alcohol abuse as a covariate, we divided the PTSD patients on the basis of a median split at 7 or more years of alcohol abuse versus less than 7 years of alcohol abuse as measured by the Addiction Severity Index and did not find a difference between the high alcohol abuse and low alcohol abuse PTSD patients in either left or right hippocampal volume. When we divided the comparison subjects on the basis of a median split of 3 or more years of alcohol abuse (high alcohol abuse group) versus less than 3 years of alcohol abuse (low alcohol abuse group), there also was no significant difference between high and low alcohol abuse groups in volume of the left or right hippocampus. When we compared right hippocampal volume in PTSD patients with and without a lifetime history of alcohol and drug dependence, there was no difference for alcohol, stimulant, cocaine, opiate, cannabis, or sedative/hypnotic/anxiolytic dependence. Similarly, there were no differences between these groups in left hippocampal volume.
TABLE 6.
Volume of the Hippocampus in Male Patients With PTSD Grouped According to Comorbid Diagnoses and in Patients and Comparison Subjects Grouped According to Level of Alcohol Abuse
| Hippocampal Volume (mm3) |
||||
|---|---|---|---|---|
| Left |
Right |
|||
| Diagnostic Groups Compared | Mean | SD | Mean | SD |
| Lifetime history of major depression | ||||
| Patients with (N=12) | 1205 | 149 | 1156 | 168 |
| Patients without (N=13) | 1158 | 149 | 1194 | 111 |
| Current dysthymia | ||||
| Patients with (N=8) | 1141 | 125 | 1205 | 136 |
| Patients without (N=17) | 1214 | 136 | 1173 | 140 |
| Alcohol abuse level | ||||
| Patientsa | ||||
| High (N=14) | 1192 | 140 | 1184 | 156 |
| Low (N=12) | 1178 | 142 | 1184 | 130 |
| Comparison subjectsb | ||||
| High (N=ll) | 1214 | 104 | 1245 | 107 |
| Low (N=ll) | 1253 | 210 | 1328 | 221 |
| Lifetime history of alcohol dependence | ||||
| Patients with (N=19) | 1181 | 125 | 1202 | 122 |
| Patients without (N=5) | 1221 | 178 | 1114 | 179 |
| Lifetime history of stimulant dependence | ||||
| Patients with (N=10) | 1154 | 168 | 1185 | 140 |
| Patients without (N=14) | 1214 | 104 | 1184 | 139 |
| Lifetime history of cocaine dependence | ||||
| Patients with (N=10) | 1176 | 170 | 1203 | 127 |
| Patients without (N=14) | 1199 | 108 | 1171 | 146 |
| Lifetime history of opiate dependence | ||||
| Patients with (N=6) | 1243 | 179 | 1253 | 120 |
| Patients without (N=18) | 1172 | 117 | 1161 | 137 |
| Lifetime history of cannabis dependence | ||||
| Patients with (N=14) | 1175 | 147 | 1213 | 138 |
| Patients without (N=10) | 1210 | 118 | 1143 | 130 |
| Lifetime history of sedative/hypnotic/anxiolytic dependence | ||||
| Patients with (N=8) | 1201 | 158 | 1231 | 126 |
| Patients without (N=16) | 1183 | 126 | 1160 | 139 |
High level was defined as 7 or more years of alcohol abuse, and low level as less than 7 years.
High level was defined as 3 or more years of alcohol abuse, and low level as less than 3 years.
DISCUSSION
Patients with combat-related PTSD had an 8.0% smaller right hippocampal volume relative to comparison subjects selected to be similar to the patients in age, sex, race, handedness, years of education, socioeconomic status, body size, and years of alcohol abuse. There was no significant difference between the PTSD patients and the comparison subjects in left hippocampal volume or in volume of the comparison brain regions measured in this study, i.e., left and right caudate and temporal lobes (minus hippocampus). Deficits in short-term verbal memory as measured by the percent retention subscale of the Wechsler Memory Scale logical component, which we previously reported to be a feature of PTSD (5), were associated with decreased right hippocampal volume in the PTSD patients but not the comparison subjects. There was no relation, however, between verbal memory and the volume of other brain structures or between visual memory and hippocampal volume in the patients or the comparison subjects, suggesting some degree of specificity of this finding.
We carefully considered the possible effects of comorbid depression and alcohol/drug abuse on hippocampal volume in PTSD. Previous studies in patients with affective disorders have found a smaller right hippocampal volume in bipolar disorder (20) but not major depression (23). In our study group we did not find a difference in right or left hippocampal volume between PTSD patients with and without a lifetime history of major depression and between PTSD patients with and without dysthymia. Some studies in animals have shown a relation between neuronal damage and alcohol, although we are not aware of any evidence of neuronal damage related to other substances or a rationale for why exposure to these substances would be expected to be associated with hippocampal neuronal damage. We therefore elected to match for alcohol abuse only. Our finding of a smaller right hippocampal volume in PTSD patients relative to comparison subjects persisted after controlling for alcohol and drug abuse in the several statistical analysis strategies outlined above.
There are several potential explanations for a smaller right hippocampal volume and possible alterations in symmetry of the hippocampus in PTSD. A small right hippocampus from the time of birth may present a pre-morbid risk factor for the development of PTSD. Extreme stress results in increased release of glucocorticoids, excitatory amino acids (36), serotonin (37), and other neurotransmitters and neuropeptides that could be associated with damage to the hippocampus (38). A kindling-type phenomenon similar to that seen in seizure disorders (39) or alterations in sex hormones, as has been hypothesized in affective disorders (20), may also represent potential mechanisms for a reduction in hippocampal volume in PTSD. Studies of glucocorticoid-mediated damage to the hippocampus associated with stress have not found evidence for an asymmetric effect of glucocorticoids on the hippocampus, although other neurotransmitters involved in stress, such as serotonin (40), have asymmetric concentrations in the brain. Alterations in the normal asymmetry of medial temporal lobe structures (24, 25, and this study) have been implicated in affective disorders (20). It is not clear from our results whether there is in fact an alteration in hippocampal symmetry in PTSD or whether there are bilateral changes that happened to be statistically significant only on the right side in this study group.
Our findings of alterations in hippocampal morphology in patients with PTSD are similar to findings in patients with schizophrenia (41). Some studies (42–45), but not all (46), have found smaller hippocampal volume in schizophrenic patients relative to comparison subjects. Some of these studies (42,44) have found a smaller left, but not right, hippocampal volume in schizophrenic patients. One study (43) found differences in hippocampal volume in monozygotic twins discordant for schizophrenia, which suggests the possibility that environmental factors (such as stress) may play a role in the etiology.
It is of interest that deficits in verbal memory in this study were correlated with smaller hippocampal volume only on the right side. It is generally felt that the left hippocampus mediates verbal memory and the right hippocampus mediates visual memory. However, patients with right hippocampal lesions have been shown to have deficits in free verbal recall of objects when compared with control subjects (47,48), while patients with left hippocampal lesions have been shown to have deficits in visuospatial performance (49). The famous patient H.M., who had bilateral hippocampal damage, had a much greater degree of verbal memory impairment than patients with left-sided hippocampal volume lesions alone (47). One recent study showed that if there is some damage to the left hippocampus, lesions of the right hippocampus are associated with deficits in verbal memory (50). In addition, positron emission tomography studies of cerebral blood flow in healthy human subjects have shown an increase in right hippocampal blood flow with verbal memory tasks involving word stem completion (51). We speculate that there are pathological processes affecting both the left and the right hippocampus, with a greater magnitude of effect on the right, resulting in a greater contribution of right hippocampal pathology to the deficits in verbal memory seen in PTSD.
Our findings may have relevance for the treatment of PTSD. The finding that PTSD patients have severe deficits in memory function (5), which the results of the current study indicate may be related to alterations in the morphology of the hippocampus, suggests that vocational rehabilitation efforts should take into consideration potential deficits in memory function. The hippocampus has also been hypothesized to play a role in binding together individual components of memories that are stored in the primary sensory neocortical areas (7). Dysfunction of the hippocampus may offer an explanation for the fragmentation of memories into single sensory phenomena that are seen clinically in patients with PTSD.
Acknowledgments
Supported by a VA Fellowship for Research in Biological Psychiatry, a VA Career Development Award to Dr. Bremner, and a grant from the National Center for Posttraumatic Stress Disorder.
The authors thank Marie Luby for assistance in data processing and analysis; Robin Greene for technical assistance in magnetic resonance imaging; Sandi Capelli, R.N., and Valinda Ouellette, R.N., for assistance in conducting the study; Viola Vaccarino, M.D., for statistical advice; and Ariel Y. Deutch, Ph.D., Robert Sapolsky, Ph.D., and John W. Mason, M.D., for discussions of the manuscript.
REFERENCES
- 1.Henderson JL, Moore M. The psychoneurosis of war. N Engl J Med. 1944;230:274–278. [Google Scholar]
- 2.Archibald HC, Tuddenham RD. Persistent stress reaction after combat. Arch Gen Psychiatry. 1965;12:475–481. doi: 10.1001/archpsyc.1965.01720350043006. [DOI] [PubMed] [Google Scholar]
- 3.Bremner JD, Steinberg M, Southwick SM, Johnson DR, Charney DS. Use of the Structured Clinical Interview for DSM-IV Dissociative Disorders for systematic assessment of dissociative symptoms in posttraumatic stress disorder. Am J Psychiatry. 1993;150:1011–1014. doi: 10.1176/ajp.150.7.1011. [DOI] [PubMed] [Google Scholar]
- 4.Sutker PB, Winstead DK, Galina ZH, Allain AN. Cognitive deficits and psychopathology among former prisoners of war and combat veterans of the Korean conflict. Am J Psychiatry. 1991;148:67–72. doi: 10.1176/ajp.148.1.67. [DOI] [PubMed] [Google Scholar]
- 5.Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthy G, Charney DS. Deficits in short-term memory in posttraumatic stress disorder. Am J Psychiatry. 1993;150:1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS, Gould EA, Sakai RR. The vulnerability of the hippocampus to protective and destructive effects of glucocorticoids in relation to stress. Br J Psychiatry. 1992;160:18–24. [PubMed] [Google Scholar]
- 7.Zola-Morgan SM, Squire LR. The primate hippocampal formation: evidence for a time-limited role in memory storage. Science. 1990;250:288–290. doi: 10.1126/science.2218534. [DOI] [PubMed] [Google Scholar]
- 8.Delaney RC, Rosen AJ, Mattson RH, Novelly RA. Memory function in focal epilepsy: a comparison of non-surgical, unilateral temporal lobe and frontal lobe samples. Cortex. 1980;16:103–117. doi: 10.1016/s0010-9452(80)80026-8. [DOI] [PubMed] [Google Scholar]
- 9.Lencz T, McCarthy G, Bronen RA, Scott TM, Inserni JA, Sass KJ, Novelly RA, Kim JH, Spencer DD. Quantitative magnetic resonance imaging studies in temporal lobe epilepsy: relationship to neuropathology and neuropsychological function. Ann Neurol. 1992;31:629–637. doi: 10.1002/ana.410310610. [DOI] [PubMed] [Google Scholar]
- 10.Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wooley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 13.Luine V, Villages M, Martinex C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 14.Sapolsky R, Pulsinelli W. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229:1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- 15.Armanini MP, Hutchins C, Stein BA, Sapolsky RM. Glucocorticoid endangerment of hippocampal neurons is NMDA-receptor dependent. Brain Res. 1990;532:7–12. doi: 10.1016/0006-8993(90)91734-x. [DOI] [PubMed] [Google Scholar]
- 16.Virgin CE, Taryn PTH, Packan DR, Tombaugh GC, Yang SH, Horner HC, Sapolsky RM. Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: implications for glucocorticoid neurotoxicity. J Neurochem. 1991;57:1422–1428. doi: 10.1111/j.1471-4159.1991.tb08309.x. [DOI] [PubMed] [Google Scholar]
- 17.Okuno T, Ito M, Konishi Y, Yoshioka M, Nakano Y. Cerebral atrophy following ACTH therapy. J Comput Assist Tomogr. 1980;4:20–23. doi: 10.1097/00004728-198002000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Bentson J, Reza M, Winter J, Wilson G. Steroids and apparent cerebral atrophy on computed tomography scans. J Comput Assist Tomogr. 1978;2:16–23. doi: 10.1097/00004728-197801000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kellner CH, Rubinow DR, Gold PW, Post RM. Relationship of cortisol hypersecretion to brain CT scan alterations in depressed patients. Psychiatry Res. 1983;8:191–197. doi: 10.1016/0165-1781(83)90062-8. [DOI] [PubMed] [Google Scholar]
- 20.Swayze VW II, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC. Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 1992;31:221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- 21.Altschuler LL, Conrad A, Hauser P, Li X, Guze BH, Denikoff K, Tourtellotte W, Post R. Reduction of temporal lobe volume in bipolar disorder: a preliminary report of magnetic resonance imaging (letter). Arch Gen Psychiatry. 1991;48:482–483. doi: 10.1001/archpsyc.1991.01810290094018. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan KRR, Doraiswamy PM, Figiel GS, Husain MM, Shaw SA, Na C, Boyko OB, McDonald WM, Nemeroff CB, Ellinwood EH., Jr Hippocampal abnormalities in depression. J Neuropsychiatry Clin Neurosci. 1991;3:387–391. doi: 10.1176/jnp.3.4.387. [DOI] [PubMed] [Google Scholar]
- 23.Axelson DA, Doraiswamy PM, McDonald WM, Boyko OB, Tupler LA, Patterson LJ, Nemeroff CB, Ellinwood EH, Jr, Krishnan KRR. Hypercortisolemia and hippocampal changes in depression. Psychiatry Res. 1993;47:163–173. doi: 10.1016/0165-1781(93)90046-j. [DOI] [PubMed] [Google Scholar]
- 24.Starkman MN, Gebarksi SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing'S syndrome. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 25.Rose RM, Poe RO, Mason JW. Psychological state and body size as determinants of 17-OHCS excretion. Arch Intern Med. 1968;121:406–413. [PubMed] [Google Scholar]
- 26.Howard JM, Olney JM, Frawley JP, Peterson RE, Smith LH, Davis JH, Guerra S, Dibrell WH. Studies of adrenal function in combat and wounded soldiers. Ann Surg. 1955;141:314–320. doi: 10.1097/00000658-195503000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spitzer RL, Williams JBW, Gibbon M. Biometrics Research. New York State Psychiatric Institute; New York: 1987. Structured Clinical Interview for DSM-III-R (SCID). [Google Scholar]
- 28.Keane TM, Caddell JM, Taylor KL. Mississippi Scale for Combat-Related Posttraumatic Stress Disorder: three studies in reliability and validity. J Consult Clin Psychol. 1988;56:85–90. doi: 10.1037//0022-006x.56.1.85. [DOI] [PubMed] [Google Scholar]
- 29.McClellan AT, Luborsky A, Cacciola J, Griffith J, Evans F, Bar HL, O'Brien CP. New data from the Addiction Severity Index: reliability and validity in three centers. J Nerv Ment Dis. 1985;73:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Keane TM, Fairbank JA, Caddell JM. Clinical evaluation of a scale to measure combat exposure. Psychol Assessment. 1989;1:53–55. [Google Scholar]
- 31.Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis. 1986;174:727–735. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Bremner JD, Southwick S, Brett E, Fontana A, Rosenheck R, Charney DS. Dissociation and posttraumatic stress disorder in Vietnam combat veterans. Am J Psychiatry. 1992;149:328–332. doi: 10.1176/ajp.149.3.328. [DOI] [PubMed] [Google Scholar]
- 33.Bronen RA, Cheung G. Relationship of hippocampus and amygdala to coronal MRI landmarks. Magn Reson Imaging. 1991;9:449–457. doi: 10.1016/0730-725x(91)90434-n. [DOI] [PubMed] [Google Scholar]
- 34.Arndt S, Cohen G, Alliger RJ, Swayze VW II, Andreasen NC. Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Res: Neuroimaging. 1991;40:79–89. doi: 10.1016/0925-4927(91)90031-k. [DOI] [PubMed] [Google Scholar]
- 35.Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- 36.Moghaddam B. Stress preferentially enhances extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- 37.Okado N, Cheng L, Tanatsugu Y, Hamada S, Hamaguchi K. Synaptic loss following removal of serotonergic fibers in newly hatched and adult chickens. J Neurobiol. 1993;24:687–698. doi: 10.1002/neu.480240512. [DOI] [PubMed] [Google Scholar]
- 38.Bremner JD, Davis M, Southwick SM, Krystal JH, Charney DS. In: Neurobiology of posttraumatic stress disorder, in American Psychiatric Press Review of Psychiatry. Oldham JM, Riba MB, Tasman A, editors. Vol. 12. American Psychiatric Press; Washington, DC: 1993. [Google Scholar]
- 39.Post RM, Rubinow DR, Ballenger JC. Conditioning and sensitization in the longitudinal course of affective illness. Br J Psychiatry. 1986;149:191–201. doi: 10.1192/bjp.149.2.191. [DOI] [PubMed] [Google Scholar]
- 40.Arato M, Frecska E, Maccrimmon DJ, Guscott R, Saxena B, Tekes K, Tothfalusi L. Serotonergic interhemispheric asymmetry: neurochemical and pharmaco-EEG evidence. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:759–764. doi: 10.1016/0278-5846(91)90004-k. [DOI] [PubMed] [Google Scholar]
- 41.Gur RE, Pearlson GD. Neuroimaging in schizophrenia research. Schizophr Bull. 1993;19:337–353. doi: 10.1093/schbul/19.2.337. [DOI] [PubMed] [Google Scholar]
- 42.Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F. Brain morphology and schizophrenia: a magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry. 1992;49:921–926. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- 43.Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DW. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- 44.Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. 1990;35:1–13. doi: 10.1016/0925-4927(90)90004-p. [DOI] [PubMed] [Google Scholar]
- 45.Bogerts B, Lieberman JA, Ashtari M, Bilder RM, Degreef G, Lerner G, Johns C, Masiar S. Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry. 1993;33:236–246. doi: 10.1016/0006-3223(93)90289-p. [DOI] [PubMed] [Google Scholar]
- 46.Zipursky RB, Marsh L, Lim KO, DeMent S, Shear PK, Sullivan EV, Murphy GM, Csernansky JG, Pfefferbaum A. Volumetric MRI assessment of temporal lobe structures in schizophrenia. Biol Psychiatry. 1994;35:501–516. doi: 10.1016/0006-3223(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 47.Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981;19:781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- 48.Tucker DM, Novelly RA, Isaac W, Spencer D. Effects of simultaneous vs sequential stimulus presentation on memory performance following temporal lobe resection in humans. Neuropsychologia. 1986;24:277–281. doi: 10.1016/0028-3932(86)90061-8. [DOI] [PubMed] [Google Scholar]
- 49.Novelly RA, Tucker DM, Spencer D. Comparison of memory function after partial versus total hippocampectomy in unilateral temporal lobectomy for psychomotor epilepsy. Epilepsia. 1983;24:259–260. [Google Scholar]
- 50.Incisa dell Rochetta A, Gadian DG, Connelly A, Polkey CE, Jackson GD, Watkins K, Johnson CL, Mishkin M, Vargha-Khadem F. Verbal memory impairment after right-temporal lobe excision: the role of abnormality on the unoperated side as revealed by [H-1]MRS and T-2 relaxometry. Proceedings of the Society for Neuroscience. 1994;2:1290. [Google Scholar]
- 51.Squire LR, Ojemann JG, Miezin FM, Petersen SE, Videen TO, Raichle ME. Activation of the hippocampus in normal humans: a functional anatomical study of memory. Proc Natl Acad Sci USA. 1992;89:1837–1841. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]