Abstract
Objective
Self-injurious behavior (SIB) is one of the most distinctive features of borderline personality disorder (BPD) and related to impulsivity and emotional dysregulation.
Method
Female patients with BPD (n = 11) and healthy controls (n = 10) underwent functional magnetic resonance imaging while listening to a standardized script describing an act of self-injury. Experimental sections of the script were contrasted to the neutral baseline section and group-specific brain activities were compared.
Results
While imagining the reactions to a situation triggering SIB, patients with BPD showed significantly less activation in the orbitofrontal cortex compared with controls. Furthermore, only patients with BPD showed increased activity in the dorsolateral prefrontal cortex during this section and a decrease in the midcingulate while imagining the self-injurious act itself.
Conclusion
This pattern of activation preliminary suggests an association with diminished emotion regulation, impulse control as well as with response selection and reappraisal during the imagination of SIB.
Keywords: borderline personality disorder, pain, impulsive behavior, self-injurious behavior
Introduction
Self-injurious behavior (SIB) is a prototypical dysfunctional behavior of patients with borderline personality disorder (BPD) (1, 2). Patients with BPD use SIB for a number of reasons, primarily for the reduction of aversive inner tension, but also for self-punishment, reduction of unpleasant feelings or to overcome dissociation (3). SIB is closely linked to emotional dysregulation, which can be viewed as a core feature of BPD (for overview see 4). Heightened emotional sensitivity together with a longer lasting emotional experience leads to elevated emotional vulnerability (5). This renders patients with BPD susceptible to context changes which eventually lead to impulsive behaviors like SIB (6, 7). Different studies have shown heightened trait – as well as state-impulsivity in patients with BPD (8, 9). Neurobiological studies in BPD found an association between impulsive behavior and deficits in serotonergic transmission as well as frontal lobe hypofunction (10–12). More specifically, impulsivity has been linked to dysfunction of the orbitofrontal cortex (OFC) in both functional imaging as well as lesion studies (13, 14). Berlin et al. (15) found striking similarities between patients with orbitofrontal lesions and patients with BPD in regard to impulsive behavior, thus closing the link between impulsivity, orbitofrontal dysfunction and BPD. In a diffusion tensor imaging study, Grant et al. (16) found frontal white matter alterations in self-injurious patients with BPD.
In the context of SIB, psychophysiological and neuroimaging studies have focused on the phenomenon of hypoalgesia. Elevated pain thresholds were found in patients with BPD (e.g. 17), which correlate with aversive inner tension (18) and increase further under subjective stress conditions (19). Pain thresholds tend to normalize in patients who terminated SIB (20). In addition, neuroimaging showed lower activation of the anterior cingulate cortex (ACC) and the amygdala concomitant with increased activation of the dorsolateral prefrontal cortex (DLPFC) in patients with BPD compared with controls in response to nociceptive stimulation, suggesting a dysfunction of brain areas mediating the affective-motivational component of pain perception (21).
The psychophysiological alterations associated with SIB itself have been investigated using script-driven imagery (22, 23). Earlier studies investigated psychophysiological correlates of emotional arousal during imagery of traumatic experiences in post-traumatic stress disorder. Bremner et al. (24) as well as Lanius et al. (25) investigated neural correlates of script-induced traumatic memories in patients with PTSD, but also used script-driven symptom provocation to induce dissociative states during functional imaging (26). So far, script-driven imagery has not been used to investigate the neural correlates of SIB, but psychophysiological changes have been assessed. Haines et al. (27) used personalized scripts of an act of self-injury to assess psychophysiological measures and found a significant decrease of heart rate and aversive inner tension in subjects displaying SIB in daily life. Welch et al. (28) found decreases in psychophysiological indicators of negative emotion during imagery of the moments after self-injury. Both studies demonstrate the negative reinforcement qualities of SIB through the reduction of aversive inner tension.
Functional imaging studies in BPD have used script-driven imagery to investigate unresolved life events or situations of abandonment and trauma. Only in borderline patients but not in controls, Beblo et al. (29) found increased activation in the insula, amygdala and the cingulate cortex during the imagination of unresolved life events in a cue-driven approach. This may indicate the patients’ effort to control the intensive emotions elicited by unresolved life events. Schmahl et al. (30) used script-driven imagery to investigate the cerebral correlates of abandonment. Patients with BPD showed increased activation in the DLPFC together with lower activation in the right dorsal ACC and the right amygdala/hippocampus compared with traumatized subjects without BPD while listening to individualized scripts of abandonment situations. In another study (31), traumatized subjects without BPD showed increased activation in the right ACC, the left OFC and the right DLPFC as well as a decreased activation in the left DLPFC in response to scripts describing abuse. In contrast, the traumatized patients with BPD did not show any activation changes in the ACC or the DLPFC during the trauma condition relative to baseline, but decreased activation of the left OFC in response to these abuse scripts.
Aims of the study
We aimed to investigate the cerebral activation pattern of SIB in patients with BPD. Therefore, we used fMRI in combination with a standardized script describing the different stages of an act of SIB. As no functional neuroimaging data on this subject were available, we used an exploratory approach.
Material and methods
Sample
Twelve female patients with BPD and twelve healthy control subjects were recruited for this study. Patients were either recruited as inpatients at the Department of Psychosomatic Medicine and Psychotherapy, Central Institute of Mental Health, Mannheim or via announcement on different borderline-specific homepages. Healthy control subjects were recruited via the municipal registration office and the local university campus. Due to fMRI movement artifacts, one patient with BPD and two control subjects had to be excluded from the functional imaging analyses. In the following, only data for the remaining ten controls and eleven patients with BPD will be reported.
All patients fulfilled DSM-IV criteria for BPD confirmed by the International Personality Disorder Examination (32). Axis I diagnoses were assessed by the Structured Clinical Interview for Axis I disorders (SCID-I, 33). Patients with current depression, alcohol or substance abuse or dependence, lifetime bipolar I disorder and schizophrenia were excluded. All patients were free of psychoactive medication for at least 2 weeks and had no history of neurological disorders or head trauma. Current and lifetime comorbidities were assessed and consisted of the following axis-I diagnoses (current/lifetime): bipolar II disorder (0/1), major depressive episode (0/8), dysthymia (1/1), panic disorder (2/2), agoraphobia (1/1), social phobia (5/5), post-traumatic stress disorder (5/5), specific phobia (1/1), anorexia nervosa (0/1), bulimia nervosa (0/2), alcohol abuse (0/1), alcohol dependence (0/1) and multiple substance dependence (0/2). Three patients were diagnosed with one or two additional axis-II disorders [avoidant personality disorder (3), paranoid personality disorder (1)]. Severity of borderline symptomatology was assessed using the Borderline Symptom List (BSL) (34, 35). All patients had a history of SIB and in all patients cutting was the most frequent form of SIB.
Healthy control subjects were also free of psychoactive medication and had no history of neurological disorder, head trauma, psychiatric illness or BPD as assessed by the SCID-I and the SCID-II screening questionnaire (36), respectively. Patients and controls were matched for gender (all female), ethnicity (all Caucasian), and age (BPD: 25.64 ± 3.83 years, controls: 25.6 ± 5.23 years; P > 0.9).
The study protocol was approved by the Ethics Committee of the University of Heidelberg. Informed written consent was obtained from each subject prior to the study.
Procedure
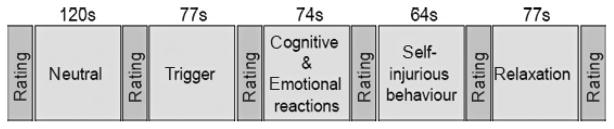
We employed script-driven imagery to induce emotional distress. One standard script describing the circumstances and execution of an act of SIB was developed in first person singular, comprising the following five sections: i) neutral sequence (baseline), ii) trigger situation, iii) emotional and cognitive reactions, iv) SIB and v) relaxation. The script was read by a professional actress and recorded. The neutral section describes a woman on a shopping tour and lasts about 120 s. The trigger situation (77 s) describes the woman watching a dispute between a mother and her child. During the following section (74 s), the emotional and cognitive reactions including the person’s ruminations concerning similar negative experiences with her mother are delineated. Subsequently, a typical act of SIB is described (64 s), including preparation and the cutting itself. The last section of the script, relaxation (77 s), includes the SIB -induced decrease in aversive inner tension.
All subjects took part in the script-driven imagery while blood-oxygen-level dependent (BOLD) signals were acquired in a 1.5 T scanner (Siemens Vision, Erlangen, Germany) applying the following protocol parameters: repetition time = 3100 ms, echo time = 60 ms, flip angle = 90°, field of view = 220 × 220 mm2, matrix = 64 × 64, slice thickness = 5 mm, slice gap = 1 mm, number of slices = 25. We acquired 183 functional volumes in the coronal plane perpendicular to the anterior–posterior commissural plane. At the beginning of the experiment, subjects were instructed to imagine the descriptions in the script as vividly as possible. At the beginning of the study as well as after each section of the script, a break was introduced, in which vividness, aversive inner tension and dissociation were assessed using Likert-Scales ranging from 0 to 9, which were projected inside the scanner together with the three items ‘vividness’, ‘aversive inner tension’, and ‘dissociation’, with 0 indicating none and 9 indicating the highest possible value for any of the parameters. During this time the scanning proceeded. Furthermore, before and after the experiment, aversive inner tension and dissociation were assessed using the Dissociation-Tension-Scale-acute (Dissoziations-Spannungs-Skala; DSS-acute; 37), a self-rating questionnaire to assess present state dissociative features as well as the current level of aversive inner tension. The study design is depicted in Fig. 1.
Fig. 1.
Study design: sections of the script and their respective length.
Image analysis and statistics
Functional data were analyzed with SPM5 (Wellcome Department of Imaging Neuroscience, University College London, UK, 2005). The first five images at the beginning of each trial were discarded to enable the signal to achieve steadystate equilibrium between radiofrequency pulse and relaxation. The fMRI time series were realigned to the mean, to correct for intra-subject ‘s head movements. Mean images were normalized to an MNI (Montreal Neurological Institute, http://www.bic.mni.mcgill.ca) echoplanar imaging template with affine registration followed by non-linear transformation with 25 mm cutoff, medium regularization and 16 iterations, resampled with trilinear interpolation and written in 3 × 3 × 3 mm3 isotropic voxels. The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each participant. Finally, images were smoothed with a Gaussian kernel of 8 mm full-width at half-maximum. The data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts.
To analyze group-specific activation patterns during the different sections of the script and between-group differences, five regressors were defined for each subject, consisting of one baseline condition (neutral script sequence) and four experimental conditions (trigger, reactions, SIB and relaxation). These regressors were convolved with a canonical hemodynamic basis function to model corresponding changes in BOLD signal. Serial autocorrelation was corrected by a first order autoregressive model. The general linear model estimated the component of variance that could be explained by each of the regressors. Subtracting the baseline condition from each experimental condition yielded the following four contrasts for each subject: i) ‘trigger situation ‘minus ‘baseline’, ii) ‘reactions’ minus ‘baseline ‘, iii) ‘self-injurious behavior’ minus ‘baseline’, iv) ‘relaxation’ minus ‘baseline’. These contrasts were subjected to intra- and inter-group comparisons. On the one hand, we compared the activation pattern under each experimental condition to the activation pattern under baseline condition for each group separately. On the other hand, we compared the two groups concerning their differential activation patterns resulting from the comparison of the experimental to the baseline conditions.
As induced aversive inner tension could influence brain activation patterns in response to the different experimental conditions, this parameter was included as a nuisance variable and specified as an additional regressor in the individual design matrix with one value of aversive inner tension per scan. To account for the changes in aversive inner tension from one section of the script to the next, we modulated a linear increase for each section, starting with the rating given after the preceding section up to the rating given after the current section, e.g. a section consisting of 25 scans and with an increase from 3 to 5 during this section would lead to an increase of 0.08 per scan.
Due to the sample size and the relatively high variability of the population compared to the single voxel signal-to-noise ratio of the measurements, inter-individual variance was not accounted for in voxel-level group analysis, and all contrast maps were computed using a fixed-effects model (38). The statistical parametric maps resulting from voxel-level analyses were thresholded after a whole-brain correction for multiple comparisons at a voxel level (P ≤ 0.05) using family-wise error correction (FWR).
To control for the effects of vividness, dissociation and aversive inner tension, a repeated measurements ANOVA was carried out. Assumption of sphericity was tested using Mauchly test. If the assumption was violated, Greenhouse–Geisser correction of degrees of freedom was applied. In the ANOVA, the Likert-scale scores (units, U) for vividness, dissociation and aversive inner tension assessed during the different sections of the script as well as scores for dissociation and aversive inner tension measured by the DSS before and after the experiment served as dependent variables. The different sections of the script and group (borderline patients vs. healthy controls) served as independent variables. Effect sizes for group differences between BPD-patients and healthy controls were calculated as Cohen’s d (based on pooled standard deviations). We conducted all analyses using SPSS statistical software (Version 14.0 for Windows; SPSS Inc., Chicago, IL, USA). A Bonferroni correction was applied to account for multiple comparisons (five analyses), resulting in a corrected significance level of P ≤ 0.01.
Results
Psychological ratings
Patients with BPD displayed medium symptom severity (BSL 1.88 ± 0.56, scaling: 0–4). At the beginning of the experiment, patients with BPD showed higher levels of aversive inner tension and dissociation (2.91 ± 1.81 U; 0.74 ± 1.38 U) than controls (0.5 ± 0.97 U; 0.14 ± 0.36 U) as measured by the DSS-acute (main effect group (aversive inner tension): F1,18 = 39.96, P ≤ 0.01; main effect group (dissociation): F1,17 = 7.56, P ≤ 0.01). Neither dissociation nor aversive inner tension had changed significantly for both groups at the end of the experiment (main effect aversive inner tension: F1,18 = 4.04, n.s.; main effect dissociation: F1,17 = 5.23, n.s.; patients with BPD: aversive inner tension 4.55 ± 2.30 U, dissociation 1.49 ± 1.03 U; controls: aversive inner tension 0.44 ± 0.52 U, dissociation 0.32 ± 0.76 U). No interaction effects were observed (aversive inner tension by group: F1,18 = 2.34, n. s.; dissociation by group: F1,17 = 3.98, n. s.). At the beginning as well as at the end of the experiment, effect sizes for aversive inner tension concerning group differences were high (d = 1.7; d = 2.5). Effect sizes for dissociation were in the medium to high range (at the beginning of the experiment: d = 0.6, at the end of the experiment: d = 1.3).
In both groups, vividness was high throughout the different sections of the script [neutral: 7.36 ± 1.29 U (BPD), 7.90 ± 1.60 U (controls); trigger: 6.27 ± 2.15 U (BPD), 7.60 ± 1.65 U (controls); reactions: 6.27 ± 2.57 U (BPD), 7.30 ± 1.70 U (controls); SIB: 7.27 ± 2.41 U (BPD), 7.00 ± 2.11 U (controls); relaxation: 7.45 ± 2.07 U (BPD), 7.10 ± 2.02 U (controls)].
There were no significant main effects for vividness (F3.1,58.9 = 1.2, n.s.) or group (F1,19 = 0.4, n.s.), and no interaction effects between group and vividness (F3.1,58.9 = 1.6, n.s.).
During the course of the experiment, dissociation was comparable for both groups [neutral: 0.4 ± 0.7 U (BPD) vs. 0.4 ± 0.8 U (controls), d = 0.0; trigger: 1.0 ± 1.3 U (BPD) vs. 0.4 ± 0.8 U (controls), d = 0.6; reactions: 0.7 ± 1.2 U (BPD) vs. 0.5 ± 0.8 U (controls), d = 0.2; SIB: 2.1 ± 2.2 U (BPD) vs. 0.6 ± 1.3 U (controls), d = 0.8; relaxation: 1.3 ± 2.1 U (BPD) vs. 0.5 ± 0.8 U (controls), d = 0.5]. There were no significant main effects for dissociation (F1.9,36.1 = 2.6, n.s.) or group (F1,19 = 2.1, n.s.) and no interaction effects (F1.9,36.1 = 1.7, n.s.).
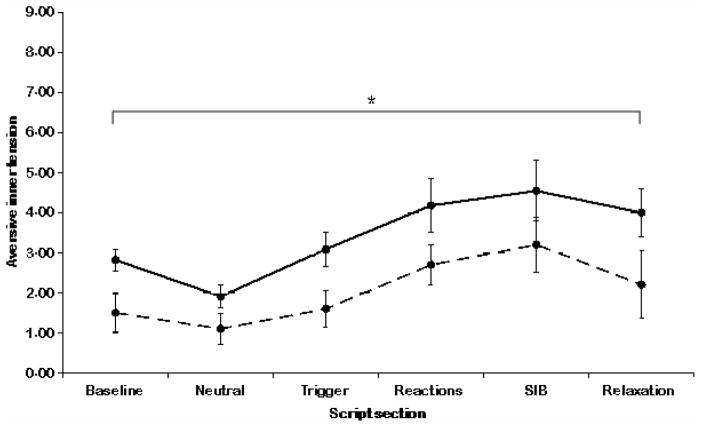
For both groups, aversive inner tension changed significantly throughout the script [neutral:1.9 ± 1.3 U (BPD) vs. 1.1 ± 0.9 U (controls), d = 0.7; trigger: 3.1 ± 1.5 U (BPD) vs. 1.6 ± 1.3 U (controls), d = 1.1; reactions: 4.2 ± 1.7 U (BPD) vs. 2.7 ± 2.1 U (controls), d = 0.8; SIB: 4.5 ± 2.3 U (BPD) vs. 3.2 ± 2.4 U (controls), d = 0.6; relaxation: 4.0 ± 2.8 U (BPD) vs. 2.2 ± 1.9 U (controls), d = 0.8]. This is indicated by a main effect for aversive inner tension (F2.3,44.5 = 9.9, P ≤ 0.01). Groups did not differ significantly (F1,19 = 4.7, n.s.). No interaction between group and aversive inner tension was observed (F2.3,44.5 = 0.4, n.s.). The ratings for aversive inner tension are shown in Fig. 2.
Fig. 2.
Ratings for aversive inner tension after baseline and each section of the script. Patients’ ratings are shown in lines drawn through, healthy controls’ ratings are shown in dashed lines. *Significant time effect P < 0.05.
Functional imaging data: intra-group comparisons
In the BPD group, the trigger situation as compared with the neutral section of the script yielded significant activation in the left medial temporal gyrus (see Table 1). Significant deactivation was found in the middle frontal gyrus, including Brodmann area (BA) 10 and parts of BA 46 and 9, the left precentral gyrus, the right posterior cingulate, the right precuneus and in the posterior cerebellum. In the control group, the trigger situation yielded no significant activations. Significant deactivations were found primarily in posterior temporal and occipital brain regions.
Table 1.
Foci of activation during the different script sections in patients with borderline personality disorder (BPD) and healthy controls
| BPD patients
|
HC
|
|||||
|---|---|---|---|---|---|---|
| Region | z-score | MNI-coordinates x, y, z | Region | z-score | MNI-coordinates x, y, z | |
| Trigger situation vs. baseline | ||||||
| Activation | l. middle temporal g., BA 39 | 5.27 | −60, −63, 9 | |||
| l. middle temporal g., BA 21 | 4.94 | −45, 9, −39 | ||||
| Deactivation | l. precentral g. | 5.50 | −60, −6, 30 | l. middle occipital g., BA 18 | 5.03 | −33, −99, −3 |
| 4.60 | −27, −12, 42 | r. medial temporal g. | 4.67 | 57, −60, −6 | ||
| r. middle frontal g., BA 10 | 4.91 | 39, 54, −9 | ||||
| 4.74 | 48, 51, 0 | |||||
| 4.68 | 39, 54, 0 | |||||
| l. middle frontal g., BA 10 | 4.68 | −36, 48, 15 | ||||
| r. posterior cingulate | 4.85 | 3, −60, 3 | ||||
| r. precuneus | 4.84 | 9, −66, 63 | ||||
| 4.84 | 12, −87, 39 | |||||
| 4.81 | 24, −27, 30 | |||||
| l. posterior cerebellum | 5.05 | −39, −72, −24 | ||||
| r. posterior cerebellum | 4.84 | 48, −54, −36 | ||||
| 4.65 | 30, −48, −42 | |||||
| posterior cerebellum | 4.70 | 0, −75, −33 | ||||
| Emotional and cognitive reactions vs. baseline | ||||||
| Activation | l. frontopolar cortex, BA 10 | 5.15 | −18, 66, 21 | |||
| posterior cingulate | 4.70 | 0, −54, 27 | ||||
| Deactivation | r. lingual g. | 5.52 | 18, −84, −3 | |||
| l. orbito- frontal g., BA 11* | 4.74 | −24, 36, −18 | ||||
| 4.61 | −33, 39, −15 | |||||
| Self-injurious behavior vs. baseline | ||||||
| Deactivation | l. fusiform g., BA 19 | 5.22 | −36, −72, −21 | |||
| r. mid-cingulate | 4.73 | 0, 21, 42 | ||||
| 4.70 | 3, 9, 45 | |||||
| Relaxation vs. baseline | ||||||
| Activation | r. temporal pole, BA 38 | 5.05 | 36, 15, −45 | |||
| r. orbito-frontal g., BA 11 | 4.62 | 36, 36, −15 | ||||
l., left; r., right; g., gyrus; BA, Brodmann area.
Significant between-group difference.
In response to the script section describing the emotional and cognitive reactions as compared with the neutral section, significant activation in the left frontopolar cortex (BA 10) and in the posterior cingulate were found in the BPD group. Significant deactivations were found in the right lingual gyrus and the left OFC (BA 11). In the control group, this comparison revealed no statistically significant BOLD signal changes.
In the BPD group, the description of SIB showed significant BOLD signal decreases in the right mid-cingulate and the left fusiform gyrus. This contrast revealed no statistically significant activation changes in the control group.
Contrasting relaxation to baseline revealed no significant activation-changes in the BPD group. In contrast, the control group showed statistically significant activation in the right temporal pole and the right orbitofrontal cortex (BA 11).
Functional imaging data: between-group comparisons
In the BPD compared with the control group, the trigger section yielded a significantly higher activation in the right medial occipital gyrus (z = 5.59; MNI coordinates, +48, −84, +9 mm). Patients with BPD showed significantly less activation in the left OFC (BA 11; z = 5.50; MNI coordinates, −33, +39, −15 mm) than controls in response to the section describing the emotional and cognitive reactions (see Fig. 3).
Fig. 3.
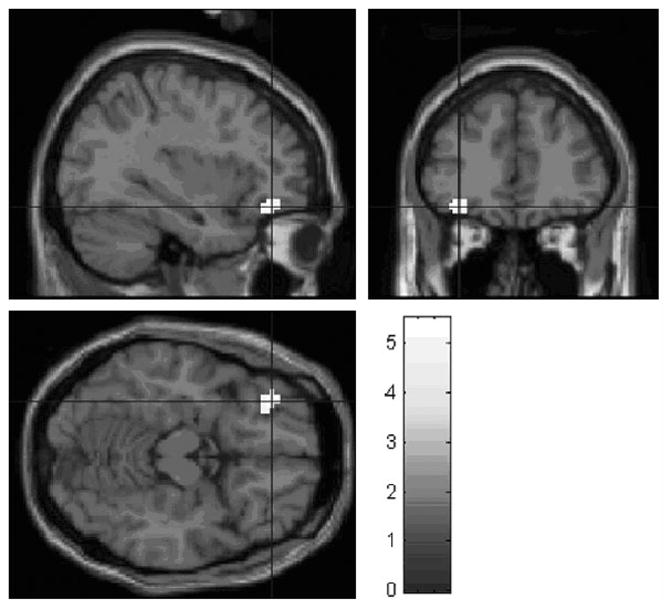
Significant BOLD signal decrease in left medial OFC (z =4.92; MNI coordinates −34 + 40 −14 mm) in the patients with BPD compared to the control group; reactions vs. neutral contrast. Bold signal data are superimposed on a single subject T1 template (SPM5; Wellcome Department of Cognitive Neurology, London, England) and displayed according to neurologic convention.
There were no significant group differences in the between-group contrasts during the SIB and the relaxation period.
Discussion
This study compared the neural activation patterns of patients with BPD and healthy controls in response to the script-driven imagery of SIB. In contrast to controls, patients with BPD showed a decrease of activation in the orbitofrontal cortex (OFC) while imagining the emotional and cognitive reactions to a stressful situation. Furthermore, only patients with BPD displayed an increase of activation in the dorsolateral prefrontal cortex (DLPFC) during this section. Imagining the self-injurious act itself elicited a significant decrease of activation in the mid-cingulate of patients with BPD, which was not found in control subjects. Patients reported increased stress levels throughout the script.
So far, SIB has been studied with psychometric instruments or psychophysiological measures only (3, 27, 28). To our knowledge, this study is among the first to investigate neural correlates of this key aspect of BPD psychopathology.
The most interesting finding of this study is the activation difference in OFC between patients with BPD and control subjects during the section describing the emotional and cognitive reactions to a situation triggering an act of SIB, which seems to be due to a stronger deactivation of the OFC in the patients with BPD. This deactivation may relate to a failure to inhibit or modulate their emotional or cognitive reactivity, which, in turn, may increase the urge for SIB as an alternative way to reduce their tension. However, this interpretation has to be taken with caution, since healthy controls do not show SIB and most probably do not feel an urge for it. Functional imaging studies in healthy humans, but also in psychiatric populations have linked deficits in response inhibition to anomalies of frontal lobe areas, especially the OFC (13, 39–41). These findings are of special importance considering the link between SIB, impulsivity and affective instability in BPD (6). In patients with impulsive–aggressive personality disorders, an abnormal function of the OFC was found in serotonergic challenge studies (42, 43) as well as an fMRI study by Coccaro et al. (44). In patients with BPD, an fMRI study by Vollm et al. (45) could demonstrate orbitofrontal dysfunction in connection with response inhibition. Considering these findings together with the observation of increased state- as well as trait-impulsivity in patients with BPD (8, 9), the attenuated OFC activity in the patients with BPD, observed in our study could indicate a dysfunction in impulse control in these patients. Failure of inhibition may directly lead to SIB, and SIB in this context, particularly in patients with BPD has been discussed as an impulsive form of self-injury (46).
Our findings of a dysfunction in the OFC are in accordance with the results of the study by Schmahl et al. (31), who found blunted orbitofrontal activation in response to trauma scripts in traumatized patients with BPD, compared to traumatized subjects without BPD. Similarities between this study and our own investigation include the use of script-driven imagery and the description of a situation triggering aversive feelings, which might explain the analogue results. In contrast to this, Beblo et al. (29) found an increase in OFC activity during script-driven imagination of unresolved life-events in patients with BPD. This discrepancy may be due to methodological differences. We employed scripts, whereas Beblo et al. (29) applied cue-driven imagery. As these authors also explicate, memory recall by cues might be determined primarily by internal processes and, thus, may enable subjects to exert control over induced emotions. By contrast, script-driven imagery provides primarily external cues, possibly rendering internal control more difficult. Attenuated orbitofrontal activity may indicate impairment in patients with BPD to initiate internal control processes when external stimulation predominates.
Furthermore, only patients with BPD but not control subjects displayed activation changes in the DLPFC. In response to the trigger situation, activation decreased in the middle frontal gyrus, including parts of BA 9, 10 and 46, whereas in response to the succeeding section describing the emotional and cognitive reactions activation increased in the frontopolar cortex. Response selection has been described as one of the primary functions of the DLPFC (47–49). The activation pattern in this area observed in our patients with BPD may be related to such executive processes. Whereas in the trigger situation sensory and cognitive processes might dominate, thus rendering response selection rather unimportant, the evaluation and selection of behavioral responses becomes relevant during the following section describing the emotional and cognitive responses to the stressful situation.
It should be taken into consideration that subjects are listening to a script and, thus, do not necessarily experience a situation triggering a personal urge to injure themselves. However, due to their personal experiences patients with BPD have more difficulties to distance themselves from the situation. This might initiate early processes of response evaluation and selection. Increased DLPFC activation in patients with BPD has also been reported in response to abuse scripts and scripts of abandonment (30, 31). Both studies confronted patients with BPD with the description of stressful situations, which would usually prompt response selection and, thus, can be compared with the DLPFC increase found in our study. Taken together, the pattern of DLPFC activation and OFC deactivation in our sample of patients with BPD could indicate early response selection and dysfunctional emotional control. The group difference may be related to the fact that the controls do not feel personally involved in the situation despite their vivid imagination and might not experience any emotional conflicts which would require impulse control or to the fact that the healthy controls are better at modulating their emotions.
A further interesting finding of this study is a deactivation in the posterior ACC during the description of SIB itself, which was only found in patients with BPD. Lack of significant between-group differences may be due to the limited sample size. The ACC is a large area that can be divided into the perigenual ACC, anterior midcingulate, and posterior midcingulate (50), which is considered to be part of the cognition division of the cingulate cortex. It is primarily involved in monitoring ongoing processing as well as evaluating the need for cognitive control (e.g. 51). A study by Ochsner et al. (52) investigated the neural bases of reappraisal, the authors found a positive correlation between ACC activation and successful reappraisal. The authors argued that successful reappraisal would depend upon monitoring for conflicts between initial emotional appraisals and cognitively restructured appraisals. Based on this assumption, the activation decrease in the ACC in our patients with BPD may indicate enhanced emotional involvement due to possible deficits in active monitoring, which may impede changing the emotional valence of the described situation. Besides, the ACC also plays a major role in pain responsiveness (for review, see 53, 54). In the context of a description of self-injury one might also speculate about ACC-mediated processes connected to pain perception in this group of patients with BPD with decreased activity possibly indicating changes in pain sensitivity. However, this interpretation can only be made with caution, as we could not assess pain perception for methodological reasons. Both factors, increased conflict monitoring and reduced pain sensitivity, could explain the change in ACC activation in these patients with BPD, but not in the sample of controls, since the latter have never experienced SIB. Although control subjects can imagine the situation vividly, they would probably neither develop an urge to react nor show any changes in pain processing. In agreement with these findings, a study by Schmahl et al. (30) has also reported decreased ACC activation in response to scripts of abandonment. An overlap between this study and our own investigation can be seen in the description of an extremely aversive situation, a condition also present in the different sections of our SIB-script.
There are several limitations to this study that should be considered. Most importantly, healthy controls do not experience SIB. Therefore activation differences may be due to differences in autobiographical memories or to differences in the urge to use SIB as a means to regulate their emotions. Second, we used fixed-effects analyses and thus the ability to generalize from the study sample to the larger population of patients with BPD is limited. However, in patient studies with relatively low sample size such as ours, fixed-effects analyses are generally accepted as a valid statistical approach, allowing to study new aspects in patient groups, where it is difficult to investigate large sample sizes. So far, conclusions can only be made for this sample and not for the whole population of borderline patients or they have to be drawn with caution. Third, we cannot determine whether our findings are related to SIB per se or to BPD features. This is also due to the fact that we used no control condition, e.g. a script of an accident or some other event as has been used in prior studies (e.g. 27). Fourth, it might have been helpful to include a low-level baseline condition like listening to a neutral story as a secondary comparison condition. This would have enabled us to analyze neutral and experimental conditions separately, thereby further clarifying the group-specific activation patterns associated with the different sections of the script. Fifth, due to the nature of the script, a single section could not be repeatedly presented, thus possibly diminishing the effect size of our results. The use of a standardized script necessarily neglects several individual triggers and methods of SIB, but it was a trade-off between individual responsivity and methodological limitations of the study. As it would have been extremely complicated to tailor individual scripts with exactly the same length for all patients, we decided to use a most prototypical event of SIB. Finally, it has to be mentioned that we only assessed white women with BPD of a narrow age range and that several co-occurrent disorders were excluded. This may also diminish the ability to generalize to a larger population. On the other hand, almost half of the patients fulfilled criteria for social phobia and PTSD. Due to limited sample size, subgroup analyses of patients with and without these two disorders were not possible. Despite these limitations, we believe that neural alterations in relation to disturbed impulse control and conflict monitoring in the context of SIB are of clinical importance in our understanding of BPD. Therefore, future studies will try to extend these findings to other groups of BPD patients. Also, we are currently conducting fMRI studies with designs which mirror SIB more closely than this script design.
Significant outcomes.
This is one of the first studies to investigate neural correlates of self-injurious behavior as a key aspect of borderline psychopathology.
Patients with borderline personality disorder (BPD) showed a decrease of activation in the orbitofrontal cortex and an increase of activation in the dorsolateral prefrontal cortex while imagining the reactions to a stressful situation.
Imagining the self-injurious act itself elicited a significant decrease of activation in the mid-cingulate of patients with BPD.
Limitations.
Fixed-effects analyses limit the ability to generalize from the study sample to the larger population of patients with borderline personality disorder (BPD).
Due to the study design, a single section of the script could not be repeatedly presented, thus possibly diminishing the effect size of our results.
As no control script was used, it cannot be determined whether our findings are related to self-injurious behavior (SIB) per se or to BPD features in general.
Acknowledgments
None.
Footnotes
Declaration of interest
None.
References
- 1.Mack J. Borderline states: A historical perspective. New York: Grune & Stratton; 1975. [Google Scholar]
- 2.Sansone RA, Wiederman MW, Sansone LA. The Self-Harm Inventory (SHI): development of a scale for identifying self-destructive behaviors and borderline personality disorder. J Clin Psychol. 1998;54:973–983. doi: 10.1002/(sici)1097-4679(199811)54:7<973::aid-jclp11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Chapman AL, Gratz KL, Brown MZ. Solving the puzzle of deliberate self-harm: the experiential avoidance model. Behav Res Ther. 2006;44:371–394. doi: 10.1016/j.brat.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Lieb K, Zanarini MC, Schmahl CG, Linehan MM, Bohus M. Borderline personality disorder. Lancet. 2004;364:459–461. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- 5.Stiglmayr CE, Grathwol T, Linehan MM, Ihorst G, Fahrenberg J, Bohus M. Aversive tension in patients with borderline personality disorder: a computer-based controlled field study. Acta Psychiatr Scand. 2005;111:372–379. doi: 10.1111/j.1600-0447.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- 6.Herpertz SC, Gretzer A, Steinmeyer EM, Muehlbauer V, Schuerkens A, Sass H. Affective instability and impulsivity in personality disorder. Results of an experimental study. J Affect Disord. 1997;44:31–37. doi: 10.1016/s0165-0327(97)01444-4. [DOI] [PubMed] [Google Scholar]
- 7.Herpertz SC, Werth U, Lukas G, et al. Emotion in criminal offenders with psychopathy and borderline personality disorder. Arch Gen Psychiatry. 2001;58:737–745. doi: 10.1001/archpsyc.58.8.737. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty DM, Bjork JM, Huckabee HCG, Moeller FG, Swann AC. Laboratory measures of aggression and impulsivity in women with borderline personality disorder. Psychiatry Res. 1999;85:315–326. doi: 10.1016/s0165-1781(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 9.Hochhausen NM, Lorenz AR, Newman JP. Specifying the impulsivity of female inmates with borderline personality disorder. J Abnorm Psychol. 2002;111:495–501. [PubMed] [Google Scholar]
- 10.Rinne T, Westenberg HGM, den Boer JA, van den Brink W. Serotonergic blunting to meta-chlorophenylpiperazine (m-CPP) highly correlates with sustained childhood abuse in impulsive and autoaggressive female borderline patients. Biol Psychiatry. 2000;47:548–556. doi: 10.1016/s0006-3223(99)00181-x. [DOI] [PubMed] [Google Scholar]
- 11.Juengling FD, Schmahl CG, Hesslinger B, et al. Positron emission tomography in female patients with Borderline personality disorder. J Psychiatr Res. 2003;37:109–115. doi: 10.1016/s0022-3956(02)00084-5. [DOI] [PubMed] [Google Scholar]
- 12.Soloff PH, Meltzer CC, Becker C, Greer PJ, Kelly TM, Constantine D. Impulsivity and prefrontal hypometabolism in borderline personality disorder. Psychiatry Res. 2003;123:153–163. doi: 10.1016/s0925-4927(03)00064-7. [DOI] [PubMed] [Google Scholar]
- 13.Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- 14.Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- 15.Berlin HA, Rolls ET, Iversen SD. Borderline personality disorder, impulsivity and the orbitofrontal cortex. Am J Psychiatry. 2005;162:2360–2373. doi: 10.1176/appi.ajp.162.12.2360. [DOI] [PubMed] [Google Scholar]
- 16.Grant JE, Correia S, Brennan-Krohn T, Malloy PF, Laidlaw DH, Schulz SC. Frontal white matter integrity in borderline personality disorder with self-injurious behavior. J Neuropsychiatry Clin Neurosci. 2007;19:383–390. doi: 10.1176/jnp.2007.19.4.383. [DOI] [PubMed] [Google Scholar]
- 17.Russ MJ, Roth SD, Lerman A, et al. Pain perception in self-injurious patients with borderline personality disorder. Biol Psychiatry. 1992;32:501–511. doi: 10.1016/0006-3223(92)90218-o. [DOI] [PubMed] [Google Scholar]
- 18.Ludaescher P, Bohus M, Lieb K, Philipsen A, Jochims A, Schmahl CG. Elevated pain thresholds correlate with dissociation and aversive arousal in patients with borderline personality disorder. Psychiatry Res. 2007;149:291–296. doi: 10.1016/j.psychres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Bohus M, Limberger MF, Ebner-Priemer UW, et al. Pain perception during self-reported distress and calmness in patients with borderline personality disorder and self-mutilating behavior. Psychiatry Res. 2000;95:251–260. doi: 10.1016/s0165-1781(00)00179-7. [DOI] [PubMed] [Google Scholar]
- 20.Ludaescher P, Greffrath W, Schmahl C, et al. A cross-sectional investigation of discontinuation of self-injury and normalizing pain perception in patients with borderline personality disorder. Acta Psychiatr Scand. doi: 10.1111/j.1600-0447.2008.01335.x. (in press) [DOI] [PubMed] [Google Scholar]
- 21.Schmahl CG, Bohus M, Esposito F, et al. Neural correlates of antinociception in borderline personality disorder. Arch Gen Psychiatry. 2006;63:659–667. doi: 10.1001/archpsyc.63.6.659. [DOI] [PubMed] [Google Scholar]
- 22.Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry. 1987;44:970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- 23.Pitman RK, Orr SP, Forgue DF, Altman B. Psychophysiologic responses to combat imagery of Vietnam veterans with posttraumatic stress disorder versus other anxiety disorders. J Abnorm Psychol. 1990;99:49–54. doi: 10.1037//0021-843x.99.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan TH, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanius RA, Williamson PC, Densmore M, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 26.Lanius RA, Williamson PC, Boksman K, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- 27.Haines J, Williams CL, Brain KL, Wilson GV. The psychophysiology of self-mutilation. J Abnorm Psychol. 1995;104:471–489. doi: 10.1037//0021-843x.104.3.471. [DOI] [PubMed] [Google Scholar]
- 28.Welch SS, Linehan MM, Sylvers P, Chittams J, Rizvi SL. Emotional responses to self-injury imagery among adults with borderline personality disorder. J Consult Clin Psychol. 2008;76:45–51. doi: 10.1037/0022-006X.76.1.45. [DOI] [PubMed] [Google Scholar]
- 29.Beblo T, Driessen M, Mertens M, et al. Functional MRI correlates of the recall of unresolved life events in borderline personality disorder. Psychol Med. 2006;36:845–856. doi: 10.1017/S0033291706007227. [DOI] [PubMed] [Google Scholar]
- 30.Schmahl CG, Elzinga BM, Vermetten E, Sanislow CA, McGlashan TH, Bremner JD. Neural correlates of memories of abandonment in women with and without borderline personality disorder. Biol Psychiatry. 2003;54:142–151. doi: 10.1016/s0006-3223(02)01720-1. [DOI] [PubMed] [Google Scholar]
- 31.Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. A positron emission tomography study of memories of childhood abuse in borderline personality disorder. Biol Psychiatry. 2004;55:759–765. doi: 10.1016/j.biopsych.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Loranger AW. International Personality Disorder Examination (IPDE): DSM-IV and ICD-10 modules. Odessa: Psychological Assessment Resources; 1999. [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for axis I DSM-IV disorders – patient edition (SCID-I/P) New York: American Psychiatric Press; 1995. [Google Scholar]
- 34.Bohus M, Limberger MF, Frank U, Sender I, Gratwohl T, Stieglitz RD. Development of the Borderline-Symptom-List. Psychother Psychosom Med Psychol. 2001;51:201–211. doi: 10.1055/s-2001-13281. [DOI] [PubMed] [Google Scholar]
- 35.Bohus M, Limberger MF, Frank U, Chapman AL, Kühler T, Stieglitz RD. Psychometric properties of the Borderline Symptom List (BSL) Psychopathology. 2007;40:126–132. doi: 10.1159/000098493. [DOI] [PubMed] [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Personality Disorders, (SCID-II) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 37.Stiglmayr CE, Braakmann D, Haaf B, Stieglitz RD, Bohus M. Development and characteristics of Dissociation-Tension-Scale acute (DSS-Acute) Psychother Psychosom Med Psychol. 2003;53:287–294. doi: 10.1055/s-2003-40495. [DOI] [PubMed] [Google Scholar]
- 38.Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- 39.Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 40.Altshuler LL, Bookheimer SY, Townsend J, et al. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Siever LJ, Buchsbaum MS, New AS, et al. d,l-fenfluramine response in impulsive personality disorder assessed with 18F fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology. 1999;20:413–423. doi: 10.1016/S0893-133X(98)00111-0. [DOI] [PubMed] [Google Scholar]
- 43.New SA, Hazlett EA, Buchsbaum MS, et al. Blunted prefrontal cortical 18flurorodeoxyglucose positron emission tomography response to meta-chlorphenylpiperazine in impulsive aggression. Arch Gen Psychiatry. 2002;59:621–629. doi: 10.1001/archpsyc.59.7.621. [DOI] [PubMed] [Google Scholar]
- 44.Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 45.Vollm B, Richardson P, Stirling J, et al. Neurobiological substrates of antisocial and borderline personality disorder: preliminary results of a functional fMRI study. Crim Behav Ment Health. 2004;14:39–54. doi: 10.1002/cbm.559. [DOI] [PubMed] [Google Scholar]
- 46.Simeon D, Hollander E. Assessment and treatment. Washington, DC: American Psychiatric Publishing; 2001. Self-injurious behaviors. [Google Scholar]
- 47.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 48.Nathaniel-James DA, Frith CD. The role of the dorsolateral prefrontal cortex: evidence from the effects of contextual constraint in a sentence completion task. Neuroimage. 2002;16:1094–1102. doi: 10.1006/nimg.2002.1167. [DOI] [PubMed] [Google Scholar]
- 49.Fleck MS, Daselaar SM, Dobbins IG, Cabeza R. Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cereb Cortex. 2006;16:1623–1630. doi: 10.1093/cercor/bhj097. [DOI] [PubMed] [Google Scholar]
- 50.Vogt BA, Berger GR, Derbyshire SWG. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 52.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 53.Davis KD. The neural circuitry of pain as explored with functional MRI. Neurol Res. 2000;22:313–317. doi: 10.1080/01616412.2000.11740676. [DOI] [PubMed] [Google Scholar]
- 54.Klossika I, Flor H, Kamping S, et al. Emotional modulation of pain: A clinical perspective. Pain. 2006;124:264–268. doi: 10.1016/j.pain.2006.08.007. [DOI] [PubMed] [Google Scholar]